Analysis of the Efficiency of Antioxidants in Inhibiting Lipid Oxidation in Terms of Characteristic Kinetic Parameters
Abstract
:1. Introduction
2. A Brief Overview of the Lipid Oxidation Reaction and Its Inhibition by Antioxidants
2.1. Addition of Chain-Breaking Antioxidants: Kinetic Effects
2.2. Inhibited Lipid Oxidation Reactions in Emulsions: Highlighting the Critical Role of the Effective Concentrations of Antioxidants in the Oil, Aqueous, and Interfacial Regions
2.3. Overview of the Pseudophase Kinetic Model to Determine the Distribution of AOs between the Oil-Interfacial and Aqueous-Interfacial Regions of the Emulsions
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Synthesis of Fatty Acid Esters
3.3. Emulsion Preparation
3.4. Monitoring the Formation of Primary Oxidation Products
3.5. Determining the Partition Constant, PWO, in Binary Oil-Water Mixtures
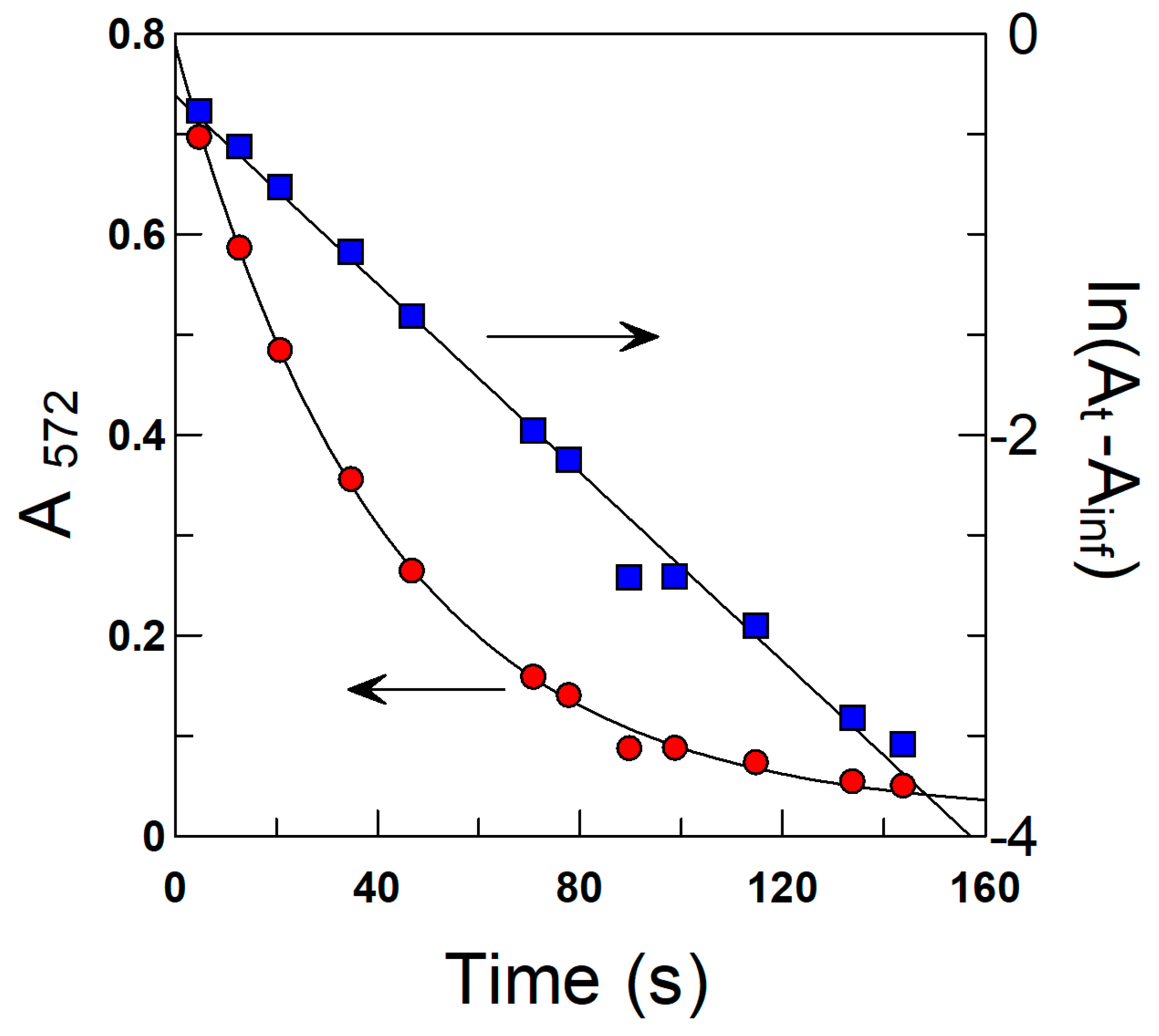
3.6. Determination of the Observed Rate Constant, kobs, for the Reaction between 16-ArN2+ and the AOs in Olive Oil Emulsions
3.7. Statistical Analysis
4. Results and Discussion
4.1. Oxidative Stability of Olive Oil-in-Water Emulsions: Antioxidant Efficiency
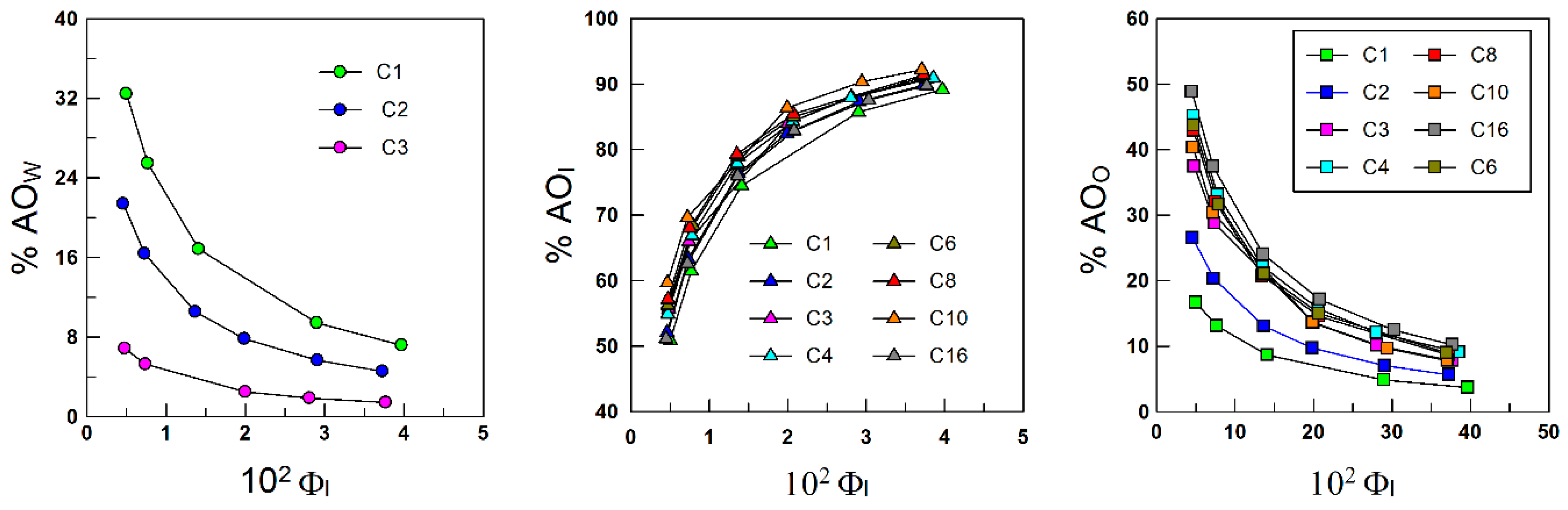
4.2. Distribution of Antioxidants between the Oil, Interfacial, and Aqueous Regions of Emulsions
4.3. Effective Concentrations of AOs in the Aqueous, Interfacial, and Oil Regions of Emulsions
4.4. Structure-Reactivity Relationships: Role of Hydrophobicity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schaich, K.M. Lipid Antioxidants: More Than Just Lipid Radical Quenchers. In Lipid Oxidation in Food and Biological Systems: A Physical Chemistry Perspective; Bravo-Diaz, C., Ed.; Springer International Publishing: Cham, Swizerland, 2022; pp. 151–184. [Google Scholar]
- Khanum, R.; Thevanayagam, H. Lipid Peroxidation: Its Effects on the Formulation and Use of Pharmaceutical Emulsions. Asian J. Pharm. Sci. 2017, 12, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Fennema, O.R. Food Chemistry, 4th ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Wu, H.; Richards, M.P. Lipid Oxidation and Antioxidant Delivery Systems in Muscle Food. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1275–1299. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.; Abdullah; Zhang, H.; Weiss, J. A Comprehensive Review on Polarity, Partitioning, and Interactions of Phenolic Antioxidants at Oil–Water Interface of Food Emulsions. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4250–4277. [Google Scholar] [CrossRef] [PubMed]
- Schaich, K.M. Lipid Oxidation: New Perspectives on an Old Reaction. In Bailey’s Industrial Oil and Fat Products; Shahidi, F., Ed.; Wiley: Hoboken, NJ, USA, 2020; pp. 1–72. [Google Scholar]
