Development of Si-Based Anodes for All-Solid-State Li-Ion Batteries
Abstract
:1. Introduction
2. Si Anodes
2.1. Si Anodes with Different Particle Sizes
2.2. Si Anodes with Different Structures
2.2.1. Porous Si Anode
2.2.2. Columnar Si Anode
2.2.3. Film Si Anode
3. Si-Based Composite Anodes
3.1. Si/C Composite Anodes
3.2. Effects of Carbon Additives for Si Anode
3.3. Li-Si Alloy Anode
3.4. Other Si-Based Composite Anodes
4. Key Factors Influencing Performance of Si in ASSBs
4.1. Interface Issues between Si and SSEs
4.2. Electrochemical–Mechanical Coupling Effects
5. Summary and Perspective
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Grey, C.P.; Hall, D.S. Prospects for lithium-ion batteries and beyond—A 2030 vision. Nat. Commun. 2020, 11, 6279. [Google Scholar] [CrossRef]
- Jiang, B.; Sun, Z.; Liu, M. China’s energy development strategy under the low-carbon economy. Energy 2010, 35, 4257–4264. [Google Scholar] [CrossRef]
- Kato, Y.; Hori, S.; Saito, T.; Suzuki, K.; Hirayama, M.; Mitsui, A.; Yonemura, M.; Iba, H.; Kanno, R. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 2016, 1, 16030. [Google Scholar] [CrossRef]
- Vedhanarayanan, B.; Seetha Lakshmi, K.C. Beyond lithium-ion: Emerging frontiers in next-generation battery technologies. Front. Batter. Electrochem. 2024, 3, 1377192. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef]
- Xiao, Q.; Fan, Y.; Wang, X.; Susantyoko, R.A.; Zhang, Q. A multilayer Si/CNT coaxial nanofiber LIB anode with a high areal capacity. Energy Environ. Sci. 2014, 7, 655–661. [Google Scholar] [CrossRef]
- Höltschi, L.; Jud, F.; Borca, C.; Huthwelker, T.; Villevieille, C.; Pelé, V.; Jordy, C.; El Kazzi, M.; Novák, P. Study of Graphite Cycling in Sulfide Solid Electrolytes. J. Electrochem. Soc. 2020, 167, 110558. [Google Scholar] [CrossRef]
- Bloi, L.M.; Hippauf, F.; Boenke, T.; Rauche, M.; Paasch, S.; Schutjajew, K.; Pampel, J.; Schwotzer, F.; Dörfler, S.; Althues, H.; et al. Mechanistic insights into the reversible lithium storage in an open porous carbon via metal cluster formation in all solid-state batteries. Carbon 2022, 188, 325–335. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, J.; Costa, C.M.; Lanceros-Mendez, S.; Zhang, Q.; Wang, W. Unveiling Challenges and Opportunities in Silicon-Based All-Solid-State Batteries: Thin-Film Bonding with Mismatch Strain. Adv. Mater. 2024, 36, 2308590. [Google Scholar] [CrossRef]
- Kang, K.; Meng, Y.S.; Breger, J.; Grey, C.P.; Ceder, G. Electrodes with high power and high capacity for rechargeable lithium batteries. Science 2006, 311, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fan, X.; Ji, X.; Chen, J.; Hou, S.; Wang, C. High-Energy Li Metal Battery with Lithiated Host. Joule 2019, 3, 732–744. [Google Scholar] [CrossRef]
- Mineral Commodity Summaries 2021; US Geological Survey: Reston, VA, USA, 2021.
