Identifying Novel Subtypes of Functional Gastrointestinal Disorder by Analyzing Nonlinear Structure in Integrative Biopsychosocial Questionnaire Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Diagnostic Questionnaires
2.3. Data Preprocessing
2.4. Comparison between Questionnaires
2.4.1. Canonical Correlation Analysis
2.4.2. Multiple Linear Regression (MLR)
2.5. Questionnaire Data-Based Clustering
2.6. FGID-Related Feature Selection
2.7. Validation of the Identified Subtypes
3. Results
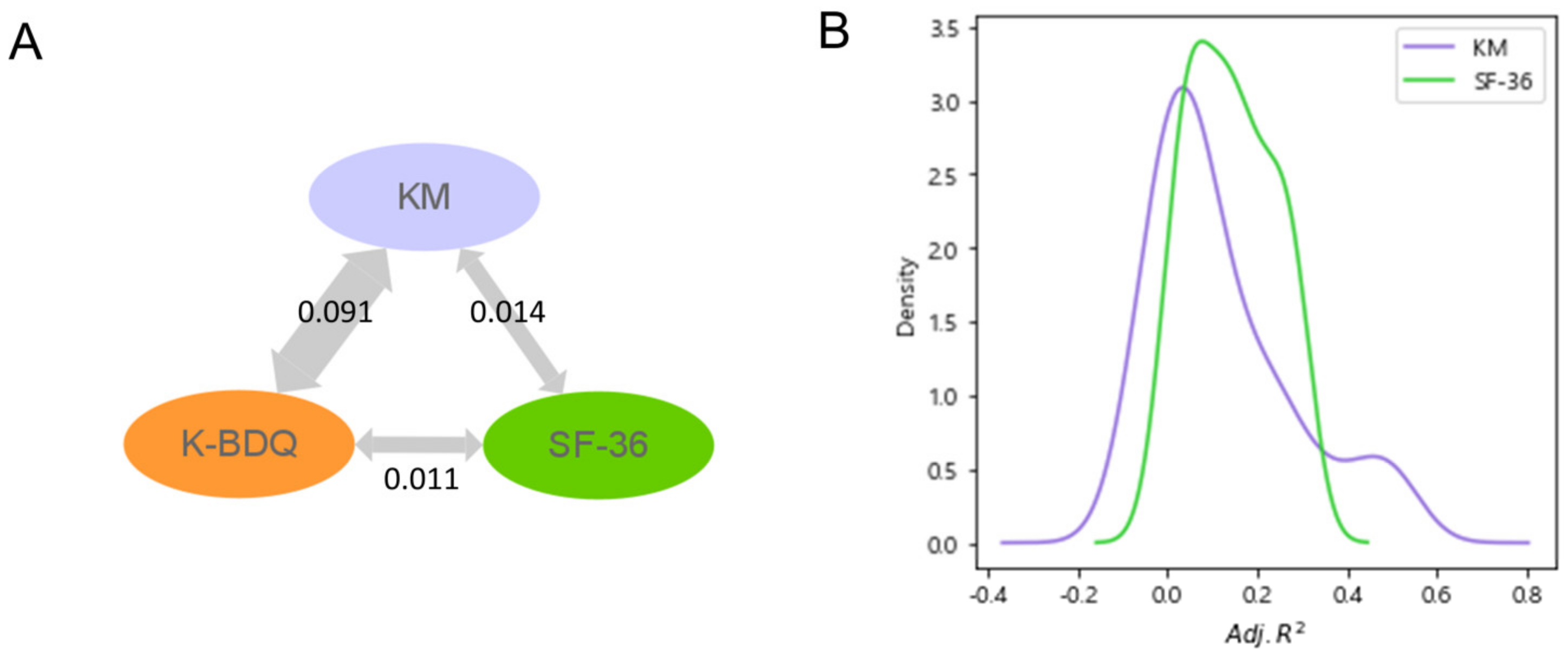
3.1. Assessing Complementary Information Obtained through KM and SF-36 Questionnaires Relative to K-BDQ
3.2. Revealing the Relationship between Complementary Information from KM and SF-36 and FGID Symptom Severity through Nonlinear Clustering Analysis
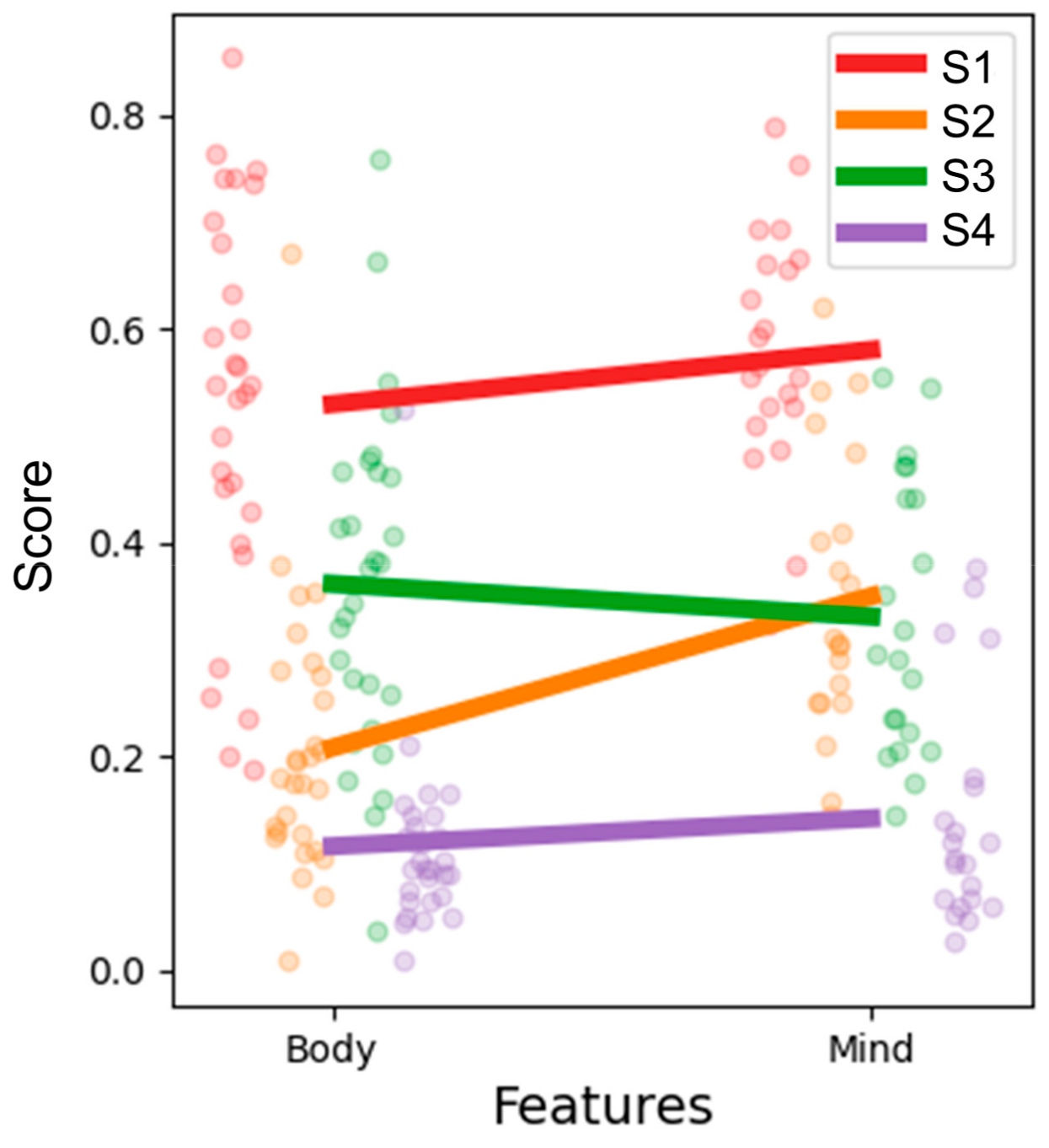
3.3. Development of a Novel Integrative Questionnaire Using the Clinically Relevant Information
3.4. Novel Subtype Identification Using the Integrative Questionnaire
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Black, C.J.; Drossman, D.A.; Talley, N.J.; Ruddy, J.; Ford, A.C. Functional gastrointestinal disorders: Advances in understanding and management. Lancet 2020, 396, 1664–1674. [Google Scholar] [CrossRef]
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114.e113. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Zhang, H.; Liu, Y.; Gao, K.; Zhang, J.; Fan, R.; Xie, S.; Xie, Z.; Wang, F.; Jiang, W. The Role of Serum Metabolomics in Distinguishing Chronic Rhinosinusitis with Nasal Polyp Phenotypes. Frontiers 2022, 7, 593976. [Google Scholar] [CrossRef]
- Van Oudenhove, L.; Levy, R.L.; Crowell, M.D.; Drossman, D.A.; Halpert, A.D.; Keefer, L.; Lackner, J.M.; Murphy, T.B.; Naliboff, B.D. Biopsychosocial Aspects of Functional Gastrointestinal Disorders. Gastroenterology 2016, 150, 1355–1367.e2. [Google Scholar] [CrossRef]
- Chang, L.; Di Lorenzo, C.; Farrugia, G.; Hamilton, F.A.; Mawe, G.M.; Pasricha, P.J.; Wiley, J.W. Functional bowel disorders: A roadmap to guide the next generation of research. Gastroenterology 2018, 154, 723–735. [Google Scholar] [CrossRef]
- Chey, W.D. Symposium report: An evidence-based approach to Ibs and Cic: Applying new advances to daily practice: A review of an adjunct clinical symposium of the American College of Gastroenterology Meeting October 16, 2016• Las Vegas, Nevada. Gastroenterol. Hepatol. 2017, 13, 1. [Google Scholar]
- Drossman, D.A. Functional gastrointestinal disorders: History, pathophysiology, clinical features, and Rome IV. Gastroenterology 2016, 150, 1262–1279.e1262. [Google Scholar] [CrossRef] [PubMed]
