Influence of Ti Vacancy Defects on the Dissolution of O-Adsorbed Ti(0001) Surface: A First-Principles Study
Abstract
:1. Introduction
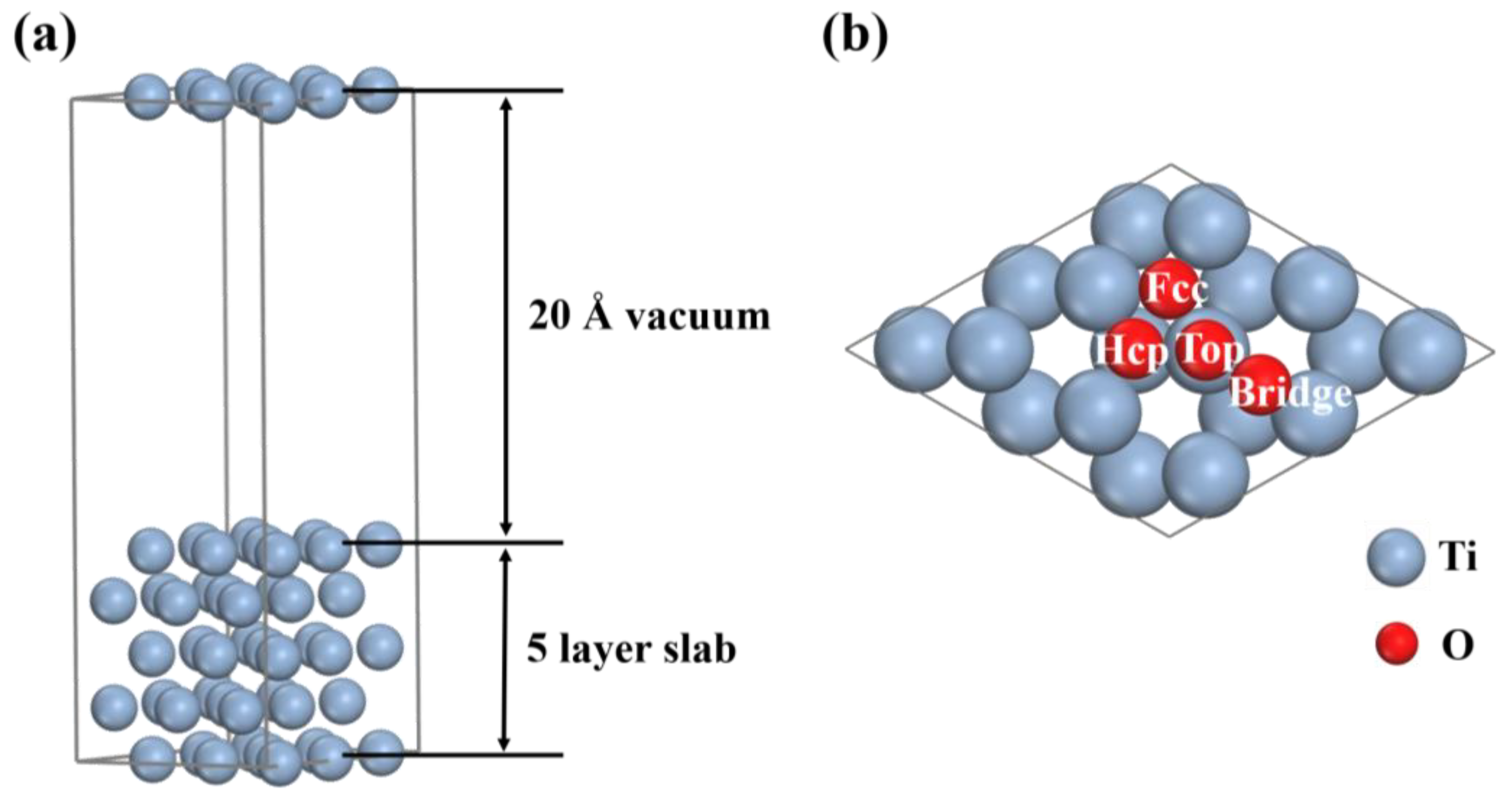
2. Computational Details
3. Results and Discussion
3.1. Adsorption Behavior of O Atom on the Ti(0001) Surface
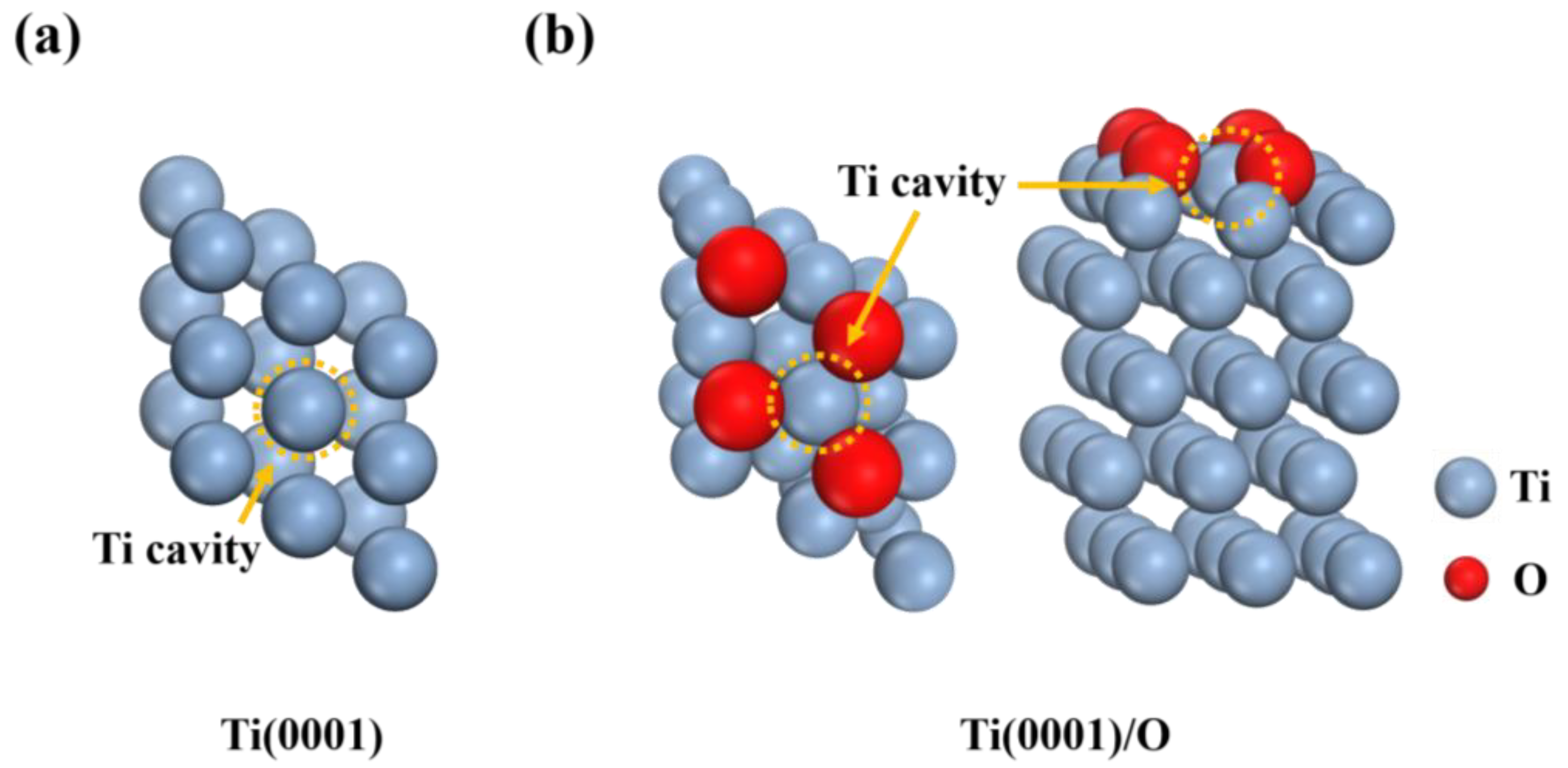
3.2. Ti Vacancy Formation Energy
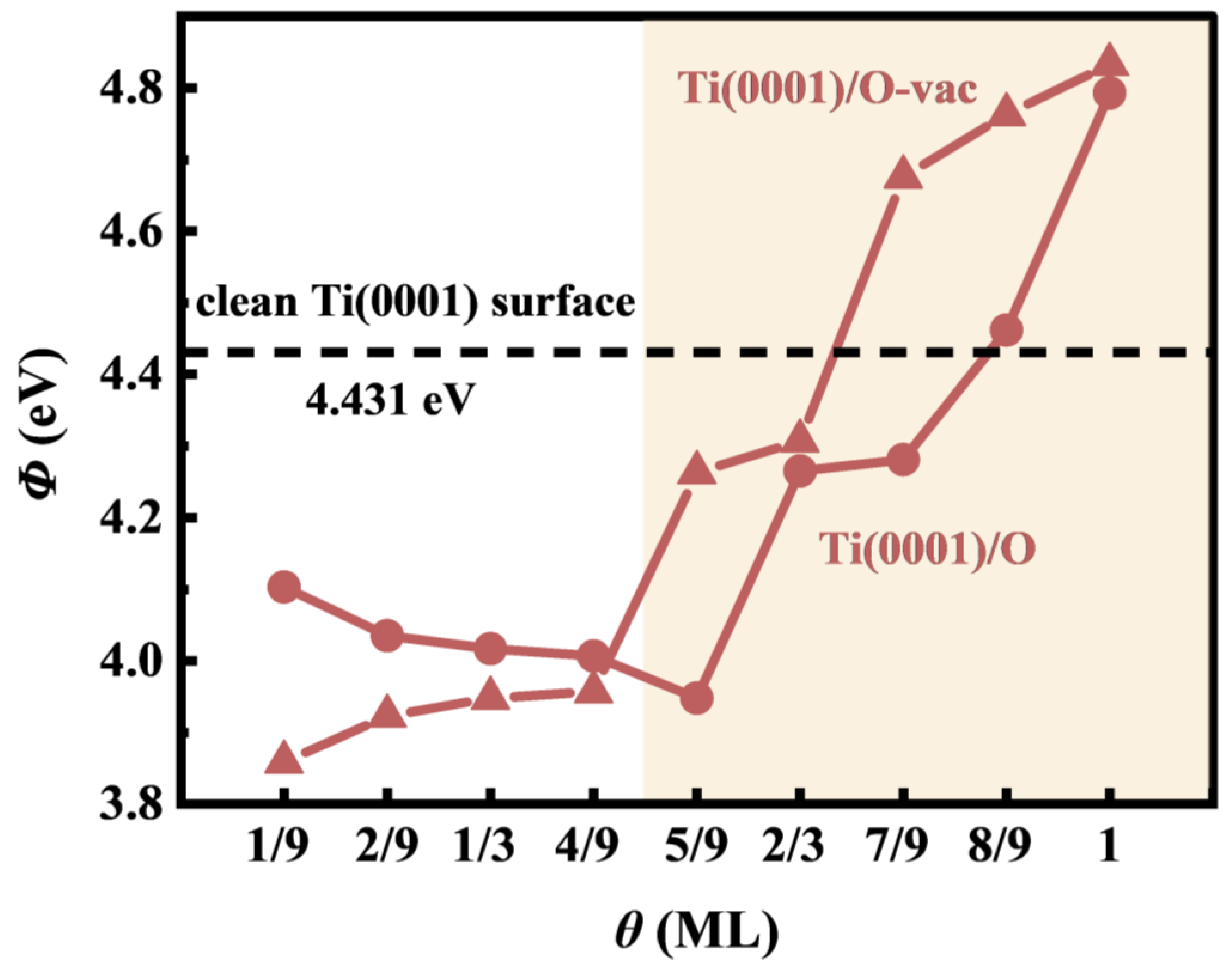
3.3. Electrochemical Potential Shift
3.4. Geometric Structures
3.5. Electronic Structures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Uwanyuze, R.S.; Alpay, S.P.; Schaffoner, S.; Sahoo, S. A first principles analysis of oxidation in titanium alloys with aluminum and vanadium. Surf. Sci. 2022, 719, 122026. [Google Scholar] [CrossRef]
- Cometa, S.; Bonifacio, M.A.; Mattioli-Belmonte, M.; Sabbatini, L.; De Giglio, E. Electrochemical Strategies for Titanium Implant Polymeric Coatings: The Why and How. Coatings 2019, 9, 268. [Google Scholar] [CrossRef]
- Huang, C.H.; Chiang, S.Y.V.; Gawkrodger, D.J. The contribution of metal allergy to the failure of metal alloy implants, with special reference to titanium: Current knowledge and controversies. Contact Dermat. 2024, 90, 201–210. [Google Scholar] [CrossRef]
- Alqahtani, A.R.; Gufran, K.; Silva, F.; Rocha, M.G.; Chang, J. A Clinical Case Report of a Potential Acute Allergic Reaction with Titanium Dental Implant. Case Rep. Dent. 2021, 2021, 5592934. [Google Scholar] [CrossRef]
- Chen, L.; Tong, Z.; Luo, H.K.; Qu, Y.; Gu, X.H.; Si, M.S. Titanium particles in peri-implantitis: Distribution, pathogenesis and prospects. Int. J. Oral Sci. 2023, 15, 49. [Google Scholar] [CrossRef]