- Nieva-Echevarría, B.; Goicoechea, E.; Guillén, M.D. Food Lipid Oxidation under Gastrointestinal Digestion Conditions: A Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 461–478. [Google Scholar] [CrossRef]
- Yang, W.; Yue, H.; Lu, G.; Wang, W.; Deng, Y.; Ma, G.; Wei, W. Advances in Delivering Oxidative Modulators for Disease Therapy. Research 2022, 2022, 9897464. [Google Scholar] [CrossRef]
- Mansoor, S.; Ali Wani, O.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive Oxygen Species in Plants: From Source to Sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef] [PubMed]
- Helberg, J.; Pratt, D.A. Autoxidation vs. Antioxidants—The Fight for Forever. Chem. Soc. Rev. 2021, 50, 7343–7358. [Google Scholar] [CrossRef]
- Fleming, E.; Luo, Y. Co-Delivery of Synergistic Antioxidants from Food Sources for the Prevention of Oxidative Stress. J. Agric. Food Res. 2021, 3, 100107. [Google Scholar] [CrossRef]
- Frankel, E. Lipid Oxidation; The Oily Press, PJ Barnes & Associates: Bridgwater, UK, 2005. [Google Scholar]
- Amft, J.; Meissner, P.M.; Steffen-Heins, A.; Hasler, M.; Stöckmann, H.; Meynier, A.; Birault, L.; Velasco, J.; Vermoesen, A.; Perez-Portabella, I.; et al. Interlaboratory Study on Lipid Oxidation During Accelerated Storage Trials with Rapeseed and Sunflower Oil Analyzed by Conjugated Dienes as Primary Oxidation Products. Eur. J. Lipid Sci. Technol. 2023, 125, 2300067. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Bravo Díaz, C.; Paiva Martins, F.; Romsted, L.S. Maxima in Antioxidant Distributions and Efficiencies with Increasing Hydrophobicity of Gallic Acid and Its Alkyl Esters. The Pseudophase Model Interpretation of the “Cut-Off Effect”. J. Agric. Food Chem. 2013, 61, 6533–6543. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.H.; Upganlawar, A.B.; Upasani, C.D. Chapter 39—Therapeutic Benefits of Phenolic Acids in Peripheral Neuropathy. In Treatments, Nutraceuticals, Supplements, and Herbal Medicine in Neurological Disorders; Martin, C.R., Patel, V.B., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 753–771. [Google Scholar]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New Progress in the Pharmacology of Protocatechuic Acid: A Compound Ingested in Daily Foods and Herbs Frequently and Heavily. Pharmacol. Res. 2020, 161, 105109. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Niu, L.; Chen, Y.; Qiu, X.; Du, T.; Zhu, M.; Wang, M.; Mo, H.; Xiao, S. Recent Advance in the Biological Activity of Chlorogenic Acid and Its Application in Food Industry. Int. J. Food Sci. Technol. 2023, 58, 4931–4947. [Google Scholar] [CrossRef]