- Frissora, C.L.; Koch, K.L. Symptom overlap and comorbidity of irritable bowel syndrome with other conditions. Curr. Gastroenterol. Rep. 2005, 7, 264–271. [Google Scholar] [CrossRef]
- Talley, N.J.; Dennis, E.H.; Schettler-Duncan, V.A.; Lacy, B.E.; Olden, K.W.; Crowell, M.D. Overlapping upper and lower gastrointestinal symptoms in irritable bowel syndrome patients with constipation or diarrhea. Am. J. Gastroenterol. 2003, 98, 2454–2459. [Google Scholar] [CrossRef]
- Talley, N.J. A unifying hypothesis for the functional gastrointestinal disorders: Really multiple diseases or one irritable gut? Rev. Gastroenterol. Disord. 2006, 6, 72–78. [Google Scholar] [PubMed]
- Van Oudenhove, L.; Holvoet, L.; Vandenberghe, J.; Vos, R.; Tack, J. Do we have an alternative for the Rome III gastroduodenal symptom-based subgroups in functional gastroduodenal disorders? A cluster analysis approach. Neurogastroenterol. Motil. 2011, 23, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, J.; Talley, N. Empiric clustering of dyspepsia into symptom subgroups: A population-based study. Scand. J. Gastroenterol. 2002, 37, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Talley, N.J.; Holtmann, G.; Agréus, L.; Jones, M. Gastrointestinal symptoms and subjects cluster into distinct upper and lower groupings in the community: A four nations study. Am. J. Gastroenterol. 2000, 95, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Koloski, N.; Jones, M.; Young, M.; Talley, N. Differentiation of functional constipation and constipation predominant irritable bowel syndrome based on Rome III criteria: A population-based study. Aliment. Pharmacol. Ther. 2015, 41, 856–866. [Google Scholar] [CrossRef]
- Zinsmeister, A.R.; Herrick, L.M.; Loftus, Y.A.S.; Schleck, C.D.; Talley, N.J. Identification and validation of functional gastrointestinal disorder subtypes using latent class analysis: A population-based study. Scand. J. Gastroenterol. 2018, 53, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Ding, L. Psychosomatic Practice in Gastroenterology: New Insights and Models from China. Psychother. Psychosom. 2022, 88, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, J.-W.; Ha, N.-Y.; Kim, J.; Ryu, H.S. Herbal therapies in functional gastrointestinal disorders: A narrative review and clinical implication. Front. Psychiatry 2020, 11, 601. [Google Scholar] [CrossRef]