- Watanabe, M.; Liu, L.P.; Ichikawa, T. Are Allergy-Induced Implant Failures Actually Hypersensitivity Reactions to Titanium? A Literature Review. Dent. J. 2023, 11, 263. [Google Scholar] [CrossRef]
- Kligman, S.; Ren, Z.; Chung, C.H.; Perillo, M.A.; Chang, Y.C.; Koo, H.; Zheng, Z.; Li, C.S. The Impact of Dental Implant Surface Modifications on Osseointegration and Biofilm Formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef]
- Xie, T.; Yang, Y.; Wang, J.Y.; Ying, T.; Xu, Z.T.; Zeng, X.Q. First-Principles Study of F and H Adsorption on Corrosion Resistance of Titanium Bipolar Plates. Metall. Mater. Trans. A 2023, 54, 3412–3416. [Google Scholar] [CrossRef]
- Tshwane, D.M.; Modiba, R.; Govender, G.; Ngoepe, P.E.; Chauke, H.R. The adsorption of halogen molecules on Ti (110) surface. J. Mater. Res. 2021, 36, 592–601. [Google Scholar] [CrossRef]
- Sahoo, S.; Alpay, S.P.; Hebert, R.J. Surface phase diagrams of titanium in Oxygen, Nitrogen and Hydrogen environments: A first principles analysis. Surf. Sci. 2018, 677, 18–25. [Google Scholar] [CrossRef]
- Greeley, J.; Norskov, J.K. Electrochemical dissolution of surface alloys in acids: Thermodynamic trends from first-principles calculations. Electrochim. Acta 2007, 52, 5829–5836. [Google Scholar] [CrossRef]
- Ma, Y.G.; Balbuena, P.B. Surface properties and dissolution trends of Pt3M alloys in the presence of adsorbates. J. Phys. Chem. C. 2008, 112, 14520–14528. [Google Scholar] [CrossRef]
