Spheroids Generated from Malignant Pleural Effusion as a Tool to Predict the Response of Non-Small Cell Lung Cancer to Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures and Reagents
2.2. Patients
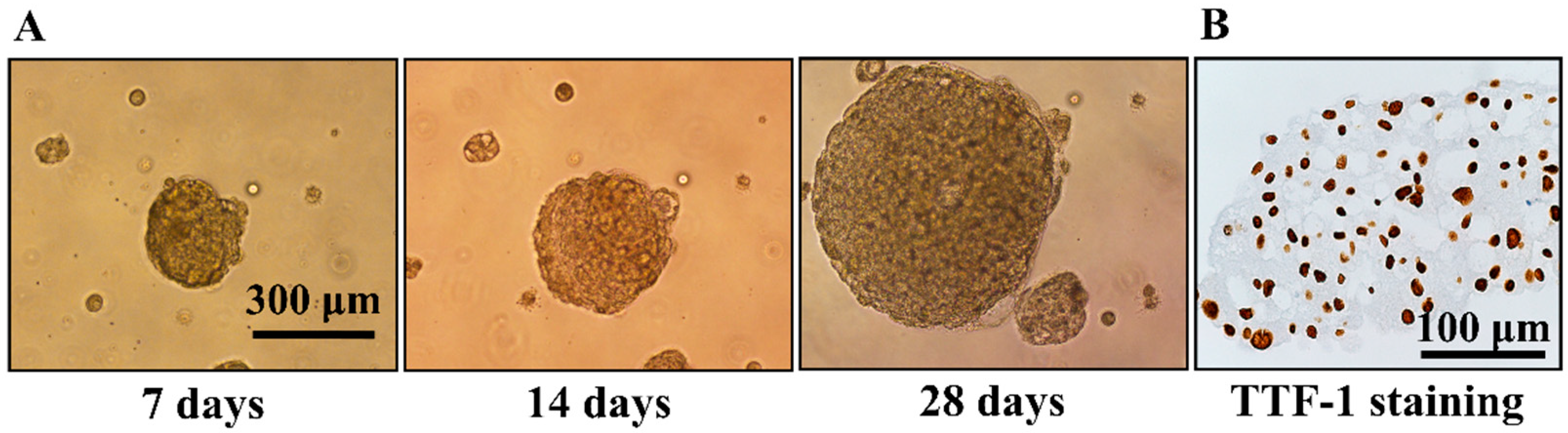
2.3. Generation of Multicellular Spheroids (Colony Formation Assay)
2.4. Immunohistochemistry Staining of TTF-1
2.5. Modified MTT Assay
2.6. Immunofluorescent Staining of Cleaved Caspase 3
3. Results
3.1. Generation of Multicellular Spheroids by NSCLC Cell Lines and Evaluation of Their Response to Gefitinib
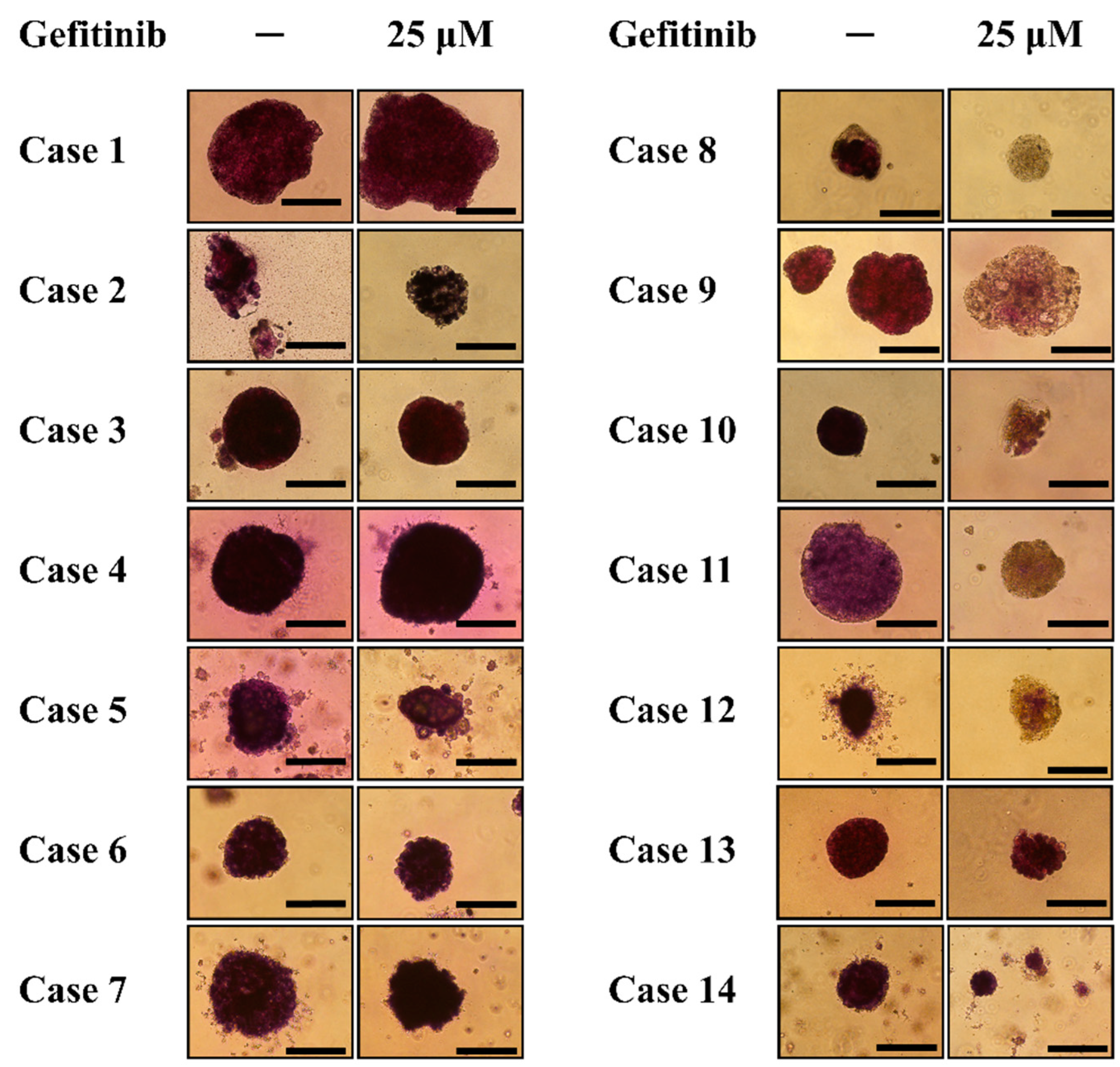
3.2. Patient Characteristics
3.3. Modified MTT Assay of Spheroids General from NSCLC-Related Malignant Pleural Effusion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Miao, D.; Zhao, J.; Han, Y.; Zhou, J.; Li, X.; Zhang, T.; Li, W.; Xia, Y. Management of locally advanced non-small cell lung cancer: State of the art and future directions. Cancer Commun. 2024, 44, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.; Kim, S.Y.; Cheng, H. Update 2020: Management of non-small cell lung cancer. Lung 2020, 198, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Bodor, J.N.; Boumber, Y.; Borghaei, H. Biomarkers for immune checkpoint inhibition in non-small cell lung cancer (nsclc). Cancer 2020, 126, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Tan, D.S.W. Targeted therapies for lung cancer patients with oncogenic driver molecular alterations. J. Clin. Oncol. 2022, 40, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Villalobos, P.; Wistuba, I.I. Lung cancer biomarkers. Hematol. Oncol. Clin. N. Am. 2017, 31, 13–29. [Google Scholar] [CrossRef]
- Soria, J.C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in untreated egfr-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Doroshow, D.B.; Sanmamed, M.F.; Hastings, K.; Politi, K.; Rimm, D.L.; Chen, L.; Melero, I.; Schalper, K.A.; Herbst, R.S. Immunotherapy in non-small cell lung cancer: Facts and hopes. Clin. Cancer Res. 2019, 25, 4592–4602. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J., Jr.; Wu, Y.L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W.; Loessner, D.; Rizzi, S.; Kaplan, D.L.; Mooney, D.J.; Clements, J.A. Can tissue engineering concepts advance tumor biology research? Trends Biotechnol. 2010, 28, 125–133. [Google Scholar] [CrossRef]
