Improving the Corrosion Resistance of Micro-Arc Oxidization Film on AZ91D Mg Alloy through Silanization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of MAO Film and Sealing Treatment
2.2. Electrochemical Tests
2.3. Salt Spray, Contact Angle Test, and Surface Characterization
3. Results
3.1. Contact Angle of MAO-Silanization Composite Film
3.2. Micro-Morphology
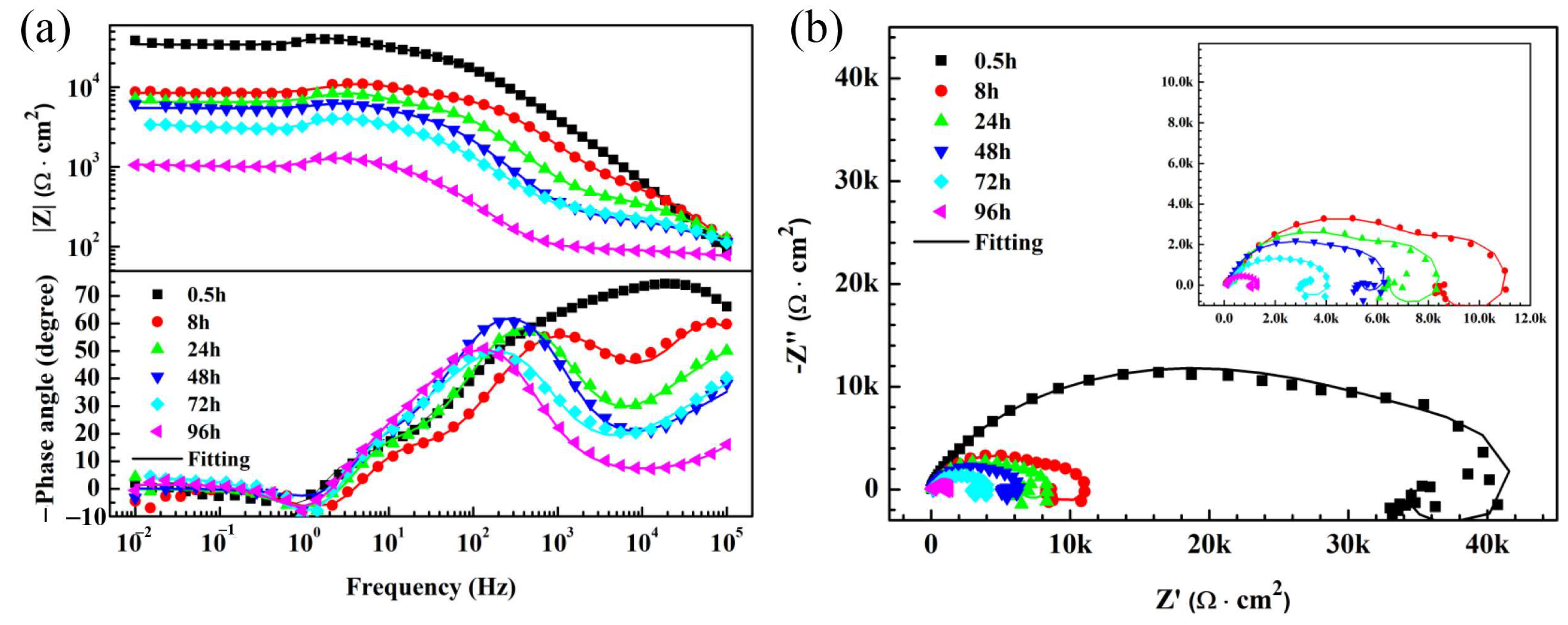
3.3. Electrochemical Measurements
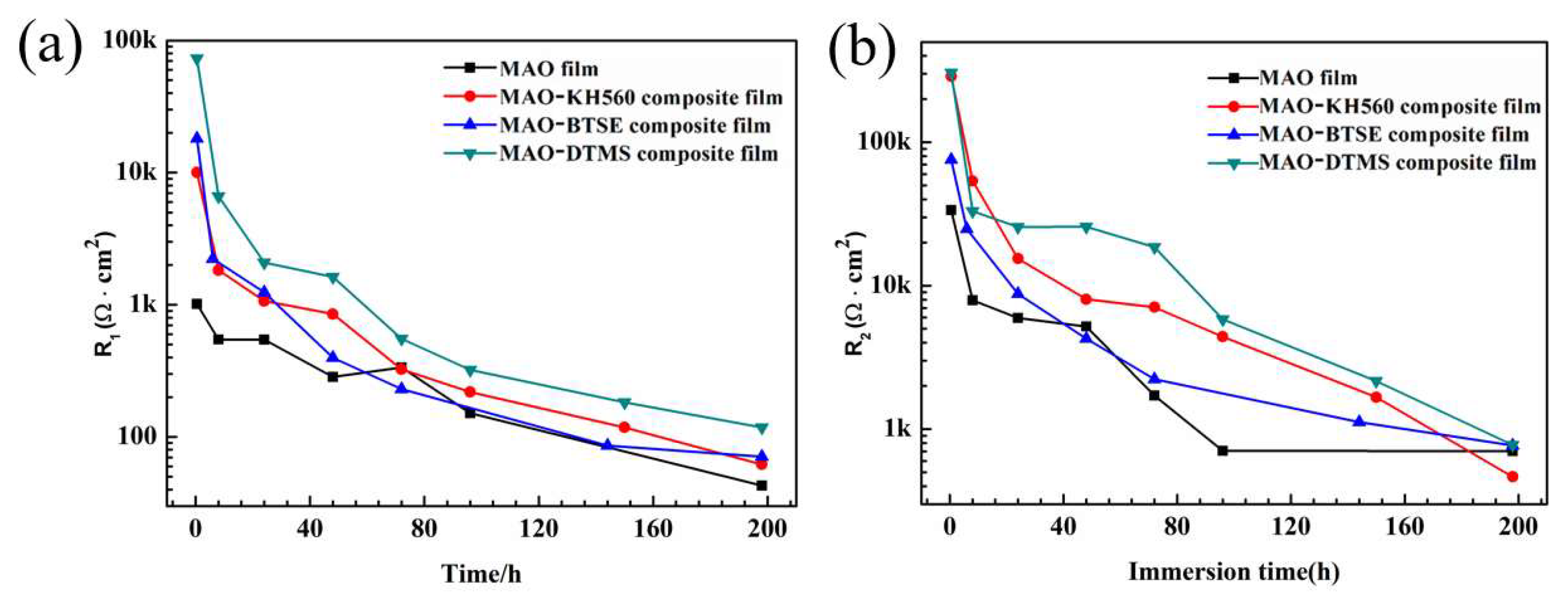
3.4. Long-Term Immersion Experiments
3.5. Long-Term Salt Spray Experiments
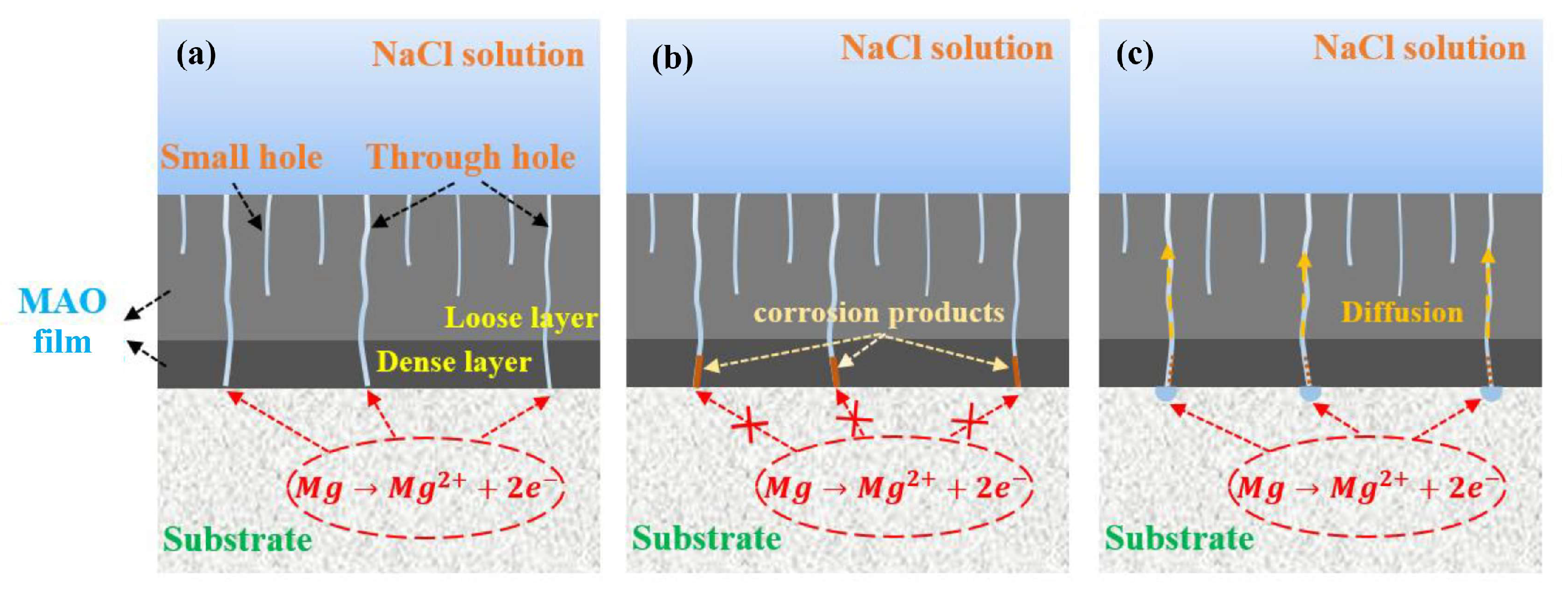
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, X.; Zhang, Q.; Mei, D.; Liu, M.; Wang, L.; Zhu, S.; Guan, S. The uniform corrosion of biodegradable Mg alloy induced by protein addition in Hanks’ balanced salt solution. Colloids Surf. A 2024, 690, 133824. [Google Scholar] [CrossRef]
- Song, Y.; Dai, J.; Sun, S. A comparative study on the corrosion behavior of AZ80 and EW75 Mg alloys in industrial atmospheric environment. Mater. Today Commun. 2024, 38, 108263. [Google Scholar] [CrossRef]
- Wu, M.; Jiang, F. Preparation, interface properties and corrosion behavior of nano-modified MAO ceramic film on 5B70 Al alloy. J. Alloys Compd. 2023, 967, 171829. [Google Scholar] [CrossRef]
- He, R.Y.; Wang, B.Y.; Xiang, J.H.; Pan, T.J. Effect of copper additive on microstructure and anti-corrosion performance of black MAO films grown on AZ91 alloy and coloration mechanism. J. Alloys Compd. 2021, 889, 161501. [Google Scholar] [CrossRef]