- Wang, X.T.; Xie, D.; Wei, L.J.; You, D.; Hou, M.X.; Leng, Y.X. DFT investigation of the dissolution trends of NiTi alloys with the B2 and B19′ phases during the initial oxidation stage. Phys. Chem. Chem. Phys. 2023, 25, 19804–19814. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.T.; Xie, D.; Liu, H.Y.; Li, Y.T.; Jing, F.J.; Leng, Y.X. Effects of oxygen adsorption on the corrosion behavior of the Ti(0001) surface: A DFT investigation. Phys. Chem. Chem. Phys. 2024, 26, 7794–7807. [Google Scholar] [CrossRef]
- Liu, J.; Fan, X.F.; Sun, C.Q.; Zhu, W.G. Oxidation of the Ti(0001) surface: Diffusion processes of oxygen from DFT. RSC Adv. 2016, 6, 71311–71318. [Google Scholar] [CrossRef]
- Fan, Y.M.; Zhuo, Y.Q.; Lou, Y.; Zhu, Z.W.; Li, L.L. SeO2 adsorption on CaO surface: DFT study on the adsorption of a single SeO2 molecule. Appl. Surf. Sci. 2017, 413, 366–371. [Google Scholar] [CrossRef]
- Soon, A.; Todorova, M.; Delley, B.; Stampfl, C. Oxygen adsorption and stability of surface oxides on Cu(111): A first-principles investigation. Phys. Rev. B 2006, 73, 165424. [Google Scholar] [CrossRef]
- Neugebauer, J.; Scheffler, M. Adsorbate-Substrate And Adsorbate-Adsorbate Interactions Of Na And K Adlayers on Al(111). Phys. Rev. B. 1992, 46, 16067–16080. [Google Scholar] [CrossRef]
- Fisher, E.S.; Renken, C.J. Single-Crystal Elastic Moduli and the hcp → bcc Transformation in Ti, Zr, and Hf. Phys. Rev. 1964, 135, A482–A494. [Google Scholar] [CrossRef]
- Huda, M.N.; Kleinman, L. Density functional calculations of the influence of hydrogen adsorption on the surface relaxation of Ti(0001). Phys. Rev. B 2005, 71, 241406. [Google Scholar] [CrossRef]
