Preparation of Hydrophilic and Fire-Resistant Phytic Acid/Chitosan/Polydopamine-Coated Expanded Polystyrene Particles by Using Coating Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
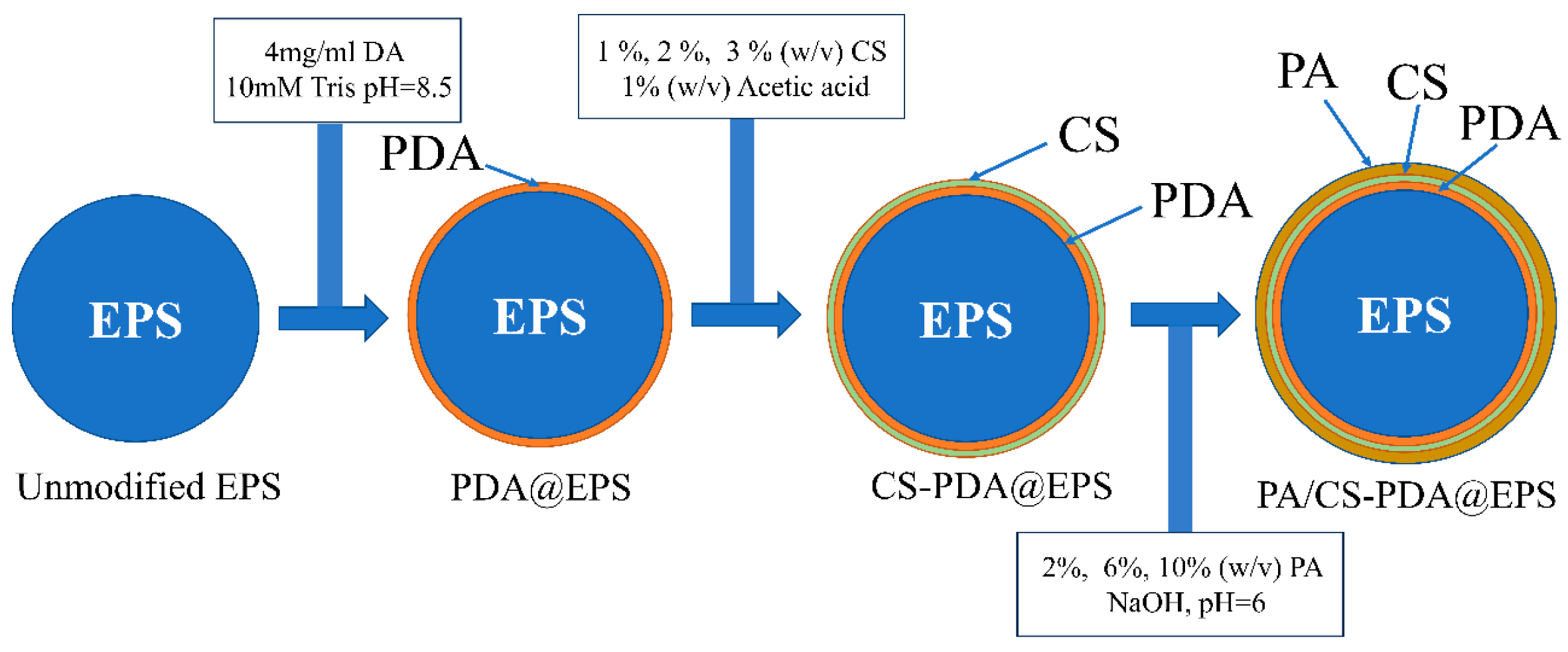
2.2. Preparation of Modified EPS Particles
2.2.1. Preparation of PDA-Coated Modified EPS Particles
2.2.2. Preparation of CS-Modified PDA@EPS Particles
2.2.3. Preparation of PA/CS-Modified PDA@EPS Particles
2.3. Characterizations
3. Results
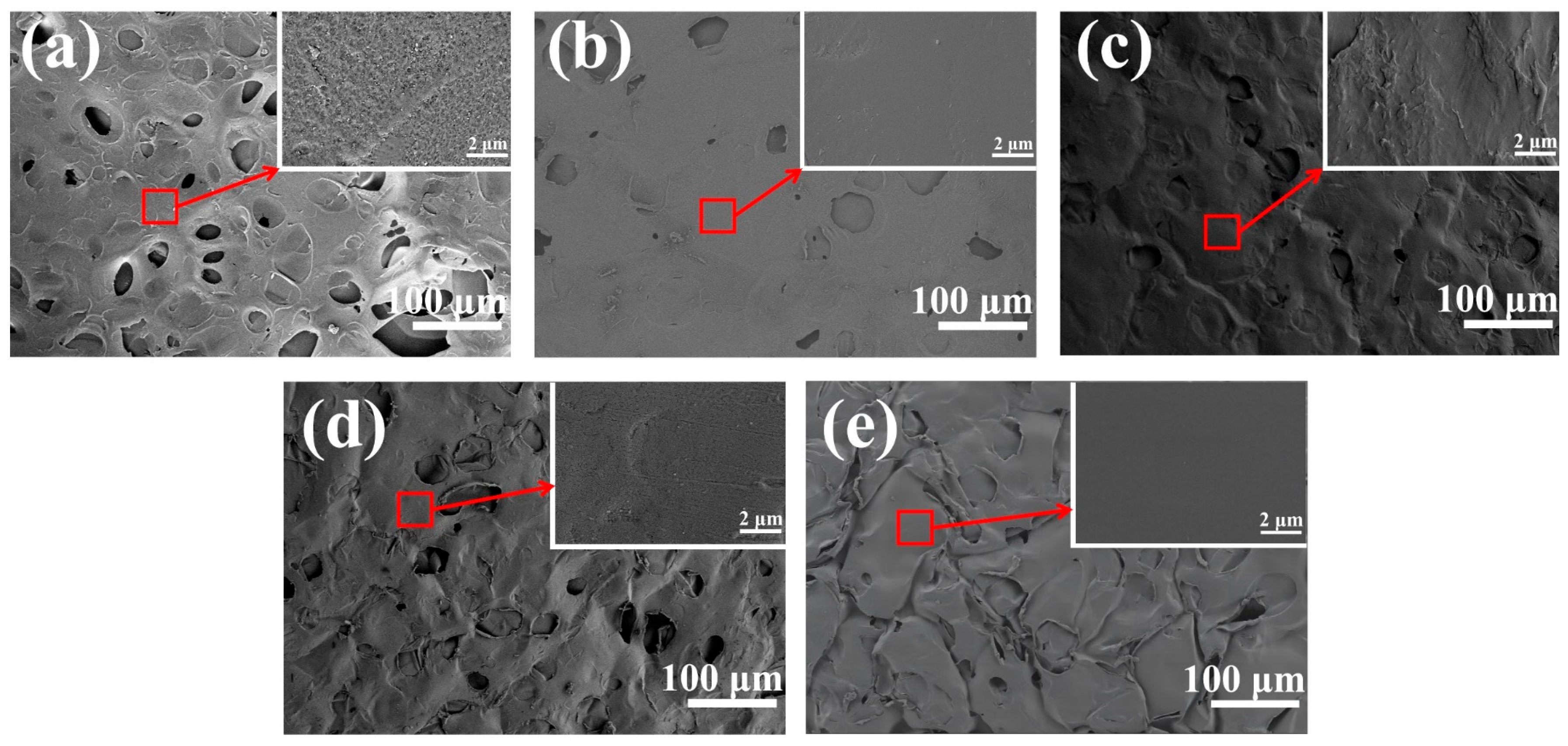
3.1. Morphology and Coating Efficiency
3.2. FTIR Analyses of EPS Samples
3.3. Water Contact Angle of EPS Samples
3.4. Thermal Stability Properties of Eps Samples
3.5. Flammability Test of EPS Samples
3.6. Water Resistance Test of EPS Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berardi, U. A cross-country comparison of the building energy consumptions and their trends. Resour. Conserv. Recycl. 2017, 123, 230–241. [Google Scholar] [CrossRef]
- Huang, Y.; Niu, J.L.; Chung, T.M. Study on performance of energy-efficient retrofitting measures on commercial building external walls in cooling-dominant cities. Appl. Energy 2013, 103, 97–108. [Google Scholar] [CrossRef]
- Shi, J.Y.; Liu, B.J.; Liu, Y.C.; Wang, E.L.; He, Z.H.; Xu, H.J.; Ren, X.D. Preparation and characterization of lightweight aggregate foamed geopolymer concretes aerated using hydrogen peroxide. Constr. Build. Mater. 2020, 256, 119442. [Google Scholar] [CrossRef]
- Rashad, A.M.; Mosleh, Y.A.; Mokhtar, M.M. Thermal insulation and durability of alkali-activated lightweight slag mortar modified with silica fume and fly ash. Constr. Build. Mater. 2024, 411, 134255. [Google Scholar] [CrossRef]
- Cavalline, T.L.; Gallegos, J.; Castrodale, R.W.; Freeman, C.; Liner, J.; Wall, J. Influence of Lightweight Aggregate Concrete Materials on Building Energy Performance. Buildings 2021, 11, 94. [Google Scholar] [CrossRef]
- Aditya, L.; Mahlia, T.M.I.; Rismanchi, B.; Ng, H.M.; Hasan, M.H.; Metselaar, H.S.C.; Muraza, O.; Aditiya, H.B. A review on insulation materials for energy conservation in buildings. Renew. Sustain. Energy Rev. 2017, 73, 1352–1365. [Google Scholar] [CrossRef]
- Hung Anh, L.D.; Pásztory, Z. An overview of factors influencing thermal conductivity of building insulation materials. J. Build. Eng. 2021, 44, 102604. [Google Scholar] [CrossRef]
- Bouzit, S.; Merli, F.; Sonebi, M.; Buratti, C.; Taha, M. Gypsum-plasters mixed with polystyrene balls for building insulation: Experimental characterization and energy performance. Constr. Build. Mater. 2021, 283, 122625. [Google Scholar] [CrossRef]
- Sayadi, A.A.; Tapia, J.V.; Neitzert, T.R.; Clifton, G.C. Effects of expanded polystyrene (EPS) particles on fire resistance, thermal conductivity and compressive strength of foamed concrete. Constr. Build. Mater. 2016, 112, 716–724. [Google Scholar] [CrossRef]
- Mehta, S.; Biederman, S.; Shivkumar, S. Thermal-Degradation of Foamed Polystyrene. J. Mater. Sci. 1995, 30, 2944–2949. [Google Scholar] [CrossRef]
