Emulsions Stabilized with an Electrostatic Complex of Quaternized Cellulose Nanofiber and Octanoyl Gelatin and the Effect of pH Value on Their Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hydrophobicised Gelatin
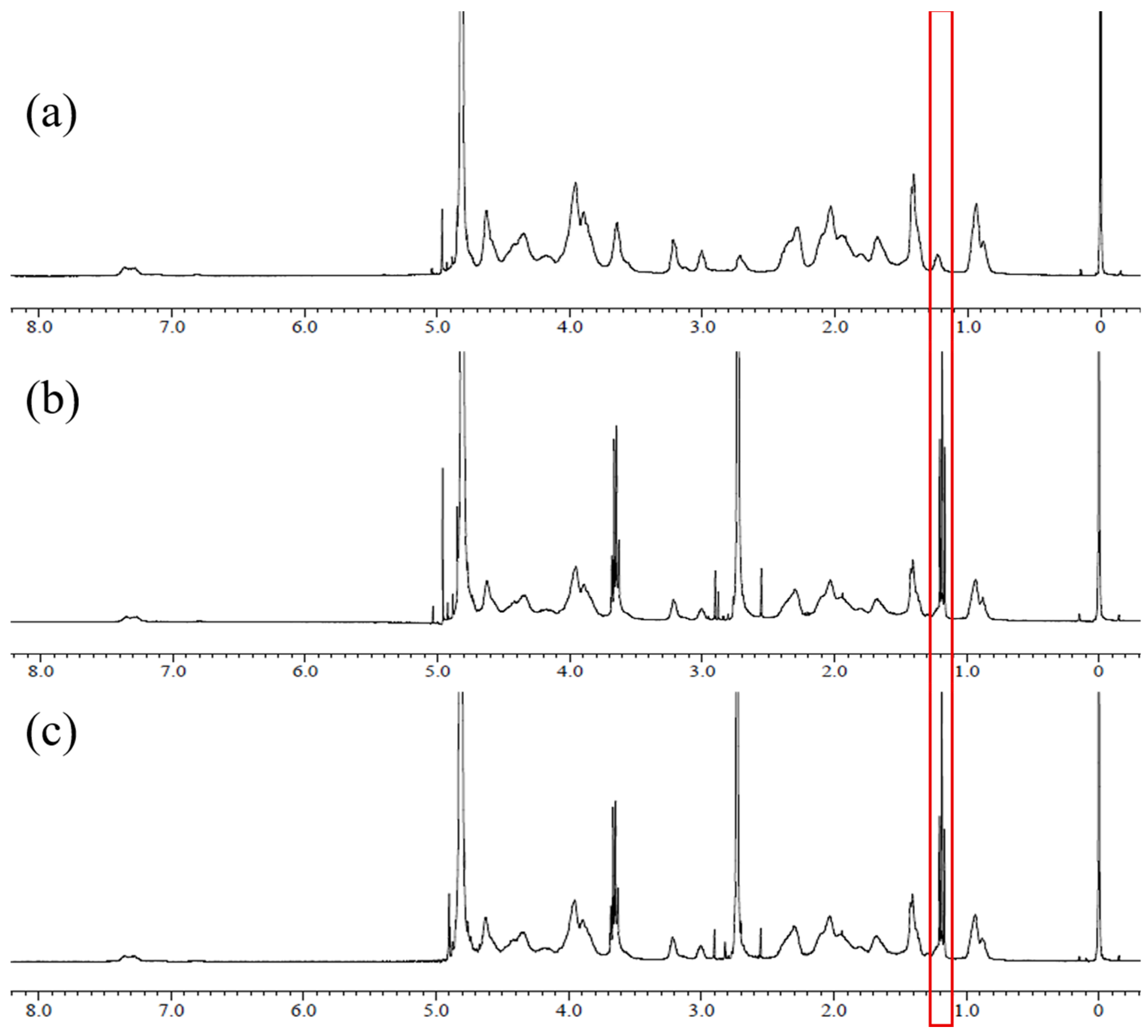
2.3. 1H NMR Spectroscopy
2.4. Measurement of Air/Water Interface Tension
2.5. Measurement of QCNF/HpGel Complexation Degree
2.6. FT−IR Spectroscopy
2.7. X−ray Diffractometry
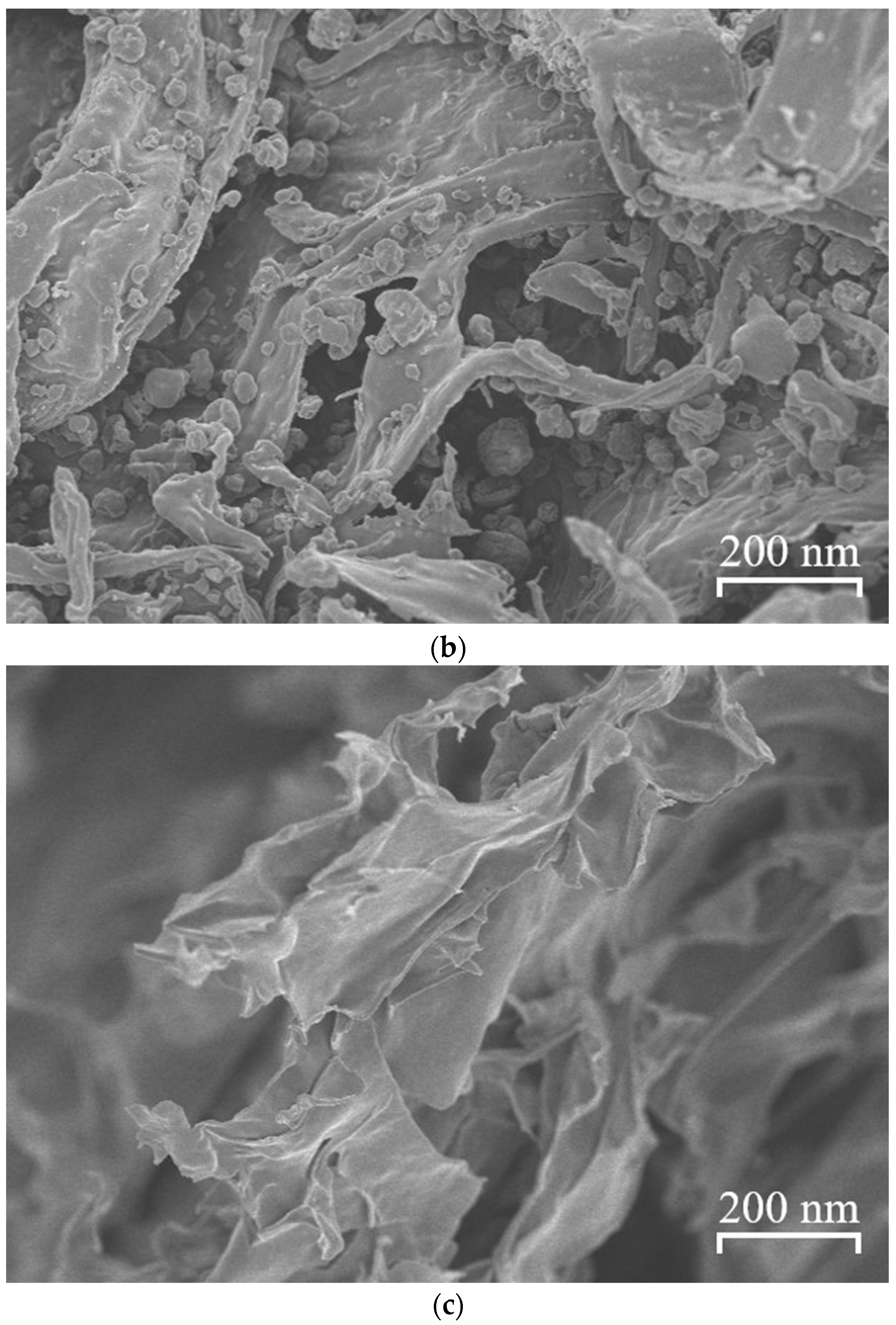
2.8. Scanning Electron Microscopy
2.9. Preparation of Emulsion Stabilized with QCNF and OC−Gel
2.10. Measurement of Size and Stability of Oil Droplets of Em(OC−Gel), Em(QCNF), and Em(QCNF/OC−Gel) by Microscopic Observation
3. Results and Discussion
3.1. 1H NMR Spectroscopy
3.2. Measurement of Air/Water Interface Tension
3.3. Measurement of QCNF/HpGel Complexation Degree
3.4. FT−IR Spectroscopy
3.5. X−ray Diffractometry
3.6. Scanning Electron Microscopy
3.7. Measurement of Size and Stability of Oil Droplets of Em(OC−Gel), Em(QCNF), and Em(QCNF/OC−Gel) by Microscopic Observation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amine, C.; Dreher, J.; Helgason, T.; Tadros, T. Investigation of emulsifying properties and emulsion stability of plant and milk proteins using interfacial tension and interfacial elasticity. Food Hydrocoll. 2014, 39, 180–186. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids as emulsifiers and emulsion stabilizers. Food Hydrocoll. 2009, 23, 1473–1482. [Google Scholar] [CrossRef]
- Nesterenko, A.; Drelich, A.; Lu, H.; Clausse, D.; Pezron, I. Influence of a mixed particle/surfactant emulsifier system on water-in-oil emulsion stability. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 49–57. [Google Scholar] [CrossRef]
- Osborn, H.T.; Akoh, C.C. Effect of emulsifier type, droplet size, and oil concentration on lipid oxidation in structured lipid-based oil-in-water emulsions. Food Chem. 2004, 84, 451–456. [Google Scholar] [CrossRef]
- Cardoso-Ugarte, G.A.; Ramírez-Corona, N.; López-Malo, A.; Palou, E.; Martín-González, M.F.S.; Jiménez-Munguía, M.T. Modeling phase separation and droplet size of W/O emulsions with oregano essential oil as a function of its formulation and homogenization conditions. J. Dispers. Sci. Technol. 2018, 39, 1065–1073. [Google Scholar] [CrossRef]
- Ushikubo, F.Y.; Cunha, R.L. Stability mechanisms of liquid water-in-oil emulsions. Food Hydrocoll. 2014, 34, 145–153. [Google Scholar] [CrossRef]