- Sankararaman, S.; Velayuthan, S.; Chen, Y.; Robertson, J.; Sferra, T.J. Role of traditional Chinese herbal medicines in functional gastrointestinal and motility disorders. Curr. Gastroenterol. Rep. 2022, 24, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Hwang, S.W.; Kim, N.; Park, J.H.; Oh, J.C.; Lee, D.H. Association between SLC6A4 serotonin transporter gene lainked polymorphic region and ADRA2A− 1291C>G and irritable bowel syndrome in Korea. J. Neurogastroenterol. Motil. 2014, 20, 388. [Google Scholar] [CrossRef]
- Hwang, S.W.; Kim, N.; Jung, H.K.; Park, J.H.; Choi, Y.J.; Kim, H.; Kim, J.; Kim, J.S.; Jung, H.C. The association of SLC 6 A 4 5-HTTLPR and TRPV1 945 G>C with functional dyspepsia in K orea. J. Gastroenterol. Hepatol. 2014, 29, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Noh, Y.W.; Jung, H.-K.; Kim, S.-E.; Jung, S.-A. Overlap of erosive and non-erosive reflux diseases with functional gastrointestinal disorders according to Rome III criteria. J. Neurogastroenterol. Motil. 2010, 16, 148. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-M.; Min, K.-J.; Park, D.-B. A study on the standardization of the hospital anxiety and depression scale for Koreans: A comparison of normal, depressed and anxious groups. J. Korean Neuropsychiatr. Assoc. 1999, 38, 289–296. [Google Scholar]
- Oh, H.-W.; Lee, J.-W.; Kim, J.-S.; Song, E.-Y.; Shin, S.-W.; Han, G.-J.; Lu, H.; Lee, J.-H. Study on the development of a standard instrument of diagnosis and assessment for spleen Qi deficiency pattern. J. Korean Med. 2014, 35, 157–170. [Google Scholar] [CrossRef]
- Lee, J.; Park, J.-W.; Ko, S.-J.; Kim, J. Development and validation of a new pattern identification scale for Stomach Qi Deficiency. Eur. J. Integr. Med. 2018, 17, 56–63. [Google Scholar] [CrossRef]
- Park, Y.-J.; Lim, J.-S.; Park, Y.-B. Development of a valid and reliable food retention questionnaire. Eur. J. Integr. Med. 2013, 5, 432–437. [Google Scholar] [CrossRef]
- Do, J.-H.; Jang, E.; Ku, B.; Jang, J.-S.; Kim, H.; Kim, J.Y. Development of an integrated Sasang constitution diagnosis method using face, body shape, voice, and questionnaire information. BMC Complement. Altern. Med. 2012, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.; Kim, J.; Kim, K.H.; Kim, J.; Kim, J.; Kim, J.; Kim, K.H. Reliability and validity analysis of a standard instrument of diagnosis and assessment for spleen Qi deficiency pattern in chronic dyspepsia patients. J. Korean Med. 2015, 36, 23–34. [Google Scholar] [CrossRef]