- Xu, Q.; Jin, C.; Griffin, G.; Jiang, Y. Fire safety evaluation of expanded polystyrene foam by multi-scale methods. J. Therm. Anal. Calorim. 2013, 115, 1651–1660. [Google Scholar] [CrossRef]
- Jin, Z.; Ma, B.; Su, Y.; Qi, H.; Lu, W.; Zhang, T. Preparation of eco-friendly lightweight gypsum: Use of beta-hemihydrate phosphogypsum and expanded polystyrene particles. Constr. Build. Mater. 2021, 297, 123837. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, Z.; Chen, T.; Zhu, Y.; Lv, Z.; Gong, X.; Niu, Y.; Ma, B. Preparation of highly dispersed expandable graphite/polystyrene composite foam via suspension polymerization with enhanced fire retardation. Carbon 2019, 146, 503–512. [Google Scholar] [CrossRef]
- Hamdani-Devarennes, S.; El Hage, R.; Dumazert, L.; Sonnier, R.; Ferry, L.; Lopez-Cuesta, J.M.; Bert, C. Water-based flame retardant coating using nano-boehmite for expanded polystyrene (EPS) foam. Prog. Org. Coat. 2016, 99, 32–46. [Google Scholar] [CrossRef]
- Wang, Y.C.; Zhao, J.P. Comparative study on flame retardancy of silica fume-based geopolymer activated by different activators. J. Alloys Compd. 2018, 743, 108–114. [Google Scholar] [CrossRef]
- Gheonea, R.; Crasmareanu, E.C.; Plesu, N.; Sauca, S.; Simulescu, V.; Ilia, G. New Hybrid Materials Synthesized with Different Dyes by Sol-Gel Method. Adv. Mater. Sci. Eng. 2017, 2017, 4537039. [Google Scholar] [CrossRef]
- Vahabi, H.; Gholami, F.; Tomas, M.; Movahedifar, E.; Yazdi, M.K.; Saeb, M.R. Hydrogel and aerogel-based flame-retardant polymeric materials: A review. J. Vinyl Addit. Technol. 2024, 30, 5–25. [Google Scholar] [CrossRef]
- Chen, M.; Tang, M.Q.; Qi, F.; Chen, X.L.; He, W.D. Microencapsulated ammonium polyphosphate and its application in the flame retardant polypropylene composites. J. Fire Sci. 2015, 33, 374–389. [Google Scholar] [CrossRef]
- Guo, W.W.; Wang, X.; Zhang, P.; Liu, J.J.; Song, L.; Hu, Y. Nano-fibrillated cellulose-hydroxyapatite based composite foams with excellent fire resistance. Carbohydr. Polym. 2018, 195, 71–78. [Google Scholar] [CrossRef]
- Varnagiris, S.; Tuckute, S.; Lelis, M.; Milcius, D. SiO2 films as heat resistant layers for protection of expandable polystyrene foam from flame torch-induced heat. J. Thermoplast. Compos. Mater. 2018, 31, 657–667. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, B.W.; Zhou, Y.X.; Essawy, H.; Zhao, C.L.; Wu, Z.G.; Zhou, X.J.; Hou, D.F.; Du, G.B. Gelatinized starch-furanic hybrid as a biodegradable thermosetting resin for fabrication of foams for building materials. Carbohydr. Polym. 2022, 298, 120157. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Wang, D.; Guo, J.; Fei, B.; Gu, X.; Li, H.; Sun, J.; Zhang, S. The preparation of starch derivatives reacted with urea-phosphoric acid and effects on fire performance of expandable polystyrene foams. Carbohydr. Polym. 2020, 233, 115841. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-E.; Yan, Y.-W.; Zhao, H.-B.; Jian, R.-K.; Wang, Y.-Z. A facile and efficient flame-retardant and smoke-suppressant resin coating for expanded polystyrene foams. Compos. Part B 2020, 185, 107797. [Google Scholar] [CrossRef]