- Semaming, Y.; Pannengpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Pharmacological Properties of Protocatechuic Acid and Its Potential Roles as Complementary Medicine. Evid.-Based Complement. Altern. Med. 2015, 2015, 593902. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; He, Z.; Liu, M.; Tan, J.; Zhang, H.; Hou, D.-X.; He, J.; Wu, S. Dietary Protocatechuic Acid Ameliorates Inflammation and up-Regulates Intestinal Tight Junction Proteins by Modulating Gut Microbiota in Lps-Challenged Piglets. J. Anim. Sci. Biotechnol. 2020, 11, 92. [Google Scholar] [CrossRef]
- Ingold, K.U.; Pratt, D.A. Advances in Radical-Trapping Antioxidant Chemistry in the 21st Century: A Kinetics and Mechanisms Perspective. Chem. Rev. 2014, 114, 9022–9046. [Google Scholar] [CrossRef] [PubMed]
- Aliaga, C.; Rezende, M.C. Location, Orientation and Buoyance Effects of Radical Probes as Studied by EPR. In Lipid Oxidation in Food and Biological Systems: A Physical Chemistry Perspective; Bravo-Diaz, C., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 133–150. [Google Scholar]
- Litwinienko, G.; Ingold, K.U. Solvent Effects on the Rates and Mechanisms of Reaction of Phenols with Free Radicals. Acc. Chem. Res. 2007, 40, 222–230. [Google Scholar] [CrossRef]
- Ingold, K.U.; Bowry, V.W.; Stocker, R.; Walling, C. Autoxidation of Lipids and Antioxidation by Alpha-Tocopherol and Ubiquinol in Homogeneous Solution and in Aqueous Dispersions of Lipids: Unrecognized Consequences of Lipid Particle Size as Exemplified by Oxidation of Human Low Density Lipoprotein. Proc. Natl. Acad. Sci. USA 1993, 90, 45–49. [Google Scholar] [CrossRef]
- Schaich, K.M.; Shahidi, F.; Zhong, Y.; Eskin, N.A.M. Chapter 11—Lipid Oxidation. In Biochemistry of Foods, 3rd ed.; Academic Press: San Diego, CA, USA, 2013; pp. 419–478. [Google Scholar]
- Culler, M.D.; Inchingolo, R.; McClements, D.J.; Decker, E.A. Impact of Polyunsaturated Fatty Acid Dilution and Antioxidant Addition on Lipid Oxidation Kinetics in Oil/Water Emulsions. J. Agric. Food Chem. 2021, 69, 750–755. [Google Scholar] [CrossRef]
- Ross, L.; Barclay, C.; Vinqvist, M.R. Phenols as Antioxidants. In The Chemistry of Phenols; Rappoport, Z., Ed.; John Wiley & Sons: West Sussex, UK, 2003. [Google Scholar]
- Bravo-Díaz, C. Advances in the Control of Lipid Peroxidation in Oil-in-Water Emulsions: Kinetic Approaches. Crit. Rev. Food Sci. Nutr. 2023, 63, 6252–6284. [Google Scholar] [CrossRef]
- Pinchuk, I.; Lichtenberg, D. Analysis of the Kinetics of Lipid Peroxidation in Terms of Characteristic Time-Points. Chem. Phys. Lipids 2014, 178, 63–76. [Google Scholar] [CrossRef]