- Lee, J.; Yim, M.H.; Kim, J.Y. Test-retest reliability of the questionnaire in the Sasang constitutional analysis tool (SCAT). Integr. Med. Res. 2018, 7, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Hotelling, H. Relations between two sets of variates. In Breakthroughs in Statistics; Springer: Berlin/Heidelberg, Germany, 1992; pp. 162–190. [Google Scholar]
- Becht, E.; McInnes, L.; Healy, J.; Dutertre, C.-A.; Kwok, I.W.; Ng, L.G.; Ginhoux, F.; Newell, E.W. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 2019, 37, 38–44. [Google Scholar] [CrossRef]
- McInnes, L.; Healy, J.; Melville, J. Umap: Uniform manifold approximation and projection for dimension reduction. arXiv 2018, arXiv:1802.03426. [Google Scholar]
- Venna, J.; Kaski, S. Neighborhood preservation in nonlinear projection methods: An experimental study. In Proceedings of the International Conference on Artificial Neural Networks, Vienna, Austria, 21–25 August 2001; Springer: Berlin/Heidelberg, Germany, 2001; pp. 485–491. [Google Scholar]
- Rousseeuw, P.J. Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987, 20, 53–65. [Google Scholar] [CrossRef]
- Xuan, N.; Julien, V.; Wales, S.; Bailey, J. Information theoretic measures for clusterings comparison: Variants, properties, normalization and correction for chance. Prop. Norm. Correct. Chance 2010, 11, 2837–2854. [Google Scholar]
- Krstajic, D.; Buturovic, L.J.; Leahy, D.E.; Thomas, S. Cross-validation pitfalls when selecting and assessing regression and classification models. J. Cheminformatics 2014, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Geurts, P.; Ernst, D.; Wehenkel, L. Extremely randomized trees. Mach. Learn. 2006, 63, 3–42. [Google Scholar] [CrossRef]
- Allaoui, M.; Kherfi, M.L.; Cheriet, A. Considerably improving clustering algorithms using UMAP dimensionality reduction technique: A comparative study. In Proceedings of the International Conference on Image and Signal Processing, Marrakesh, Morocco, 4–6 June 2020; Springer: Cham, Switzerland, 2020; pp. 317–325. [Google Scholar]
- Fikree, A.; Byrne, P. Management of functional gastrointestinal disorders. Clin. Med. 2021, 21, 44. [Google Scholar] [CrossRef]
- Aboubakr, A.; Cohen, M.S. Functional bowel disease. Clin. Geriatr. Med. 2021, 37, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Berens, S.; Engel, F.; Gauss, A.; Tesarz, J.; Herzog, W.; Niesler, B.; Stroe-Kunold, E.; Schaefert, R. Patients with multiple functional gastrointestinal disorders (FGIDs) show increased illness severity: A cross-sectional study in a tertiary care FGID specialty clinic. Gastroenterol. Res. Pract. 2020, 2020, 9086340. [Google Scholar] [CrossRef] [PubMed]