- Cao, B.; Yu, T.; Sun, J.; Gu, X.Y.; Liu, X.D.; Li, H.F.; Fei, B.; Zhang, S. Improving the fire performance and smoke suppression of expandable polystyrene foams by coating with multi-dimensional carbon nanoparticles. J. Appl. Polym. Sci. 2020, 137, 49227. [Google Scholar] [CrossRef]
- Wang, L.Y.; Wang, C.; Liu, P.W.; Jing, Z.J.; Ge, X.S.; Jiang, Y.J. The flame resistance properties of expandable polystyrene foams coated with a cheap and effective barrier layer. Constr. Build. Mater. 2018, 176, 403–414. [Google Scholar] [CrossRef]
- Jia, Y.; Luo, B.; Lee, S.H.; Huang, H.; Wu, Z.; Zhou, B.; Zhou, X.; Zhang, J. Facile preparation of high-performance plywood adhesive from gelatinized corn starch crosslinked with ammonium dihydrogen phosphate. Int. J. Biol. Macromol. 2024, 256, 128548. [Google Scholar] [CrossRef]
- Takigami, H.; Watanabe, M.; Kajiwara, N. Destruction behavior of hexabromocyclododecanes during incineration of solid waste containing expanded and extruded polystyrene insulation foams. Chemosphere 2014, 116, 24–33. [Google Scholar] [CrossRef]
- Hou, M.M.; Wang, Y.; Zhao, H.X.; Zhang, Q.N.; Xie, Q.; Zhang, X.J.; Chen, R.Z.; Chen, J.W. Halogenated flame retardants in building and decoration materials in China: Implications for human exposure via inhalation and dust ingestion. Chemosphere 2018, 203, 291–299. [Google Scholar] [CrossRef]
- van der Veen, I.; de Boer, J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012, 88, 1119–1153. [Google Scholar] [CrossRef]
- Huo, S.Q.; Song, P.A.; Yu, B.; Ran, S.Y.; Chevali, V.S.; Liu, L.; Fang, Z.P.; Wang, H. Phosphorus-containing flame retardant epoxy thermosets: Recent advances and future perspectives. Prog. Polym. Sci. 2021, 114, 101366. [Google Scholar] [CrossRef]
- Ji, W.F.; Yao, Y.; Guo, J.; Fei, B.; Gu, X.Y.; Li, H.F.; Sun, J.; Zhang, S. Toward an understanding of how red phosphorus and expandable graphite enhance the fire resistance of expandable polystyrene foams. J. Appl. Polym. Sci. 2020, 137, 49045. [Google Scholar] [CrossRef]
- Yan, Y.W.; Huang, J.Q.; Guan, Y.H.; Shang, K.; Jian, R.K.; Wang, Y.Z. Flame retardance and thermal degradation mechanism of polystyrene modified with aluminum hypophosphite. Polym. Degrad. Stab. 2014, 99, 35–42. [Google Scholar] [CrossRef]
- Shao, X.M.; Du, Y.Q.; Zheng, X.F.; Wang, J.C.; Wang, Y.C.; Zhao, S.; Xin, Z.X.; Li, L. Reduced fire hazards of expandable polystyrene building materials via intumescent flame-retardant coatings. J. Mater. Sci. 2020, 55, 7555–7572. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Hiziroglu, S.; Waisurasingha, C.; Kasemsiri, P. Properties of Wood Flour/Expanded Polystyrene Waste Composites Modified With Diammonium Phosphate Flame Retardant. Polym. Compos. 2015, 36, 604–612. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Zhang, X.H.; Liu, F.; Chen, S.; Qi, G.R. Novel flame retardant thermosets from nitrogen-containing and phosphorus-containing epoxy resins cured with dicyandiamide. J. Appl. Polym. Sci. 2007, 106, 2391–2397. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.S.; Liu, Y.; Wang, Q. Properties and mechanisms of different guanidine flame retardant wood pulp paper. J. Anal. Appl. Pyrolysis 2017, 128, 224–231. [Google Scholar] [CrossRef]