- Osswald, A.; Hedrich, V.; Sommergruber, W. 3d-3 tumor models in drug discovery for analysis of immune cell infiltration. Methods Mol. Biol. 2019, 1953, 151–162. [Google Scholar] [PubMed]
- Atat, O.E.; Farzaneh, Z.; Pourhamzeh, M.; Taki, F.; Abi-Habib, R.; Vosough, M.; El-Sibai, M. 3d modeling in cancer studies. Human Cell 2022, 35, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.K.; Veena, M.S.; Oh, S.; Huang, G.; Srivatsan, E.; Huang, M.; Sharma, S.; Batra, R.K. The malignant pleural effusion as a model to investigate intratumoral heterogeneity in lung cancer. PLoS ONE 2009, 4, e5884. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Liu, Z.; Sa, J.K.; Shin, S.; Wang, J.; Bordyuh, M.; Cho, H.J.; Elliott, O.; Chu, T.; Choi, S.W.; et al. Pharmacogenomic landscape of patient-derived tumor cells informs precision oncology therapy. Nat. Genet. 2018, 50, 1399–1411. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.; Kuraguchi, M.; Xu, M.; Portell, A.J.; Taus, L.; Diala, I.; Lalani, A.S.; Choi, J.; Chambers, E.S.; Li, S.; et al. Use of ex vivo patient-derived tumor organotypic spheroids to identify combination therapies for her2 mutant non-small cell lung cancer. Clin. Cancer Res. 2020, 26, 2393–2403. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.C.; Jin, X.; Wang, Y.; Wang, K. Role of epidermal growth factor receptor in lung cancer and targeted therapies. Am. J. Cancer Res. 2017, 7, 187–202. [Google Scholar] [PubMed]
- Ziogas, D.C.; Tsiara, A.; Tsironis, G.; Lykka, M.; Liontos, M.; Bamias, A.; Dimopoulos, M.A. Treating alk-positive non-small cell lung cancer. Ann. Transl. Med. 2018, 6, 141. [Google Scholar] [CrossRef] [PubMed]
- VanderLaan, P.A.; Rangachari, D.; Majid, A.; Parikh, M.S.; Gangadharan, S.P.; Kent, M.S.; McDonald, D.C.; Huberman, M.S.; Kobayashi, S.S.; Costa, D.B. Tumor biomarker testing in non-small-cell lung cancer: A decade of change. Lung Cancer 2018, 116, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Tan, E.H.; O’Byrne, K.; Zhang, L.; Hirsh, V.; Boyer, M.; Yang, J.C.; Mok, T.; Lee, K.H.; Lu, S.; et al. Afatinib versus gefitinib in patients with egfr mutation-positive advanced non-small-cell lung cancer: Overall survival data from the phase iib lux-lung 7 trial. Ann. Oncol. 2017, 28, 270–277. [Google Scholar] [CrossRef]