- Schaich, K.M. Lipid Oxidation: Theoretical Aspects. In Bailey’s Industrial Oil and Fat Products; Shahidi, F., Ed.; Wiley & Sons: New York, NY, USA, 2005; pp. 269–355. [Google Scholar]
- Suhag, R.; Ferrentino, G.; Morozova, K.; Zatelli, D.; Scampicchio, M.; Amorati, R. Antioxidant Efficiency and Oxidizability of Mayonnaise by Oximetry and Isothermal Calorimetry. Food Chem. 2024, 433, 137274. [Google Scholar] [CrossRef]
- Costa, M.; Freiría-Gándara, J.; Losada-Barreiro, S.; Paiva-Martins, F.; Aliaga, C.; Bravo-Díaz, C. Interfacial Kinetics in Olive Oil-in-Water Nanoemulsions: Relationships between Rates of Initiation of Lipid Peroxidation, Induction Times and Effective Interfacial Antioxidant Concentrations. J. Colloid Interface Sci. 2021, 604, 248–259. [Google Scholar] [CrossRef]
- Costa, M.; Freiría-Gándara, J.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Effects of Droplet Size on the Interfacial Concentrations of Antioxidants in Fish and Olive Oil-in-Water Emulsions and Nanoemulsions and on Their Oxidative Stability. J. Colloid Interface Sci. 2020, 562, 352–362. [Google Scholar] [CrossRef]
- Jodko-Piórecka, J.; Cedrowski, J.; Litwinienko, G. Physico-Chemical Principles of Antioxidant Action, Including Solvent and Matrix Dependence and Interfacial Phenomena. In Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications; Apak, R., Capanoglu, E., Shahidi, F., Eds.; Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 225–272. [Google Scholar]
- Waldeck, A.R.; Stocker, R. Radical-Initiated Lipid Peroxidation in Low Density Lipoproteins: Insights Obtained from Kinetic Modeling. Chem. Res. Toxicol. 1996, 9, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Dar, A.A.; Bravo-Diaz, C.; Nazir, N.; Romsted, L.S. Chemical Kinetic and Chemical Trapping Methods: Unique Approaches for Determining Respectively the Antioxidant Distributions and Interfacial Molarities of Water, Counter-Anions, and Other Weakly Basic Nucleophiles in Association Colloidss. Curr. Opin. Colloid Interface Sci. 2017, 32, 84–93. [Google Scholar] [CrossRef]
- Bravo-Díaz, C.; Romsted, L.S.; Liu, C.; Losada-Barreiro, S.; Pastoriza-Gallego, M.J.; Gao, X.; Gu, Q.; Krishnan, G.; Sánchez-Paz, V.; Zhang, Y.; et al. To Model Chemical Reactivity in Heterogeneous Emulsions, Think Homogeneous Microemulsions. Langmuir 2015, 31, 8961–8979. [Google Scholar] [CrossRef]
- Farhoosh, R. A Reconsidered Approach Providing Kinetic Parameters and Rate Constants to Analyze the Oxidative Stability of Bulk Lipid Systems. Food Chem. 2020, 327, 127088. [Google Scholar] [CrossRef] [PubMed]