- Meng, X.Z.; Li, X.R.; Li, F.; Yan, H.J.; Zhang, Q.H.; Wu, L.K.; Di Tommaso, D.; Cao, F.H. Molecular Insights into the Stability of Titanium in Electrolytes Containing Chlorine and Fluorine Ions. Langmuir 2023, 39, 17853–17861. [Google Scholar] [CrossRef] [PubMed]
- Feibelman, P.J. Relaxation of hcp(0001) surfaces: A chemical view. Phys. Rev. B 1996, 53, 13740–13746. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Terakura, K. Plane-wave-basis pseudopotential calculations of the surface relaxations of Ti(0001) and Zr(0001). Phys. Rev. B 1997, 56, 9282–9285. [Google Scholar] [CrossRef]
- Hanson, D.M.; Stockbauer, R.; Madey, T.E. Photon-stimulated desorption and other spectroscopic studies of the interaction of oxygen with a titanium (001) surface. Phys. Rev. B 1981, 24, 5513–5521. [Google Scholar] [CrossRef]
- Kang, Q.X.; Wang, G.F.; Liu, Q.; Sui, X.C.; Liu, Y.K.; Chen, Y.Q.; Luo, S.Y.; Li, Z.L. Theoretical research for oxidation mechanism of α-Ti: A combination of DFT and ab initio molecular dynamics study. Vacuum 2021, 193, 110522. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Wang, X.Y.; Duan, Y.H.; Peng, M.J. Revealing boron adsorption on the α-Ti(0001) surface by first-principles calculations. Philos. Mag. 2022, 102, 1873–1890. [Google Scholar] [CrossRef]
- Wen, P.C.; Yuan, L.J.; Tao, R.; Li, J.; Li, D. First-principles investigation of interaction between surface oxygen and other alloy atoms in Ti(0001) for designing high-temperature titanium alloy. Appl. Surf. Sci. 2022, 604, 154535. [Google Scholar] [CrossRef]