- Gong, Y.; Geng, J.; Huang, J.; Chen, Z.; Wang, M.; Chen, D.; Wang, H. Self-healing performance and corrosion resistance of novel CeO2-sealed MAO film on aluminum alloy. Surf. Coat. Tech. 2021, 417, 127208. [Google Scholar] [CrossRef]
- Lee, H.-B.; Sheu, H.-H.; Jian, J.-S.; Hsiao, R.-C. Study on the Characteristics of MAO/Polymer/Ni Three-Layer Composite Film formed on AZ31 Magnesium Alloy. Int. J. Electrochem. Sci. 2021, 16, 211246. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Lee, J.-L.; Jian, S.-Y.; Chen, C.-A.; Aktug, S.L.; Ger, M.-D. The effect of fluoride on the formation of an electroless Ni–P plating film on MAO-coated AZ31B magnesium alloy. J. Mater. Res. Technol. 2022, 19, 542–556. [Google Scholar] [CrossRef]
- Zhu, J.; Jia, H.; Liao, K.; Li, X. Improvement on corrosion resistance of micro-arc oxidized AZ91D magnesium alloy by a pore-sealing coating. J. Alloys Compd. 2021, 889, 161460. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Si, N.-C.; Xia, Y.-P.; Li, L. Influence of nano-SiC on microstructure and property of MAO coating formed on AZ91D magnesium alloy. Trans. Nonferrous Met. Soc. China 2015, 25, 1926–1934. [Google Scholar] [CrossRef]
- Cui, L.; Wang, Y.; Hu, L.; Gao, L.; Du, B.; Wei, Q. Mechanism of Pb (II) and methylene blue adsorption onto magnetic carbonate hydroxyapatite/graphene oxide. RSC Adv. 2015, 5, 9759–9770. [Google Scholar] [CrossRef]