- López de Arbina, A.; Losada-Barreiro, S.; Rezende, M.C.; Vidal, M.; Aliaga, C. The Location of Amphiphobic Antioxidants in Micellar Systems: The Diving-Swan Analogy. Food Chem. 2019, 279, 288–293. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A. Lipid Oxidation in Oil-in-Water Emulsions: Impact of Molecular Environment on Chemical Reactions in Heterogeneous Food Systems. JFS Concise Rev. Food Sci. 2000, 65, 1270–1282. [Google Scholar] [CrossRef]
- Laguerre, M.; Bily, A.; Roller, M.; Birtić, S. Mass Transport Phenomena in Lipid Oxidation and Antioxidation. Annu. Rev. Food Sci. Technol. 2017, 8, 391–411. [Google Scholar] [CrossRef] [PubMed]
- Romsted, L.S.; Bravo-Díaz, C. Modelling Chemical Reactivity in Emulsions. Curr. Opin. Colloid Interface Sci. 2013, 18, 3–14. [Google Scholar] [CrossRef]
- Bravo-Díaz, C.; Romsted, L.S.; Losada-Barreiro, S.; Paiva-Martins, F. Using a Pseudophase Model to Determine AO Distributions in Emulsions: Why Dynamic Equilibrium Matters. Eur. J. Lipid Sci. Technol. 2017, 119, 1600277. [Google Scholar] [CrossRef]
- Costa, M.; Paiva-Martins, F.; Losada-Barreiro, S.; Bravo-Díaz, C. Modeling Chemical Reactivity at the Interfaces of Emulsions: Effects of Partitioning and Temperature. Molecules 2021, 26, 4703. [Google Scholar] [CrossRef]
- Oehlke, K.; Garamus, V.; Heins, A.; Stöckman, H.; Schwarz, K. The Partitioning of Emulsifiers in O/W Emulsions: A Comparative Study of Sans, Ultrafiltration and Dialysis. J. Colloid Interface Sci. 2008, 322, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Heins, A.; Sokolowski, T.; Stöckmann, T.; Schwarz, K. Investigating the Location of Propyl Gallate at Surfaces and Its Chemical Microenvironment by 1H NMR. Lipids 2007, 42, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Stöckman, H.; Schwarz, K.; Huynh-Ba, T. The Influence of Various Emulsifiers on the Partitioning and Antioxidant Activity of Hydrobenzoic acids and Their Derivatives in Oil-in-Water Emulsions. J. Am. Oil Chem. Soc. 2000, 77, 535–542. [Google Scholar] [CrossRef]
- Sørensen, A.-D.M.; Villeneuve, P.; Jacobsen, C. Alkyl Caffeates as Antioxidants in O/W Emulsions: Impact of Emulsifier Type and Endogenous Tocopherols. Eur. J. Lipid Sci. Technol. 2017, 119, 1600276. [Google Scholar] [CrossRef]
- Huang, S.W.; Frankel, E.N.; Aeschbach, R.; German, J.B. Partition of Selected Antioxidants in Corn Oil-Water Model Systems. J. Agric. Food Chem. 1997, 45, 1991–1994. [Google Scholar] [CrossRef]
- Jacobsen, C.; Schwarz, K.; Stöckmann, H.; Meyer, A.S.; Adler-Nissen, J. Partitioning of Selected Antioxidants in Mayonnaise. J. Agric. Food. Chem. 1999, 47, 3601–3610. [Google Scholar] [CrossRef]
- Costa, M.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Polyphenolic Antioxidants in Lipid Emulsions: Partitioning Effects and Interfacial Phenomena. Foods 2021, 10, 539. [Google Scholar] [CrossRef]