- Ruan, C.P.; Ai, K.L.; Li, X.B.; Lu, L.H. A Superhydrophobic Sponge with Excellent Absorbency and Flame Retardancy. Angew. Chem. Int. Ed. 2014, 53, 5556–5560. [Google Scholar] [CrossRef]
- Chen, M.J.; Shao, Z.B.; Wang, X.L.; Chen, L.; Wang, Y.Z. Halogen-Free Flame-Retardant Flexible Polyurethane Foam with a Novel Nitrogen-Phosphorus Flame Retardant. Ind. Eng. Chem. Res. 2012, 51, 9769–9776. [Google Scholar] [CrossRef]
- Castellano, A.; Colleoni, C.; Iacono, G.; Mezzi, A.; Plutino, M.R.; Malucelli, G.; Rosace, G. Synthesis and characterization of a phosphorous/nitrogen based sol-gel coating as a novel halogen- and formaldehyde-free flame retardant finishing for cotton fabric. Polym. Degrad. Stab. 2019, 162, 148–159. [Google Scholar] [CrossRef]
- Zhu, Z.M.; Xu, Y.J.; Liao, W.; Xu, S.M.; Wang, Y.Z. Highly Flame Retardant Expanded Polystyrene Foams from Phosphorus-Nitrogen-Silicon Synergistic Adhesives. Ind. Eng. Chem. Res. 2017, 56, 4649–4658. [Google Scholar] [CrossRef]
- Maqsood, M.; Seide, G. Biodegradable Flame Retardants for Biodegradable Polymer. Biomolecules 2020, 10, 1038. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, A.; Cheng, Y.; Li, M.; Cui, Y.; Li, Z. Recent advances in biomass phytic acid flame retardants. Polym. Test. 2023, 124, 108100. [Google Scholar] [CrossRef]
- Taib, M.; Antov, P.; Savov, V.; Fatriasari, W.; Madyaratri, E.W.; Wirawan, R.; Osvaldová, L.M.; Hua, L.S.; Ghani, M.A.A.; Al Edrus, S.; et al. Current progress of biopolymer-based flame retardant. Polym. Degrad. Stab. 2022, 205, 110153. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Jin, X.; Li, H.; Sun, J.; Gu, X. The novel application of chitosan: Effects of cross-linked chitosan on the fire performance of thermoplastic polyurethane. Carbohydr. Polym. 2018, 189, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gu, X.; Sun, J.; Zhang, S. Preparation and characterization of chitosan derivatives and their application as flame retardants in thermoplastic polyurethane. Carbohydr. Polym. 2017, 167, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.Z.; Wang, X.Y.; Li, Y.T.; Tan, S.M.; Zhao, S.; Li, L. Synthesis of bio-based toughening phosphorus-nitrogen flame retardant and study on high toughness EPS flame retardant insulation sheet. Polym. Degrad. Stab. 2023, 218, 110560. [Google Scholar] [CrossRef]
- Qin, M.; Hu, X.; Guo, J. Preparation of a New Type of Expansion Flame Retardant and Application in Polystyrene. Coatings 2023, 13, 733. [Google Scholar] [CrossRef]
- Nabipour, H.; Wang, X.; Song, L.; Hu, Y. A fully bio-based coating made from alginate, chitosan and hydroxyapatite for protecting flexible polyurethane foam from fire. Carbohydr. Polym. 2020, 246, 116641. [Google Scholar] [CrossRef]