- Duan, Y.H.; Sun, Y.; Zhou, S.G. Different coverages of fluorine adsorption on Mg(0001) surface. Comput. Mater. Sci. 2013, 72, 81–85. [Google Scholar] [CrossRef]
- Zhao, J.P.; Xu, Y.L.; Liu, S.H.; Ding, X.D. The effect of oxygen-containing species on corrosion behavior of Ta(110) surface: A DFT study with an experimental verification. Appl. Surf. Sci. 2022, 586, 152810. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, W.G.; Pan, Y.; Pan, Y.F.; Liu, T.; Zhou, X.G.; Shi, D.N.; Shi, Y.G.; Wei, X.Y. Formation and binding energies of vacancies in the Al(111) surface: Density functional theory calculations confirm simple bond model. Surf. Sci. 2015, 637, 85–89. [Google Scholar] [CrossRef]
- Gupta, S.S.; van Huis, M.A.; Dijkstra, M.; Sluiter, M.H.F. Depth dependence of vacancy formation energy at (100), (110), and (111) Al surfaces: A first-principles study. Phys. Rev. B 2016, 93, 085432. [Google Scholar] [CrossRef]
- Cao, C.N. Principles of Electrochemistry of Corrosion, 3rd ed.; Chemical Industrial Press: Beijing, China, 2008. [Google Scholar]
- Yan, C.; Zeng, Q.F.; He, W.J.; Zhu, J.N. First-principles investigation on the adsorption and dissociation of O and H O molecules on the Ni-rich TiNi alloy surface. Appl. Surf. Sci. 2020, 534, 147570. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Population Analysis on LCAO–MO Molecular Wave Functions. I. J. Chem. Phys. 1955, 23, 1833–1840. [Google Scholar] [CrossRef]
- Da Silva, J.L.F. All-electron first-principles calculations of clean surface properties of low-Miller-index Al surfaces. Phys. Rev. B 2005, 71, 195416. [Google Scholar] [CrossRef]