- Park, K.; Tan, E.H.; O’Byrne, K.; Zhang, L.; Boyer, M.; Mok, T.; Hirsh, V.; Yang, J.C.; Lee, K.H.; Lu, S.; et al. Afatinib versus gefitinib as first-line treatment of patients with egfr mutation-positive non-small-cell lung cancer (lux-lung 7): A phase 2b, open-label, randomised controlled trial. Lancet Oncol. 2016, 17, 577–589. [Google Scholar] [CrossRef]
- Ahn, M.J.; Tsai, C.M.; Shepherd, F.A.; Bazhenova, L.; Sequist, L.V.; Hida, T.; Yang, J.C.H.; Ramalingam, S.S.; Mitsudomi, T.; Janne, P.A.; et al. Osimertinib in patients with t790m mutation-positive, advanced non-small cell lung cancer: Long-term follow-up from a pooled analysis of 2 phase 2 studies. Cancer 2019, 125, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.L.; Lin, J.J.; Shaw, A.T. Alk-positive lung cancer: A moving target. Nat. Cancer 2023, 4, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.J.; Mok, T.; Kim, D.W.; Wu, Y.L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; et al. First-line crizotinib versus chemotherapy in alk-positive lung cancer. N. Engl. J. Med. 2014, 371, 2167–2177. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.C.; Tan, D.S.W.; Chiari, R.; Wu, Y.L.; Paz-Ares, L.; Wolf, J.; Geater, S.L.; Orlov, S.; Cortinovis, D.; Yu, C.J.; et al. First-line ceritinib versus platinum-based chemotherapy in advanced alk-rearranged non-small-cell lung cancer (ascend-4): A randomised, open-label, phase 3 study. Lancet 2017, 389, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.W.; Ou, S.I.; Perol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib versus crizotinib in untreated alk-positive non-small-cell lung cancer. N. Engl. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Kim, H.R.; Ahn, M.J.; Yang, J.C.; Han, J.Y.; Lee, J.S.; Hochmair, M.J.; Li, J.Y.; Chang, G.C.; Lee, K.H.; et al. Brigatinib versus crizotinib in alk-positive non-small-cell lung cancer. N. Engl. J. Med. 2018, 379, 2027–2039. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Bauer, T.M.; de Marinis, F.; Felip, E.; Goto, Y.; Liu, G.; Mazieres, J.; Kim, D.W.; Mok, T.; Polli, A.; et al. First-line lorlatinib or crizotinib in advanced alk-positive lung cancer. N. Engl. J. Med. 2020, 383, 2018–2029. [Google Scholar] [CrossRef]
- So, W.V.; Dejardin, D.; Rossmann, E.; Charo, J. Predictive biomarkers for pd-1/pd-l1 checkpoint inhibitor response in nsclc: An analysis of clinical trial and real-world data. J. Immunother. Cancer 2023, 11, e006464. [Google Scholar] [CrossRef] [PubMed]