- Liu, R.; Xu, D.; Liu, Y.; Wu, L.; Yong, Q.; Xie, Z.-H. Enhanced corrosion protection for MAO coating on magnesium alloy by the synergism of LDH doping with deposition of 8HQ inhibitor film. Ceram. Int. 2023, 49, 30039–30048. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, S.; Wang, Q.; Ramachandran, C.S. Effect of hydrothermal treatment on the surface characteristics and bioactivity of HAP based MAO coating on Ti-6Al-4V alloy. Surf. Coat. Tech. 2023, 464, 129566. [Google Scholar] [CrossRef]
- Ma, F.C.; Liu, P.; Chen, Y.; Li, W.; Liu, X.K.; Chen, X.H.; He, D.H. Various Morphologies Hydroxyapatite Crystals on Ti MAO Film Prepared by Hydrothermal Treatment. Phys. Procedia 2013, 50, 442–448. [Google Scholar] [CrossRef]
- Chen, C.-A.; Jian, S.-Y.; Lu, C.-H.; Lee, C.-Y.; Aktuğ, S.L.; Ger, M.-D. Evaluation of microstructural effects on corrosion behavior of AZ31B magnesium alloy with a MAO coating and electroless Ni-P plating. J. Mater. Res. Technol. 2020, 9, 13902–13913. [Google Scholar] [CrossRef]
- Li, W.; Tian, A.; Li, T.; Zhao, Y.; Chen, M. Ag/ZIF-8/Mg-Al LDH composite coating on MAO pretreated Mg alloy as a multi-ion-release platform to improve corrosion resistance, osteogenic activity, and photothermal antibacterial properties. Surf. Coat. Tech. 2023, 464, 129555. [Google Scholar] [CrossRef]
- Yang, W.; Gao, Y.; Xu, D.; Zhao, J.; Ke, P.; Wang, A. Bactericidal abilities and in vitro properties of diamond-like carbon films deposited onto MAO-treated titanium. Mater. Lett. 2019, 244, 155–158. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, X.; Jiang, Y.; Yang, L.; Liu, H.H.; Song, Z. Development and characterization of MAO/PLA-nHA nanocomposite coatings on pure zinc for orthopedic applications. Surf. Coat. Tech. 2024, 478, 130452. [Google Scholar] [CrossRef]