- Bennett, E.; Piesse, C.; Palmer, K.; Badcock, C.; Tennant, C.; Kellow, J. Functional gastrointestinal disorders: Psychological, social, and somatic features. Gut 1998, 42, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Varis, K. Psychosomatic factors in gastrointestinal disorders. Ann. Clin. Res. 1987, 19, 135–142. [Google Scholar] [PubMed]
- Franks, I. Psychological factors in functional dyspepsia—Keeping an open mind. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 488. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rico, M.; Alvarado, J.M. A machine learning approach for studying the comorbidities of complex diagnoses. Behav. Sci. 2019, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, H.; Zhang, Y.; Zhang, T.; Gong, J.; Wei, Y.; Duan, Y.-G.; Shu, M.; Yang, Y.; Wu, D.; et al. Dimensionality reduction by UMAP reinforces sample heterogeneity analysis in bulk transcriptomic data. Cell Rep. 2021, 36, 109442. [Google Scholar] [CrossRef] [PubMed]
- Weijler, L.; Kowarsch, F.; Wödlinger, M.; Reiter, M.; Maurer-Granofszky, M.; Schumich, A.; Dworzak, M.N. UMAP Based Anomaly Detection for Minimal Residual Disease Quantification within Acute Myeloid Leukemia. Cancers 2022, 14, 898. [Google Scholar] [CrossRef] [PubMed]
- Miwa, H.; Kusano, M.; Arisawa, T.; Oshima, T.; Kato, M.; Joh, T.; Suzuki, H.; Tominaga, K.; Nakada, K.; Nagahara, A. Evidence-based clinical practice guidelines for functional dyspepsia. J. Gastroenterol. 2015, 50, 125–139. [Google Scholar] [CrossRef]
- Aziz, I.; Palsson, O.S.; Törnblom, H.; Sperber, A.D.; Whitehead, W.E.; Simrén, M. Epidemiology, clinical characteristics, and associations for symptom-based Rome IV functional dyspepsia in adults in the USA, Canada, and the UK: A cross-sectional population-based study. Lancet Gastroenterol. Hepatol. 2018, 3, 252–262. [Google Scholar] [CrossRef] [PubMed]



| Feature Name | Importance | Feature Name | Importance |
|---|---|---|---|
| Feeling of being slowed down | 0.0997 | Softer stool with abdomen pain | 0.0074 |
| Worrying thoughts in mind | 0.0918 | Decrease in the amount of food one eats | 0.0069 |
| Feeling like butterflies in the stomach | 0.0559 | Feeling of incomplete emptying | 0.0066 |
| Sudden feelings of panic | 0.0450 | Feeling calm | 0.0061 |
| Looking forward to things with enjoyment | 0.0425 | Abdomen pain disappearing after a bowel movement | 0.0060 |
| Bloating | 0.0363 | Vomiting | 0.0054 |
| Getting frightened feeling | 0.0351 | Food coma | 0.0053 |