- Chen, H.B.; Shen, P.; Chen, M.J.; Zhao, H.B.; Schiraldi, D.A. Highly Efficient Flame Retardant Polyurethane Foam with Alginate/Clay Aerogel Coating. ACS Appl. Mater. Interfaces 2016, 8, 32557–32564. [Google Scholar] [CrossRef]
- Cho, J.H.; Vasagar, V.; Shanmuganathan, K.; Jones, A.R.; Nazarenko, S.; Ellison, C.J. Bioinspired Catecholic Flame Retardant Nanocoating for Flexible Polyurethane Foams. Chem. Mater. 2015, 27, 6784–6790. [Google Scholar] [CrossRef]
- Yang, W.; Wu, S.; Yang, W.; Chun-Yin Yuen, A.; Zhou, Y.; Yeoh, G.; Boyer, C.; Wang, C.H. Nanoparticles of polydopamine for improving mechanical and flame-retardant properties of an epoxy resin. Compos. Part B 2020, 186, 107828. [Google Scholar] [CrossRef]
- Furtado, L.M.; Ando, R.A.; Petri, D.F.S. Polydopamine-coated cellulose acetate butyrate microbeads for caffeine removal. J. Mater. Sci. 2019, 55, 3243–3258. [Google Scholar] [CrossRef]
- Huang, D.; Zheng, Y.; Quan, Q. Enhanced mechanical properties and UV shield of carboxymethyl cellulose films with polydopamine-modified natural fibre-like palygorskite. Appl. Clay Sci. 2019, 183, 105314. [Google Scholar] [CrossRef]
- Yassin, M.A.; Gad, A.A.M. Immobilized Enzyme on Modified Polystyrene Foam Waste: A Biocatalyst for Wastewater Decolorization. J. Environ. Chem. Eng. 2020, 8, 104435. [Google Scholar] [CrossRef]
- Jin, Z.; Xiao, Y.; Xu, Z.; Zhang, Z.; Wang, H.; Mu, X.; Gui, Z. Dopamine-modified poly(styrene) nanospheres as new high-speed adsorbents for copper-ions having enhanced smoke-toxicity-suppression and flame-retardancy. J. Colloid Interface Sci. 2021, 582, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Hecker, M.; Ting, M.S.H.; Malmström, J. Simple coatings to render polystyrene protein resistant. Coatings 2018, 8, 55. [Google Scholar] [CrossRef]
- Huang, B.-H.; Li, S.-Y.; Chiou, Y.-J.; Chojniak, D.; Chou, S.-C.; Wong, V.C.M.; Chen, S.-Y.; Wu, P.-W. Electrophoretic fabrication of a robust chitosan/polyethylene glycol/polydopamine composite film for UV-shielding application. Carbohydr. Polym. 2021, 273, 118560. [Google Scholar] [CrossRef] [PubMed]
- Stephy, A.; Antony, A.M.; Francis, T. Thermal Degradation Kinetics of Chitosan/Phytic Acid Polyelectrolyte Complex as Investigated by Thermogravimetric Analysis. J. Appl. Polym. Sci. 2023, 31, 210–220. [Google Scholar] [CrossRef]
- Deak, N.A.; Johnson, L.A. Fate of phytic acid in producing soy protein ingredients. J. Am. Oil Chem. Soc. 2007, 84, 369–376. [Google Scholar] [CrossRef]
- Sim, G.Y.; Lee, S.U.; Lee, J.W. Enhanced extraction of phytic acid from rice hulls with enzymatic treatment and production of ethanol from reducing sugars in hydrolyzed rice hulls after extraction of phytic acid. Lwt-Food Sci. Technol. 2020, 133, 110111. [Google Scholar] [CrossRef]
- Bloot, A.P.M.; Kalschne, D.L.; Amaral, J.A.S.; Baraldi, I.J.; Canan, C. A Review of Phytic Acid Sources, Obtention, and Applications. Food Rev. Int. 2023, 39, 73–92. [Google Scholar] [CrossRef]
- Cheng, X.W.; Guan, J.P.; Yang, X.H.; Tang, R.C.; Yao, F. A bio-resourced phytic acid/chitosan polyelectrolyte complex for the flame retardant treatment of wool fabric. J. Clean. Prod. 2019, 223, 342–349. [Google Scholar] [CrossRef]