- Oh, P.; Yun, J.; Choi, J.H.; Saqib, K.S.; Embleton, T.J.; Park, S.; Lee, C.; Ali, J.; Ko, K.; Cho, J. Development of High-Energy Anodes for All-Solid-State Lithium Batteries Based on Sulfide Electrolytes. Angew. Chem. Int. Ed. Engl. 2022, 61, 202201249. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shao, B.; Wang, Y.; Han, F. Solid-state silicon anode with extremely high initial coulombic efficiency. Energy Environ. Sci. 2023, 16, 1569–1580. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T.; Kumar, J. Effect of Pressure on Lithium Metal Deposition and Stripping against Sulfide-Based Solid Electrolytes. ACS Appl. Mater. Interfaces 2020, 12, 34771–34776. [Google Scholar] [CrossRef] [PubMed]
- Doux, J.M.; Nguyen, H.; Tan, D.H.S.; Banerjee, A.; Wang, X.; Wu, E.A.; Jo, C.; Yang, H.; Meng, Y.S. Stack Pressure Considerations for Room-Temperature All-Solid-State Lithium Metal Batteries. Adv. Energy Mater. 2019, 10, 1903253. [Google Scholar] [CrossRef]
- Hopcroft, M.A.; Nix, W.D.; Kenny, T.W. What is the Young’s Modulus of Silicon? J. Microelectromechan. Syst. 2010, 19, 229–238. [Google Scholar] [CrossRef]
- Cao, D.; Sun, X.; Li, Y.; Anderson, A.; Lu, W.; Zhu, H. Long-Cycling Sulfide-Based All-Solid-State Batteries Enabled by Electrochemo-Mechanically Stable Electrodes. Adv. Mater. 2022, 34, 2200401. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-Y.; Xu, Q.; Li, G.; Yin, Y.-X.; Wan, L.-J.; Guo, Y.-G. Research progress regarding Si-based anode materials towards practical application in high energy density Li-ion batteries. Mater. Chem. Front. 2017, 1, 1691–1708. [Google Scholar] [CrossRef]
- Tan, D.H.S.; Chen, Y.T.; Yang, H.; Bao, W.; Sreenarayanan, B.; Doux, J.M.; Li, W.; Lu, B.; Ham, S.Y.; Sayahpour, B.; et al. Carbon-free high-loading silicon anodes enabled by sulfide solid electrolytes. Science 2021, 373, 1494–1499. [Google Scholar] [CrossRef]
- Trevey, J.; Jang, J.S.; Jung, Y.S.; Stoldt, C.R.; Lee, S.-H. Glass-ceramic Li2S-P2S5 electrolytes prepared by a single step ball billing process and their application for all-solid-state lithium-ion batteries. Electrochem. Commun. 2009, 11, 1830–1833. [Google Scholar] [CrossRef]
- Yamamoto, M.; Terauchi, Y.; Sakuda, A.; Takahashi, M. Slurry mixing for fabricating silicon-composite electrodes in all-solid-state batteries with high areal capacity and cycling stability. J. Power Sources 2018, 402, 506–512. [Google Scholar] [CrossRef]
- Wang, X.; He, K.; Li, S.; Zhang, J.; Lu, Y. Realizing high-performance all-solid-state batteries with sulfide solid electrolyte and silicon anode: A review. Nano Res. 2022, 16, 3741–3765. [Google Scholar] [CrossRef]
- Lewis, J.A.; Cavallaro, K.A.; Liu, Y.; McDowell, M.T. The promise of alloy anodes for solid-state batteries. Joule 2022, 6, 1418–1430. [Google Scholar]
- Huo, H.; Janek, J. Silicon as Emerging Anode in Solid-State Batteries. ACS Energy Lett. 2022, 7, 4005–4016. [Google Scholar]
- Liu, H.; Sun, Q.; Zhang, H.; Cheng, J.; Li, Y.; Zeng, Z.; Zhang, S.; Xu, X.; Ji, F.; Li, D.; et al. The application road of silicon-based anode in lithium-ion batteries: From liquid electrolyte to solid-state electrolyte. Energy Storage Mater. 2023, 55, 244–263. [Google Scholar] [CrossRef]