- Capek, I. Degradation of kinetically-stable o/w emulsions. Adv. Colloid Interface Sci. 2004, 107, 125–155. [Google Scholar] [CrossRef] [PubMed]
- Wardhono, E.Y.; Zafimahova-Ratisbonne, A.; Saleh, K.; Clausse, D.; Lanoiselle, J.-L. W/O Emulsion Destabilization and Release of a Polysaccharide Entrapped in the Droplets. J. Dispers. Sci. Technol. 2016, 37, 1581–1589. [Google Scholar] [CrossRef]
- Burgos-Díaz, C.; Wandersleben, T.; Marqués, A.M.; Rubilar, M. Multilayer emulsions stabilized by vegetable proteins and polysaccharides. Curr. Opin. Colloid Interface Sci. 2016, 25, 51–57. [Google Scholar] [CrossRef]
- Maier, C.; Zeeb, B.; Weiss, J. Investigations into aggregate formation with oppositely charged oil-in-water emulsions at different pH values. Colloids Surf. B Biointerfaces 2014, 117, 368–375. [Google Scholar] [CrossRef]
- Bobin, M.-F.; Michel, V.; Martini, M.-C. Study of formulation and stability of emulsions with polymeric emulsifiers. Colloids Surf. A Physicochem. Eng. Asp. 1999, 152, 53–58. [Google Scholar] [CrossRef]
- Xu, Y.-T.; Wang, Y.-H.; Chen, F.-P.; Tang, C.-H. Whether ovalbumin performs as a particulate or polymeric emulsifier is largely determined by pH. Food Hydrocoll. 2020, 103, 105694. [Google Scholar] [CrossRef]
- Kang, M.K.; Kim, J.-C. Preparations and temperature- and pH-dependent release property of ethylcellulose microcapsules containing N-isopropylacrylamide copolymer. J. Appl. Polym. Sci. 2010, 118, 421–427. [Google Scholar] [CrossRef]
- Yari, S.; Nasirpour, A.; Fathi, M. Effect of Polymer Concentration and Acidification Time on Olive Oil Microcapsules Obtained by Complex Coacervation. Appl. Food Biotechnol. 2016, 3, 53–58. [Google Scholar] [CrossRef]
- Loxley, A.; Vincent, B. Preparation of Poly(methylmethacrylate) Microcapsules with Liquid Cores. J. Colloid Interface Sci. 1998, 208, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Aghbashlo, M.; Mobli, H.; Madadlou, A.; Rafiee, S. The correlation of wall material composition with flow characteristics and encapsulation behavior of fish oil emulsion. Food Res. Int. 2012, 49, 379–388. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, J.-C. Oxidation–Responsive Emulsions Stabilized with Poly(Vinyl Pyrrolidone-co-allyl Phenyl Sulfide). Polymers 2020, 12, 498. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Quinlan, P.J.; Tam, K.C. Stimuli-responsive Pickering emulsions: Recent advances and potential applications. Soft Matter. 2015, 11, 3512–3529. [Google Scholar] [CrossRef] [PubMed]