- Gompelmann, D.; Sarova, P.; Mosleh, B.; Papaporfyriou, A.; Oberndorfer, F.; Idzko, M.; Hoda, M.A. Pd-l1 assessment in lung cancer biopsies-pitfalls and limitations. Int. J. Biol. Markers 2024, 39, 3–8. [Google Scholar] [CrossRef]
- Addeo, A.; Friedlaender, A.; Banna, G.L.; Weiss, G.J. Tmb or not tmb as a biomarker: That is the question. Crit. Rev. Oncol. Hematol. 2021, 163, 103374. [Google Scholar] [CrossRef]
- Di Liello, R.; Ciaramella, V.; Barra, G.; Venditti, M.; Della Corte, C.M.; Papaccio, F.; Sparano, F.; Viscardi, G.; Iacovino, M.L.; Minucci, S.; et al. Ex vivo lung cancer spheroids resemble treatment response of a patient with nsclc to chemotherapy and immunotherapy: Case report and translational study. ESMO Open 2019, 4, e000536. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Okami, J.; Okuyama, H.; Kumagai, T.; Uchida, J.; Kondo, J.; Takehara, T.; Nishizawa, Y.; Imamura, F.; Higashiyama, M.; et al. Spheroid culture of primary lung cancer cells with neuregulin 1/her3 pathway activation. J. Thorac. Oncol. 2013, 8, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Taverna, J.A.; Hung, C.N.; Williams, M.; Williams, R.; Chen, M.; Kamali, S.; Sambandam, V.; Chiu, C.H.-L.; Osmulski, P.A.; Gaczynska, M.E.; et al. Ex vivo drug testing of patient-derived lung organoids to predict treatment responses for personalized medicine. Lung Cancer 2024, 190, 107533. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Renna, M.; Park, S.J.; Menzies, F.M.; Ricketts, T.; Fullgrabe, J.; Ashkenazi, A.; Frake, R.A.; Lombarte, A.C.; Bento, C.F.; et al. Contact inhibition controls cell survival and proliferation via yap/taz-autophagy axis. Nat. Commun. 2018, 9, 2961. [Google Scholar] [CrossRef] [PubMed]
- Borowicz, S.; Van Scoyk, M.; Avasarala, S.; Rathinam, M.K.K.; Tauler, J.; Bikkavilli, R.K.; Winn, R.A. The soft agar colony formation assay. J. Vis. Exp. 2014, 92, e51998. [Google Scholar]
- van Tonder, A.; Joubert, A.M.; Cromarty, A.D. Limitations of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2h-tetrazolium bromide (mtt) assay when compared to three commonly used cell enumeration assays. BMC Res. Notes 2015, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Huyck, L.; Ampe, C.; Van Troys, M. The xtt cell proliferation assay applied to cell layers embedded in three-dimensional matrix. Assay Drug Dev. Technol. 2012, 10, 382–392. [Google Scholar] [CrossRef]