- Romsted, L.S.; Bravo-Díaz, C. Determining Antioxidant Distributions in Intact Emulsions by Kinetic Methods: Application of Pseudophase Models. In Lipid Oxidation in Food and Biological Systems: A Physical Chemistry Perspective; Bravo-Diaz, C., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 31–48. [Google Scholar]
- Doyle, M.P.; Bryker, W.J. Alkyl-Nitrite-Metal Halide Deamination Reactions. 6. Direct Synthesis of Arene Diazonium Tetrafluorborates Salts from Aromatic Amines, ter-Butyl Nitrite and Borom Trifluoride Ethearate in Anhydrous Media. J. Org. Chem. 1979, 44, 1572–1574. [Google Scholar] [CrossRef]
- Gordon, M.H.; Paiva-Martins, F.; Almeida, M. Antioxidant Activity of Hydroxytyrosol Acetate Compared to That of Other Olive Oil Polyphenols. J. Agric. Food. Chem. 2001, 49, 2480–2485. [Google Scholar] [CrossRef]
- Reis, A.; de Freitas, V. When Polyphenols Meet Lipids: Challenges in Membrane Biophysics and Opportunities in Epithelial Lipidomics. Food Chem. 2020, 333, 127509. [Google Scholar] [CrossRef] [PubMed]
- Adefegha, S.A.; Oboh, G.; Ejakpovi, I.I.; Oyeleye, S.I. Antioxidant and Antidiabetic Effects of Gallic and Protocatechuic Acids: A Structure–Function Perspective. Comp. Clin. Pathol. 2015, 24, 1579–1585. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A Review on Protocatechuic Acid and Its Pharmacological Potential. ISRN Pharmacol. 2014, 2014, 952943. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A. Chapter 42—Bioavailability of Dietary Anthocyanins and Hydroxycinnamic Acids. In Polyphenols in Human Health and Disease; Academic Press: San Diego, CA, USA, 2014; pp. 561–576. [Google Scholar]
- Reis, B.; Martins, M.; Barreto, B.; Milhazes, N.; Garrido, E.M.; Silva, P.; Garrido, J.; Borges, F. Structure–Property–Activity Relationship of Phenolic Acids and Derivatives. Protocatechuic Acid Alkyl Esters. J. Agric. Food Chem. 2010, 58, 6986. [Google Scholar] [CrossRef]
- Yuji, H.; Weiss, J.; Villeneuve, P.; López-Giraldo, L.J.; Figueroa-Espinoza, M.J.; Decker, E.A. Ability of Surface-Active Antioxidants to Inhibit Lipid Oxidation in Oil-in-Water Emulsion. J. Agric. Food Chem. 2007, 55, 11052–11056. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Losada-Barreiro, S.; Vicente, A.; Bravo-Díaz, C.; Paiva-Martins, F. Unexpected Antioxidant Efficiency of Chlorogenic Acid Phenolipids in Fish Oil-in-Water Nanoemulsions: An Example of How Relatively Low Interfacial Concentrations Can Make Antioxidants to Be Inefficient. Molecules 2022, 27, 861. [Google Scholar] [CrossRef]