- Brugger, B.; Richtering, W. Magnetic, Thermosensitive Microgels as Stimuli-Responsive Emulsifiers Allowing for Remote Control of Separability and Stability of Oil in Water-Emulsions. Adv. Mater. 2007, 19, 2973–2978. [Google Scholar] [CrossRef]
- Shahid, S.; Gurram, S.R.; Basavaraj, M.G. Doubly pH Responsive Emulsions by Exploiting Aggregation of Oppositely Charged Nanoparticles and Polyelectrolytes. Langmuir 2018, 34, 5060–5071. [Google Scholar] [CrossRef]
- Seo, S.R.; Lee, H.Y.; Kim, J.-C. Thermo- and pH-Responsiveness of Emulsions Stabilized with Acidic Thermosentive Polymers. J. Dispers. Sci. Technol. 2013, 34, 1280–1285. [Google Scholar] [CrossRef]
- Seo, S.R.; Kim, J.-C. Emulsions Stabilized with poly(Hydroxyethyl Acrylate-co-Coumaryl Acrylate-co-2-Ethylhexyl acrylate). J. Macromol. Sci. Part A 2013, 50, 855–860. [Google Scholar] [CrossRef]
- Kwon, K.; Kim, J.-C. Emulsion stabilized with disulfide proteinoid and its stability in reducing condition. J. Dispers. Sci. Technol. 2018, 39, 333–340. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, J.-C. Oil-in-gold nanoparticle solution emulsion stabilized with amphiphilic polymers and its stability under NIR irradiation. J. Dispers. Sci. Technol. 2018, 39, 961–969. [Google Scholar] [CrossRef]
- Yoshizawa, K.; Taguchi, T. Bonding behavior of hydrophobically modified gelatin films on the intestinal surface. J. Bioact. Compat. Polym. 2014, 29, 560–571. [Google Scholar] [CrossRef]
- Chen, X.; Mizuta, R.; Fukata, N.; Taguchi, T. Design of bio-inspired adhesive surface composed of hexanoyl group-modified gelatin and silicon nanowire. Colloids Surf. B Biointerfaces 2019, 178, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Toledano, O.; Magdassi, S. Emulsification and Foaming Properties of Hydrophobically Modified Gelatin. J. Colloid Interface Sci. 1998, 200, 235–240. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Z.; Zou, Y.; Yu, J.; Liu, L.; Fan, Y. Comparison of Cellulose and Chitin Nanofibers on Pickering Emulsion Stability—Investigation of Size and Surface Wettability Contribution. Int. J. Biol. Macromol. 2023, 235, 123754. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-H.; Chen, K.-M. Preparation and surface activity of gelatin derivative surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2006, 272, 8–14. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, J.; Zhang, Y.; Wang, X.; Lorenzo, J.M.; Zhong, J. Gelatins as emulsifiers for oil-in-water emulsions: Extraction, chemical composition, molecular structure, and molecular modification. Trends Food Sci. Technol. 2020, 106, 113–131. [Google Scholar] [CrossRef]