- Trasatti, S. Structure of the metal/electrolyte solution Interface: New data for theory. Electrochim. Acta 1991, 36, 1657–1658. [Google Scholar] [CrossRef]
- Singh, R.; Dahotre, N.B. Corrosion degradation and prevention by surface modification of biometallic materials. J. Mater. Sci: Mater. Med. 2007, 18, 725–751. [Google Scholar] [CrossRef]










| This Work | Experiments | Other Calculations | |
|---|---|---|---|
| Lattice (Å) | a = b = 2.94 c = 4.66 c/a = 1.585 | a = 2.95, c/a = 1.587 [19] | GGA-PBE: a = b = 2.92, c = 4.63 [1] c/a = 1.580 [20] a = b = 2.94, c = 4.63 [21] |
| c/a = 1.586 [20] | LDA: c/a = 1.580 [22] c/a = 1.594 [23] | ||
| c/a = 1.588 [22] | |||
| Work function (eV) | 4.431 | 4.6 ± 0.2 [24] | GGA-PBE: 4.48 [25], 4.42 [20] |
| 4.45 [23] | LDA: 4.64 [22], 4.75 [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Xie, D.; Jing, F.; Ma, D.; Leng, Y. Influence of Ti Vacancy Defects on the Dissolution of O-Adsorbed Ti(0001) Surface: A First-Principles Study. Metals 2024, 14, 573. https://doi.org/10.3390/met14050573
Wang X, Xie D, Jing F, Ma D, Leng Y. Influence of Ti Vacancy Defects on the Dissolution of O-Adsorbed Ti(0001) Surface: A First-Principles Study. Metals. 2024; 14(5):573. https://doi.org/10.3390/met14050573
Chicago/Turabian StyleWang, Xiaoting, Dong Xie, Fengjuan Jing, Donglin Ma, and Yongxiang Leng. 2024. "Influence of Ti Vacancy Defects on the Dissolution of O-Adsorbed Ti(0001) Surface: A First-Principles Study" Metals 14, no. 5: 573. https://doi.org/10.3390/met14050573