- Li, M.-E.; Zhao, H.-B.; Cheng, J.-B.; Wang, T.; Fu, T.; Zhang, A.-N.; Wang, Y.-Z. An Effective Green Porous Structural Adhesive for Thermal Insulating, Flame-Retardant, and Smoke-Suppressant Expandable Polystyrene Foam. Engineering 2022, 17, 151–160. [Google Scholar] [CrossRef]
- Laufer, G.; Kirkland, C.; Morgan, A.B.; Grunlan, J.C. Intumescent multilayer nanocoating, made with renewable polyelectrolytes, for flame-retardant cotton. Biomacromolecules 2012, 13, 2843–2848. [Google Scholar] [CrossRef]
- van den Broek, L.A.; Knoop, R.J.; Kappen, F.H.; Boeriu, C.G. Chitosan films and blends for packaging material. Carbohydr. Polym. 2015, 116, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Sivanesan, I.; Gopal, J.; Muthu, M.; Shin, J.; Mari, S.; Oh, J. Green Synthesized Chitosan/Chitosan Nanoforms/Nanocomposites for Drug Delivery Applications. Polymers 2021, 13, 2256. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Riva, R.; Ragelle, H.; des Rieux, A.; Duhem, N.; Jérôme, C.; Préat, V. Chitosan and Chitosan Derivatives in Drug Delivery and Tissue Engineering. Adv. Polym. Sci. 2011, 244, 19–44. [Google Scholar]
- Chen, C.; Gu, X.; Jin, X.; Sun, J.; Zhang, S. The effect of chitosan on the flammability and thermal stability of polylactic acid/ammonium polyphosphate biocomposites. Carbohydr. Polym. 2017, 157, 1586–1593. [Google Scholar] [CrossRef]
- Lee, D.W.; Lim, H.; Chong, H.N.; Shim, W.S. Advances in chitosan material and its hybrid derivatives: A review. Open Biomed. J. 2009, 1, 10–20. [Google Scholar] [CrossRef]
- Cheng, X.; Shi, L.; Fan, Z.; Yu, Y.; Liu, R. Bio-based coating of phytic acid, chitosan, and biochar for flame-retardant cotton fabrics. Polym. Degrad. Stab. 2022, 199, 109898. [Google Scholar] [CrossRef]
- Fang, Y.; Sun, W.; Li, J.; Liu, H.; Liu, X. Eco-friendly flame retardant and dripping-resistant of polyester/cotton blend fabrics through layer-by-layer assembly fully bio-based chitosan/phytic acid coating. Int. J. Biol. Macromol. 2021, 175, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Spriano, S.; Riccucci, G.; Örlygsson, G.; Ng, C.H.; Vernè, E.; Sehn, F.P.; de Oliveira, P.T.; Ferraris, S. Coating of bioactive glasses with chitosan: The effects of the glass composition and coating method on the surface properties, including preliminary in vitro results. Surf. Coat. Technol. 2023, 470, 129824. [Google Scholar] [CrossRef]
- Xie, L.; Liu, Y.; Zhang, W.; Xu, S. A dopamine/tannic-acid-based co-deposition combined with phytic acid modification to enhance the anti-fouling property of RO membrane. Membranes 2021, 11, 342. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Zhang, T.; Luo, Y.X.; Wang, Y.X.; Li, J.C.; Ye, T.; Guo, R.F.; Song, P.A.; Zhou, J.; Wang, H. Multifunctional polyurethane sponge coatings with excellent flame retardant, antibacterial, compressible, and recyclable properties. Compos. Part B 2021, 215, 108785. [Google Scholar] [CrossRef]