- Silva, R.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Partitioning and Antioxidative Effect of Protocatechuates in Soybean Oil Emulsions: Relevance of Emulsifier Concentration. Eur. J. Lipid Sci. Technol. 2017, 119, 1600274. [Google Scholar] [CrossRef]
- Costa, M.; Paiva-Martins, F.; Bravo-Díaz, C.; Losada-Barreiro, S. Control of Lipid Oxidation in Oil-in Water Emulsions: Effects of Antioxidant Partitioning and Surfactant Concentration. In Lipid Oxidation in Food and Biological Systems: A Physical Chemistry Perspective; Bravo-Diaz, C., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 201–216. [Google Scholar]
- Laguerre, M.; López-Giraldo, L.J.; Lecomte, J.; Figueroa-Espinoza, M.J.; Baréa, B.; Weiss, J.; Decker, E.A.; Villeneuve, P. Chain Length Affects Antioxidant Properties of Chlorogenate Esters in Emulsion: The Cut-Off Theory Behind the Polar Paradox. J. Agric. Food Chem 2009, 57, 11335–11342. [Google Scholar] [CrossRef] [PubMed]
- Laguerre, M.M.; López-Giraldo, L.J.; Lecomte, J.; Figueroa-Espinoza, M.J.; Baréa, B.; Weiss, J.; Decker, E.A.; Villeneuve, P. Relationship between Hydrophobicity and Antioxidant Ability of “Phenolipids” in Emulsion: A parabolic Effect of the Chain Lenght of Rosmarinate Esters. J. Agric. Food Chem. 2010, 58, 2869–2876. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Trujillo, M.; Pereira-Caro, G.; Madrona, A.; Cert, A.; Espartero, J.L. New Lipophilic Tyrosyl Esters. Comparative Antioxidant Evaluation with Hydroxytyrosyl Esters. J. Agric. Food Chem. 2008, 53, 10960–10966. [Google Scholar] [CrossRef] [PubMed]
- Medina, I.; Lois, S.; Alcántara, D.; Lucas, L.; Morales, J.C. Effect of Lipophilization of Hydroxytyrosol on Its Antioxidant Activity in Fish Oils and Fish Oil-in-Water Emulsions. J. Agric. Food Chem. 2009, 57, 9773–9779. [Google Scholar] [CrossRef] [PubMed]
- Berton-Carabin, C.C.; Ropers, M.-H.; Genot, C. Lipid Oxidation in Oil-in-Water Emulsions: Involvement of the Interfacial Layer. Compr. Rev. Food Sci. Food Safety 2014, 13, 945–977. [Google Scholar] [CrossRef]
- Berton, C.; Ropers, M.H.; Viau, M.; Genot, C. Contribution of the interfacial layer to the protection of emulsified lipids against oxidation. J. Agric. Food Chem. 2011, 59, 5052–5061. [Google Scholar] [CrossRef] [PubMed]
- Kahveci, D.; Laguerre, M.; Villeneuve, P. 7—Phenolipids as New Antioxidants: Production, Activity, and Potential Applications. In Polar Lipids; Elsevier: Amsterdam, The Netherlands, 2015; pp. 185–214. [Google Scholar]
- Boozer, C.E.; Hammond, G.S.; Hamilton, C.E.; Sen, J.N. Air Oxidation of Hydrocarbons.1 II. The Stoichiometry and Fate of Inhibitors in Benzene and Chlorobenzene. J. Am. Chem. Soc. 1955, 77, 3233–3237. [Google Scholar] [CrossRef]