- Cao, H.; Fang, M.; Jia, W.; Liu, X.; Xu, Q. Remarkable improvement of corrosion resistance of silane composite coating with Ti3C2Tx MXene on copper. Compos. Part B 2022, 228, 109427. [Google Scholar] [CrossRef]
- Huang, J.; Dun, Y.; Wan, Q.; Wu, Z.; Zhao, X.; Tang, Y.; Zhang, X.; Zuo, Y. Improved corrosion resistance of MAO coating on Mg-Li alloy by RGO modified silanization. J. Alloys Compd. 2022, 929, 167283. [Google Scholar] [CrossRef]
- Han, J.; Liu, E.; Zhou, Y.; Zhao, S.; Yan, H.; Hu, C.; Kang, J.; Han, Q.; Su, Y. Robust superhydrophobic film on aluminum alloy prepared with TiO2/SiO2-silane composite film for efficient self-cleaning, anti-corrosion and anti-icing. Mater. Today Commun. 2023, 34, 105085. [Google Scholar] [CrossRef]
- Chen, X.; Li, G.; Lian, J.; Jiang, Q. Study of the formation and growth of tannic acid based conversion coating on AZ91D magnesium alloy. Surf. Coat. Tech. 2009, 204, 736–747. [Google Scholar] [CrossRef]
- Mandelli, A.; Bestetti, M.; Da Forno, A.; Lecis, N.; Trasatti, S.; Trueba, M. A composite coating for corrosion protection of AM60B magnesium alloy. Surf. Coat. Tech. 2011, 205, 4459–4465. [Google Scholar] [CrossRef]
- ASTM-B117; Standard Practice for Operating Salt Spray (Fog) Apparatus. ASTM Standard: West Conshohocken, PA, USA, 2019.