- Song, A.; Zhang, W.; Guo, H.; Dong, L.; Jin, T.; Shen, C.; Xie, K. A Review on the Features and Progress of Silicon Anodes-Based Solid-State Batteries. Adv. Energy Mater. 2023, 13, 2301464. [Google Scholar]
- Zhan, X.; Li, M.; Li, S.; Pang, X.; Mao, F.; Wang, H.; Sun, Z.; Han, X.; Jiang, B.; He, Y.-B.; et al. Challenges and opportunities towards silicon-based all-solid-state batteries. Energy Storage Mater. 2023, 61, 102875. [Google Scholar] [CrossRef]
- Sun, Z.; Yin, Q.; Chen, H.; Li, M.; Zhou, S.; Wen, S.; Pan, J.; Zheng, Q.; Jiang, B.; Liu, H.; et al. Building better solid-state batteries with silicon-based anodes. Interdiscip. Mater. 2023, 2, 635–663. [Google Scholar] [CrossRef]
- Dunlap, N.A.; Kim, S.; Jeong, J.J.; Oh, K.H.; Lee, S.-H. Simple and inexpensive coal-tar-pitch derived Si-C anode composite for all-solid-state Li-ion batteries. Solid State Ion. 2018, 324, 207–217. [Google Scholar] [CrossRef]
- Trevey, J.E.; Rason, K.W.; Stoldt, C.R.; Lee, S.-H. Improved Performance of All-Solid-State Lithium-Ion Batteries Using Nanosilicon Active Material with Multiwalled-Carbon-Nanotubes as a Conductive Additive. Electrochem. Solid State Lett. 2010, 13, A154–A157. [Google Scholar] [CrossRef]
- Rana, M.; Rudel, Y.; Heuer, P.; Schlautmann, E.; Rosenbach, C.; Ali, M.Y.; Wiggers, H.; Bielefeld, A.; Zeier, W.G. Toward Achieving High Areal Capacity in Silicon-Based Solid-State Battery Anodes: What Influences the Rate-Performance? ACS Energy Lett. 2023, 8, 3196–3203. [Google Scholar] [CrossRef]
- Liang, J.; Xiao, K.; Fang, R.; Rawal, A.; Lennon, A.; Wang, D.-W. High volumetric capacity nanoparticle electrodes enabled by nanofluidic fillers. Energy Storage Mater. 2021, 43, 202–211. [Google Scholar] [CrossRef]
- Gueon, D.; Ju, M.Y.; Moon, J.H. Complete encapsulation of sulfur through interfacial energy control of sulfur solutions for high-performance Li-S batteries. Proc. Natl. Acad. Sci. USA 2020, 117, 12686–12692. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Lakhnot, A.S.; Bhimani, K.; Sharma, S.; Mahajani, V.; Panchal, R.A.; Kamble, M.; Han, F.; Wang, C.; Koratkar, N. Nanostructuring versus microstructuring in battery electrodes. Nat. Rev. Mater. 2022, 7, 736–746. [Google Scholar] [CrossRef]
- Xu, J.; Liu, L.; Yao, N.; Wu, F.; Li, H.; Chen, L. Liquid-involved synthesis and processing of sulfide-based solid electrolytes, electrodes, and all-solid-state batteries. Mater. Today Nano 2019, 8, 100048. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, H.A.; Song, Y.B.; Park, J.W.; Lee, S.-M.; Jung, Y.S. Sheet-type Li6PS5Cl-infiltrated Si anodes fabricated by solution process for all-solid-state lithium-ion batteries. J. Power Sources 2019, 426, 143–150. [Google Scholar] [CrossRef]
- Miyazaki, R.; Ohta, N.; Ohnishi, T.; Takada, K. Anode properties of silicon-rich amorphous silicon suboxide films in all-solid-state lithium batteries. J. Power Sources 2016, 329, 41–49. [Google Scholar] [CrossRef]