| Burning pain in the chest (heartburn) | 0.0316 | Bodily pain interfering with normal work | 0.0053 |
| Feeling cheerful | 0.0285 | Having energy | 0.0052 |
| Being able to feel relaxed | 0.0255 | Tenderness below sternum | 0.0049 |
| Feeling tense | 0.0253 | Bodily pain | 0.0048 |
| Enjoying things I used to enjoy | 0.0250 | Nausea | 0.0047 |
| Feeling restless | 0.0217 | Frequent indigestion | 0.0045 |
| Food getting stuck in the chest | 0.0191 | Unhappy person | 0.0045 |
| Decrease in concentration | 0.0172 | Harder stool with abdomen pain | 0.0044 |
| Bloated stomach | 0.0169 | Unable to be as careful as usual (emotional) | 0.0043 |
| Feeling lethargic | 0.0157 | Loss of appetite | 0.0043 |
| Being able to laugh | 0.0127 | General health | 0.0039 |
| Feeling so down | 0.0119 | Full of pep | 0.0038 |
| Having lost interest in appearance | 0.0119 | Mushy or watery stools | 0.0038 |
| Acid reflux | 0.0115 | Feeling worn out | 0.0037 |
| Bloated stomach after meals | 0.0099 | Pale face | 0.0036 |
| Nausea | 0.0095 | Weight loss | 0.0035 |
| No energy to talk | 0.0088 | Indigestion | 0.0035 |
| Listless body | 0.0081 | Acid reflux | 0.0034 |
| Variable Name | S1 | S2 | S3 | S4 | |
|---|---|---|---|---|---|
| Body-symptom-related questions | Feeling of being slowed down | 0.60 | 0.13 | 0.48 | 0.10 |
| Bloating | 0.63 | 0.20 | 0.39 | 0.10 | |
| Burning pain in the chest (heartburn) | 0.59 | 0.13 | 0.38 | 0.09 | |
| Feeling of being slowed down | 0.75 | 0.38 | 0.41 | 0.17 | |
| Nausea | 0.40 | 0.07 | 0.16 | 0.05 | |
| Acid reflux | 0.47 | 0.11 | 0.27 | 0.06 | |
| Softer stool with abdomen pain | 0.39 | 0.13 | 0.23 | 0.09 | |
| Harder stool with abdomen pain | 0.20 | 0.09 | 0.20 | 0.10 | |
| Vomiting | 0.26 | 0.01 | 0.04 | 0.01 | |
| Mushy or watery stools | 0.28 | 0.14 | 0.15 | 0.09 | |
| Abdomen pain disappearing after a bowel movement | 0.24 | 0.14 | 0.21 | 0.10 | |
| Feeling of incomplete emptying | 0.19 | 0.11 | 0.18 | 0.05 | |
| Indigestion | 0.76 | 0.20 | 0.66 | 0.21 | |
| Bloated stomach | 0.74 | 0.28 | 0.48 | 0.15 | |
| Feeling lethargic | 0.70 | 0.25 | 0.42 | 0.12 | |
| Bloated stomach after meals | 0.74 | 0.29 | 0.52 | 0.16 | |
| Listless body | 0.74 | 0.35 | 0.47 | 0.15 | |
| Tenderness below sternum | 0.55 | 0.18 | 0.47 | 0.13 | |
| Decrease in the amount of food one eats | 0.68 | 0.36 | 0.55 | 0.17 | |
| No energy to talk | 0.57 | 0.21 | 0.29 | 0.05 | |
| Nausea | 0.50 | 0.11 | 0.27 | 0.08 | |
| Pale face | 0.46 | 0.17 | 0.35 | 0.05 | |
| Weight loss | 0.43 | 0.32 | 0.38 | 0.07 | |