- Auweter, H.; André, V.; Horn, D.; Lüddecke, E. The function of gelatin in controlled precipitation processes of nanosize particles. J. Dispers. Sci. Technol. 1998, 19, 163–184. [Google Scholar] [CrossRef]
- Sewald, L.; Claaßen, C.; Götz, T.; Claaßen, M.H.; Truffault, V.; Tovar, G.E.M.; Southan, A.; Borchers, K. Beyond the Modification Degree: Impact of Raw Material on Physicochemical Properties of Gelatin Type A and Type B Methacryloyls. Macromol. Biosci. 2018, 18, 1800168. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Zhou, W.; Zhang, Z.; Zhang, B. Effects of Relative Molecular Weight Distribution and Isoelectric Point on the Swelling Behavior of Gelatin Films. Front. Chem. 2022, 10, 857976. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, G.; Shi, B. Physicochemical properties of collagen, gelatin and collagen hydrolysate derived from bovine limed split wastes. J. Soc. Leather Technol. Chem. 2006, 90, 23–28. [Google Scholar]
- Park, S.H.; Zhao, F.; Park, S.C.; Kim, J.C. Gelatin-Loaded Cubosomes Stabilized with Hydrophobically Modified Quaternized Cellulose Nanofiber and Their PH-Dependent Release Property. J. Biomater. Appl. 2021, 35, 1109–1118. [Google Scholar] [CrossRef]
- Zhao, Y.; Khalid, N.; Shu, G.; Neves, M.A.; Kobayashi, I.; Nakajima, M. Complex Coacervates from Gelatin and Octenyl Succinic Anhydride Modified Kudzu Starch: Insights of Formulation and Characterization. Food Hydrocoll. 2019, 86, 70–77. [Google Scholar] [CrossRef]
- Bian, H.; Yang, Y.; Tu, P.; Chen, J.Y. Value-Added Utilization of Wheat Straw: From Cellulose and Cellulose Nanofiber to All-Cellulose Nanocomposite Film. Membranes 2022, 12, 475. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, C.; Zhao, H.; Wang, J.; Yin, C.; Zhang, L.; Zhao, Y. Eects of Cellulose Nanocrystals and Cellulose Nanofibers on the Structure and Properties of Polyhydroxybutyrate Nanocomposites. Polymers 2019, 11, 2063. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Estaca, J.; Montero, P.; Fernández-Martín, F.; Gómez-Guillén, M.C. Physico-chemical and film-forming properties of bovine-hide and tuna-skin gelatin: A comparative study. J. Food Eng. 2009, 90, 480–486. [Google Scholar] [CrossRef]
- Hoque, M.S.; Benjakul, S.; Prodpran, T. Effect of heat treatment of film-forming solution on the properties of film from cuttlefish (Sepia pharaonis) skin gelatin. J. Food Eng. 2010, 96, 66–73. [Google Scholar] [CrossRef]