- Shi, C.; Zhang, L.; Zhao, J.; Tian, L.; Wang, S.; Liu, X.; Liu, G.; Shao, Y. Characterization and performance of organic-inorganic composite zinc phosphate with nano-sheet structure synthesized by a composite reaction of sol-gel with silane modification. Surf. Interfaces 2024, 47, 104225. [Google Scholar] [CrossRef]
- Wang, G.; Guo, L.; Ruan, Y.; Zhao, G.; Zhang, X.; Liu, Y.; Kim, D.-E. Improved wear and corrosion resistance of alumina alloy by MAO and PECVD. Surf. Coat. Tech. 2024, 479, 130556. [Google Scholar] [CrossRef]
- Yang, C.; Wang, C.; Zhao, X.; Shen, Z.; Wen, M.; Zhao, C.; Sheng, L.; Wang, Y.; Xu, D.; Zheng, Y.; et al. Superhydrophobic surface on MAO-processed AZ31B alloy with zinc phosphate nanoflower arrays for excellent corrosion resistance in salt and acidic environments. Mater. Des. 2024, 239, 112769. [Google Scholar] [CrossRef]
- Pinto, R.; Carmezim, M.J.; Ferreira, M.G.S.; Montemor, M.F. A two-step surface treatment, combining anodisation and silanisation, for improved corrosion protection of the Mg alloy WE54. Prog. Org. Coat. 2010, 69, 143–149. [Google Scholar] [CrossRef]
- Yang, Z.; Che, J.; Zhang, Z.; Yu, L.; Hu, M.; Sun, W.; Gao, W.; Fan, J.; Wang, L.; Liu, G. High-efficiency graphene/epoxy composite coatings with outstanding thermal conductive and anti-corrosion performance. Compos. Part A 2024, 181, 108152. [Google Scholar] [CrossRef]
- Wang, S.; Ma, X.; Bai, J.; Niu, J.; Ma, R.; Du, A.; Zhao, X.; Fan, Y.; Li, G. Study on the structure and corrosion behavior of hot-dipped Zn–6Al–3Mg alloy coating in chlorine-containing environment. Corros. Sci. 2024, 231, 112001. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Deng, Y.Z.; Shao, Y.W.; Wang, F.H. New sealing treatment of microarc oxidation coating. Surf. Eng. 2014, 30, 31–35. [Google Scholar] [CrossRef]
- Yang, H.; Li, S.; Liang, Z. Anodized oxidative electrosynthesis of magnesium silicate whiskers. Int. J. Electrochem. Sci. 2013, 8, 9332–9337. [Google Scholar] [CrossRef]
- Meng, G.; Dou, B.; Zhang, T.; Liu, B.; Wang, F. Growth Behaviors of Layered Double Hydroxide on Microarc Oxidation Film and Anti-Corrosion Performances of the Composite Film. J. Electrochem. Soc. 2016, 163, C917. [Google Scholar]
- Zanotto, F.; Grassi, V.; Frignani, A.; Zucchi, F. Protection of the AZ31 magnesium alloy with cerium modified silane coatings. Mater. Chem. Phys. 2011, 129, 1–8. [Google Scholar] [CrossRef]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Yin, H.; Xu, Z.; Shao, Y.; Wang, Y. Improving the Corrosion Resistance of Micro-Arc Oxidization Film on AZ91D Mg Alloy through Silanization. Metals 2024, 14, 569. https://doi.org/10.3390/met14050569
Liu J, Yin H, Xu Z, Shao Y, Wang Y. Improving the Corrosion Resistance of Micro-Arc Oxidization Film on AZ91D Mg Alloy through Silanization. Metals. 2024; 14(5):569. https://doi.org/10.3390/met14050569
Chicago/Turabian StyleLiu, Junchi, Hang Yin, Zhengyi Xu, Yawei Shao, and Yanqiu Wang. 2024. "Improving the Corrosion Resistance of Micro-Arc Oxidization Film on AZ91D Mg Alloy through Silanization" Metals 14, no. 5: 569. https://doi.org/10.3390/met14050569