| Acid reflux | 0.55 | 0.21 | 0.33 | 0.14 | |
| Frequent indigestion | 0.54 | 0.20 | 0.41 | 0.13 | |
| Food coma | 0.53 | 0.18 | 0.32 | 0.10 | |
| Bodily pain | 0.57 | 0.28 | 0.46 | 0.12 | |
| Bodily pain interfering with normal work | 0.45 | 0.18 | 0.26 | 0.07 | |
| General health | 0.85 | 0.67 | 0.76 | 0.53 | |
| Mind-symptom-related questions | Worrying thoughts in mind | 0.63 | 0.36 | 0.35 | 0.12 |
| Feeling cheerful | 0.69 | 0.54 | 0.44 | 0.17 | |
| Enjoying things I used to enjoy | 0.60 | 0.40 | 0.24 | 0.05 | |
| Feeling like butterflies in the stomach | 0.53 | 0.25 | 0.27 | 0.05 | |
| Having lost interest in appearance | 0.48 | 0.37 | 0.21 | 0.07 | |
| Feeling tense | 0.56 | 0.27 | 0.30 | 0.10 | |
| Looking forward to things with enjoyment | 0.56 | 0.30 | 0.21 | 0.06 | |
| Sudden feelings of panic | 0.53 | 0.31 | 0.29 | 0.08 | |
| Getting frightened feeling | 0.51 | 0.25 | 0.24 | 0.07 | |
| Being able to feel relaxed | 0.59 | 0.41 | 0.38 | 0.18 | |
| Being able to laugh | 0.38 | 0.25 | 0.15 | 0.10 | |
| Feeling restless | 0.32 | 0.15 | 0.18 | 0.03 | |
| Decrease in concentration | 0.69 | 0.34 | 0.48 | 0.12 | |
| Loss of appetite | 0.56 | 0.31 | 0.47 | 0.11 | |
| Unable to be as careful as usual (emotional) | 0.67 | 0.16 | 0.20 | 0.06 | |
| Feeling worn out | 0.49 | 0.29 | 0.32 | 0.14 | |
| Having energy | 0.76 | 0.55 | 0.56 | 0.32 | |
| Feeling so down | 0.54 | 0.21 | 0.22 | 0.13 | |
| Full of pep | 0.79 | 0.62 | 0.55 | 0.38 | |
| Feeling calm | 0.66 | 0.48 | 0.47 | 0.31 | |
| Unhappy person | 0.66 | 0.51 | 0.44 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-Y.; Bae, H.; Jeong, H.-Y.; Lee, J.Y.; Kwon, Y.-K.; Kim, C.-E. Identifying Novel Subtypes of Functional Gastrointestinal Disorder by Analyzing Nonlinear Structure in Integrative Biopsychosocial Questionnaire Data. J. Clin. Med. 2024, 13, 2821. https://doi.org/10.3390/jcm13102821
Park S-Y, Bae H, Jeong H-Y, Lee JY, Kwon Y-K, Kim C-E. Identifying Novel Subtypes of Functional Gastrointestinal Disorder by Analyzing Nonlinear Structure in Integrative Biopsychosocial Questionnaire Data. Journal of Clinical Medicine. 2024; 13(10):2821. https://doi.org/10.3390/jcm13102821
Chicago/Turabian StylePark, Sa-Yoon, Hyojin Bae, Ha-Yeong Jeong, Ju Yup Lee, Young-Kyu Kwon, and Chang-Eop Kim. 2024. "Identifying Novel Subtypes of Functional Gastrointestinal Disorder by Analyzing Nonlinear Structure in Integrative Biopsychosocial Questionnaire Data" Journal of Clinical Medicine 13, no. 10: 2821. https://doi.org/10.3390/jcm13102821