- Xu, B.T.; Jin, D.Z.; Yu, Y.; Zhang, Q.; Weng, W.J.; Ren, K.X.; Tai, Y.L. Nanoclay-reinforced alginate aerogels: Preparation and properties. Rsc Adv. 2024, 14, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Lazar, S.; Kolibaba, T.J.; Shen, R.Q.; Quan, Y.F.; Wang, Q.S.; Chiang, H.C.; Palen, B.; Grunlan, J.C. Environmentally Benign and Self-Extinguishing Multilayer Nanocoating for Protection of Flammable Foam. ACS Appl. Mater. Interfaces 2020, 12, 49130–49137. [Google Scholar] [CrossRef]
- Gonçalves, O.H.; Leimann, F.V.; de Araújo, P.H.H.; Machado, R.A.F. Expansion of core–shell PS/PMMA particles. J. Appl. Polym. Sci. 2013, 130, 4521–4527. [Google Scholar] [CrossRef]
- Han, D.; Zhao, H.; Gao, L.; Qin, Z.; Ma, J.; Han, Y.; Jiao, T. Preparation of carboxymethyl chitosan/phytic acid composite hydrogels for rapid dye adsorption in wastewater treatment. Colloids Surf. A 2021, 628, 127355. [Google Scholar] [CrossRef]
- Li, S.; Zhao, F.; Wang, X.; Liu, Z.; Guo, J.; Li, Y.; Tan, S.; Xin, Z.; Zhao, S.; Li, L. A green flame retardant coating based on one-step aqueous complexation of phytic acid and urea for fabrication of lightweight and high toughness flame retardant EPS insulation board. Polym. Degrad. Stab. 2024, 219, 110597. [Google Scholar] [CrossRef]
- Bhoite, S.P.; Kim, J.; Jo, W.; Bhoite, P.H.; Mali, S.S.; Park, K.H.; Hong, C.K. Expanded Polystyrene Beads Coated with Intumescent Flame Retardant Material to Achieve Fire Safety Standards. Polymers 2021, 13, 2662. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Fu, J.; Liu, S. Facile preparation of Au nanoparticle-embedded polydopamine hollow microcapsule and its catalytic activity for the reduction of methylene blue. J. Macromol. Sci. Part A Pure Appl. Chem. 2019, 56, 1104–1113. [Google Scholar] [CrossRef]
- MohammadAlizadeh, A.; Elmi, F. Flame retardant and superoleophilic polydopamine/chitosan-graft (g)-octanal coated polyurethane foam for separation oil/water mixtures. Int. J. Biol. Macromol. 2024, 259, 129237. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Wang, M.; Chen, D. Preparation of polydopamine-modified graphene oxide/chitosan aerogel for uranium (VI) adsorption. Ind. Eng. Chem. Res. 2018, 57, 8472–8483. [Google Scholar] [CrossRef]
- Han, G.; Liu, S.; Pan, Z.; Lin, Y.; Ding, S.; Li, L.; Luo, B.; Jiao, Y.; Zhou, C. Sulfonated chitosan and phosphorylated chitosan coated polylactide membrane by polydopamine-assisting for the growth and osteogenic differentiation of MC3T3-E1s. Carbohydr. Polym. 2020, 229, 115517. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef]
- Omar, H.; Fardous, R.; Alhindi, Y.M.; Aodah, A.H.; Alyami, M.; Alsuabeyl, M.S.; Alghamdi, W.M.; Alhasan, A.H.; Almalik, A. α1-acid glycoprotein-decorated hyaluronic acid nanoparticles for suppressing metastasis and overcoming drug resistance breast cancer. Biomedicines 2022, 10, 414. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Song, S.K.; Dong, L.B.; Guo, J.Z.; Wang, J.C.; Tan, S.M.; Li, Y.T.; Shen, M.; Zhao, S.; Li, L.; et al. Bio-based phytic acid and urea interfacial layer by layer assembly for flame-retardant cotton. Polym. Degrad. Stab. 2023, 216, 110479. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Pan, Y.T.; Yáñez, A.P.; Hu, S.; Zhang, X.Q.; Wang, R.; Wang, D.Y. Polydopamine induced natural fiber surface functionalization: A way towards flame retardancy of flax/poly(lactic acid) biocomposites. Compos. Part B 2018, 154, 56–63. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Zhu, L.; Zhang, H.; Zhu, B.; Xu, Y. Antifouling and antimicrobial polymer membranes based on bioinspired polydopamine and strong hydrogen-bonded poly (N-vinyl pyrrolidone). ACS Appl. Mater. Interfaces 2013, 5, 12895–12904. [Google Scholar] [CrossRef] [PubMed]
- Kumar Kundu, C.; Wang, W.; Zhou, S.; Wang, X.; Sheng, H.; Pan, Y.; Song, L.; Hu, Y. A green approach to constructing multilayered nanocoating for flame retardant treatment of polyamide 66 fabric from chitosan and sodium alginate. Carbohydr. Polym. 2017, 166, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.W.; Guan, J.P.; Tang, R.C.; Liu, K.Q. Phytic acid as a bio-based phosphorus flame retardant for poly(lactic acid) nonwoven fabric. J. Clean. Prod. 2016, 124, 114–119. [Google Scholar] [CrossRef]
- Faravelli, T.; Pinciroli, M.; Pisano, F.; Bozzano, G.; Dente, M.; Ranzi, E. Thermal degradation of polystyrene. J. Anal. Appl. Pyrolysis 2001, 60, 103–121. [Google Scholar] [CrossRef]






| Sample | Initial Weight (g) | Final Weight (g) after Drying | Weight of Materials Coated on EPS (g)/Efficiency |
|---|---|---|---|
| PDA@EPS | 8 | 8.22 | 0.22 (2.75%) |
| 1CS-PDA@EPS | 8 | 8.47 | 0.47 (5.91%) |
| 2CS-PDA@EPS | 8 | 9.48 | 1.48 (18.54%) |
| 3CS-PDA@EPS | 8 | 10.29 | 2.29 (28.58%) |
| 2PA/3CS-PDA@EPS | 8 | 9.27 | 1.27 (15.9%) |
| 6PA/3CS-PDA@EPS | 8 | 10.38 | 2.38 (29.77%) |
| 10PA/3CS-PDA@EPS | 8 | 11.16 | 3.16 (39.45%) |
| Sample | T10% (°C) | T50% (°C) | Tmax (°C) | DTGmax (%/°C) | Residue (wt%) at 800 °C |
|---|---|---|---|---|---|
| EPS | 350 | 395 | 404 | −1.88 | 0.17 |
| PDA@EPS | 368 | 407 | 414 | −1.68 | 1.11 |
| 3CS-PDA@EPS | 314 | 388 | 396 | −1.2 | 2.23 |
| 2PA/3CS-PDA@EPS | 285 | 381 | 397 | −0.86 | 13.1 |
| 6PA/3CS-PDA@EPS | 273 | 383 | 386 | −0.8 | 16.76 |
| 10PA/3CS-PDA@EPS | 132 | 385 | 381 | −0.59 | 24.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, W.; Huang, D.; Qiang, X.; Liu, W. Preparation of Hydrophilic and Fire-Resistant Phytic Acid/Chitosan/Polydopamine-Coated Expanded Polystyrene Particles by Using Coating Method. Coatings 2024, 14, 574. https://doi.org/10.3390/coatings14050574
Tang W, Huang D, Qiang X, Liu W. Preparation of Hydrophilic and Fire-Resistant Phytic Acid/Chitosan/Polydopamine-Coated Expanded Polystyrene Particles by Using Coating Method. Coatings. 2024; 14(5):574. https://doi.org/10.3390/coatings14050574
Chicago/Turabian StyleTang, Wenjie, Dajian Huang, Xiaohu Qiang, and Wang Liu. 2024. "Preparation of Hydrophilic and Fire-Resistant Phytic Acid/Chitosan/Polydopamine-Coated Expanded Polystyrene Particles by Using Coating Method" Coatings 14, no. 5: 574. https://doi.org/10.3390/coatings14050574