- Cao, N.; Fu, Y.; He, J. Preparation and physical properties of soy protein isolate and gelatin composite films. Food Hydrocoll. 2007, 21, 1153–1162. [Google Scholar] [CrossRef]
- Goi, Y.; Fujisawa, S.; Saito, T.; Yamane, K.; Kuroda, K.; Isogai, A. Dual Functions of TEMPO-Oxidized Cellulose Nanofibers in Oil-in-Water Emulsions: A Pickering Emulsifier and a Unique Dispersion Stabilizer. Langmuir 2019, 35, 10920–10926. [Google Scholar] [CrossRef]
- Costa, A.L.R.; Gomes, A.; Tibolla, H.; Menegalli, F.C.; Cunha, R.L. Cellulose nanofibers from banana peels as a Pickering emulsifier: High-energy emulsification processes. Carbohydr. Polym. 2018, 194, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, W.; Jia, H.; Gao, G.; Wang, X.; Zhang, X.; Wang, Y. Using Cellulose Nanofibers and Its Palm Oil Pickering Emulsion as Fat Substitutes in Emulsified Sausage. J. Food Sci. 2018, 83, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Tanpichai, S.; Biswas, S.K.; Witayakran, S.; Yano, H. Optically transparent tough nanocomposites with a hierarchical structure of cellulose nanofiber networks prepared by the Pickering emulsion method. Compos. Part A Appl. Sci. Manuf. 2020, 132, 105811. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Rao, C.M. The role of surface charge in the desolvation process of gelatin: Implications in nanoparticle synthesis and modulation of drug release. Int. J. Nanomed. 2017, 12, 795–808. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Lu, P.-L.; Chen, K.-H.; Tabata, Y.; Hsiue, G.-H. Effect of Charge and Molecular Weight on the Functionality of Gelatin Carriers for Corneal Endothelial Cell Therapy. Biomacromolecules 2006, 7, 1836–1844. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, H.K.; Park, S.C.; Kim, J.-C. Emulsions Stabilized with an Electrostatic Complex of Quaternized Cellulose Nanofiber and Octanoyl Gelatin and the Effect of pH Value on Their Stability. Appl. Sci. 2024, 14, 4122. https://doi.org/10.3390/app14104122
Son HK, Park SC, Kim J-C. Emulsions Stabilized with an Electrostatic Complex of Quaternized Cellulose Nanofiber and Octanoyl Gelatin and the Effect of pH Value on Their Stability. Applied Sciences. 2024; 14(10):4122. https://doi.org/10.3390/app14104122
Chicago/Turabian StyleSon, Hyeon Ki, Soo Chan Park, and Jin-Chul Kim. 2024. "Emulsions Stabilized with an Electrostatic Complex of Quaternized Cellulose Nanofiber and Octanoyl Gelatin and the Effect of pH Value on Their Stability" Applied Sciences 14, no. 10: 4122. https://doi.org/10.3390/app14104122