- Rolver, M.G.; Elingaard-Larsen, L.O.; Pedersen, S.F. Assessing cell viability and death in 3d spheroid cultures of cancer cells. J. Vis. Exp. 2019, 148, e59714. [Google Scholar]



| Case | Gender | Age | Cell Type | EGFR Mutation | TKI Treatment | Response to TKI |
|---|---|---|---|---|---|---|
| 1 | Female | 80.6 | Adenocarcinoma | Wild type | No | Not Applicable |
| 2 | Male | 82.7 | Adenocarcinoma | Wild type | No | Not Applicable |
| 3 | Male | 61.0 | Adenocarcinoma | Wild type | Erlotinib | Disease progression |
| 4 | Female | 79.0 | Adenocarcinoma | Wild type | No | Not Applicable |
| 5 | Female | 49.6 | Adenocarcinoma | Wild type | No | Not Applicable |
| 6 | Male | 63.0 | Adenocarcinoma | Wild type | No | Not Applicable |
| 7 | Female | 74.5 | Adenocarcinoma | Exon 20 insertion | No | Not Applicable |
| 8 | Male | 77.6 | Adenocarcinoma | L861Q | Afatinib | Partial response |
| 9 | Male | 53.5 | Adenocarcinoma | L858R | Erlotinib | Partial response |
| 10 | Female | 77.9 | Adenocarcinoma | L858R | Erlotinib | Partial response |
| 11 | Male | 59.2 | Adenocarcinoma | L858R | Gefitinib | Partial response |
| 12 | Male | 80.4 | Adenocarcinoma | L858R | Afatinib | Partial response |
| 13 | Female | 73.6 | Adenocarcinoma | Exon 19 deletion | Erlotinib | Disease progression |
| 14 | Female | 82.4 | Adenocarcinoma | Exon 19 deletion | Gefitinib | Disease progression |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.-M.; Fang, Y.-H.; Lin, C.-M.; Chen, M.-F.; Lin, C.-L. Spheroids Generated from Malignant Pleural Effusion as a Tool to Predict the Response of Non-Small Cell Lung Cancer to Treatment. Diagnostics 2024, 14, 998. https://doi.org/10.3390/diagnostics14100998
Yang T-M, Fang Y-H, Lin C-M, Chen M-F, Lin C-L. Spheroids Generated from Malignant Pleural Effusion as a Tool to Predict the Response of Non-Small Cell Lung Cancer to Treatment. Diagnostics. 2024; 14(10):998. https://doi.org/10.3390/diagnostics14100998
Chicago/Turabian StyleYang, Tsung-Ming, Yu-Hung Fang, Chieh-Mo Lin, Miao-Fen Chen, and Chun-Liang Lin. 2024. "Spheroids Generated from Malignant Pleural Effusion as a Tool to Predict the Response of Non-Small Cell Lung Cancer to Treatment" Diagnostics 14, no. 10: 998. https://doi.org/10.3390/diagnostics14100998