- Löpez-Giraldo, L.J.; Laguerre, M.; Lecomte, J.; Figueroa-Espinoza, M.J.; Baréa, B.; Weiss, J.; Decker, E.A.; Villeneuve, P. Kinetic and Stoichiometry of the Reaction of Chlorogenic Acid and Its Alkyl Esters against the DPPH Radical. J. Agric. Food Chem. 2011, 57, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Villaño, D.; Fernández-Pachón, M.S.; Moyá, M.L.; Troncoso, A.M.; García-Parrilla, M.C. Radical Scavenging Ability of Polyphenolic Compounds Towards Dpph Free Radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef]
- Angeli, L.; Morozova, K.; Scampicchio, M. A Kinetic-Based Stopped-Flow Dpph• Method. Sci. Rep. 2023, 13, 7621. [Google Scholar] [CrossRef]
- Meireles, M.; Losada-Barreiro, S.; Costa, M.; Paiva-Martins, F.; Bravo-Díaz, C.; Monteiro, L.S. Control of Antioxidant Efficiency of Chlorogenates in Emulsions: Modulation of Antioxidant Interfacial Concentrations. J. Sci. Food Agric. 2019, 99, 3917–3925. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Okamoto, Y.; Kawabata, J. Effects of Alcoholic Solvents on Antiradical Abilities of Protocatechuic Acid and Its Alkyl Esters. Biosci. Biotechnol. Biochem. 2004, 68, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, T.; Ishida, T.; Ueyama, H.; Hayashida, H.; Ishimaru, T.; Nishiyama, T. Comparison of the Efficiencies of the Fused Heterocyclic Compounds, 9h-Xanthene-2,7-Diols, and Related Chain-Breaking Phenolic Antioxidants. Bull. Chem. Soc. Jpn. 1996, 69, 1713–1717. [Google Scholar] [CrossRef]
- Litwinienko, G.; Ingold, K.U. Abnormal Solvent Effects on Hydrogen Atom Abstraction. 2. Resolution of the Curcumin Antioxidant Controversy. The Role of Sequential Proton Loss Electron Transfer. J. Org. Chem. 2004, 69, 5888–5896. [Google Scholar] [CrossRef]
- Berton-Carabin, C.C.; Sagis, L.; Schroën, K. Formation, Structure, and Functionality of Interfacial Layers in Food Emulsions. Annu. Rev. Food Sci. Technol. 2018, 9, 551–587. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Analysis of the Efficiency of Antioxidants in Inhibiting Lipid Oxidation in Terms of Characteristic Kinetic Parameters. Antioxidants 2024, 13, 593. https://doi.org/10.3390/antiox13050593
Losada-Barreiro S, Paiva-Martins F, Bravo-Díaz C. Analysis of the Efficiency of Antioxidants in Inhibiting Lipid Oxidation in Terms of Characteristic Kinetic Parameters. Antioxidants. 2024; 13(5):593. https://doi.org/10.3390/antiox13050593
Chicago/Turabian StyleLosada-Barreiro, Sonia, Fátima Paiva-Martins, and Carlos Bravo-Díaz. 2024. "Analysis of the Efficiency of Antioxidants in Inhibiting Lipid Oxidation in Terms of Characteristic Kinetic Parameters" Antioxidants 13, no. 5: 593. https://doi.org/10.3390/antiox13050593