- Yamamoto, M.; Terauchi, Y.; Sakuda, A.; Kato, A.; Takahashi, M. Effects of volume variations under different compressive pressures on the performance and microstructure of all-solid-state batteries. J. Power Sources 2020, 473, 228595. [Google Scholar] [CrossRef]
- Dunlap, N.A.; Kim, J.; Guthery, H.; Jiang, C.-S.; Morrissey, I.; Stoldt, C.R.; Oh, K.H.; Al-Jassim, M.; Lee, S.-H. Towards the Commercialization of the All-Solid-State Li-ion Battery: Local Bonding Structure and the Reversibility of Sheet-Style Si-PAN Anodes. J. Electrochem. Soc. 2020, 167, 060522. [Google Scholar] [CrossRef]
- Stephan, A.K. Perfect combination: Solid-state electrolytes and silicon anodes? Joule 2021, 5, 3074–3075. [Google Scholar] [CrossRef]
- Feng, K.; Ahn, W.; Lui, G.; Park, H.W.; Kashkooli, A.G.; Jiang, G.; Wang, X.; Xiao, X.; Chen, Z. Implementing an in-situ carbon network in Si/reduced graphene oxide for high performance lithium-ion battery anodes. Nano Energy 2016, 19, 187–197. [Google Scholar] [CrossRef]
- Okuno, R.; Yamamoto, M.; Kato, A.; Terauchi, Y.; Takahashi, M. Microstructures of nanoporous-Si composite anodes in sulfide-based all-solid-state lithium-ion batteries. IOP Conf. Ser. Mater. Sci. Eng. 2019, 625, 012012. [Google Scholar] [CrossRef]
- Okuno, R.; Yamamoto, M.; Terauchi, Y.; Takahashi, M. Stable cyclability of porous Si anode applied for sulfide-based all-solid-state batteries. Energy Procedia 2019, 156, 183–186. [Google Scholar] [CrossRef]
- Okuno, R.; Yamamoto, M.; Kato, A.; Takahashi, M. High Cycle Stability of Nanoporous Si Composites in All-solid-state Lithium-ion Batteries. J. Electrochem. Soc. 2022, 169, 080502. [Google Scholar] [CrossRef]
- Okuno, R.; Yamamoto, M.; Kato, A.; Takahashi, M. Stable Cyclability Caused by Highly Dispersed Nanoporous Si Composite Anodes with Sulfide-based Solid Electrolyte. J. Electrochem. Soc. 2020, 167, 140522. [Google Scholar] [CrossRef]
- Cangaz, S.; Hippauf, F.; Reuter, F.S.; Doerfler, S.; Abendroth, T.; Althues, H.; Kaskel, S. Enabling High-Energy Solid-State Batteries with Stable Anode Interphase by the Use of Columnar Silicon Anodes. Adv. Energy Mater. 2020, 10, 2001320. [Google Scholar] [CrossRef]
- Sakabe, J.; Ohta, N.; Ohnishi, T.; Mitsuishi, K.; Takada, K. Porous amorphous silicon film anodes for high-capacity and stable all-solid-state lithium batteries. Commun. Chem. 2018, 1, 24. [Google Scholar] [CrossRef]
- Fridman, K.; Sharabi, R.; Elazari, R.; Gershinsky, G.; Markevich, E.; Salitra, G.; Aurbach, D.; Garsuch, A.; Lampert, J. A new advanced lithium ion battery: Combination of high performance amorphous columnar silicon thin film anode. Electrochem. Commun. 2013, 33, 31–34. [Google Scholar] [CrossRef]
- Wada, M.; Yin, J.; Tanabe, E.; Kitano, Y.; Tanase, S.; Kajita, O.; Sakai, T. Nanocomposite Anode Materials for Li-ion Batteries. Electrochemistry 2003, 71, 1064–1066. [Google Scholar] [CrossRef]
- Piwko, M.; Thieme, S.; Weller, C.; Althues, H.; Kaskel, S. Enabling electrolyte compositions for columnar silicon anodes in high energy secondary batteries. J. Power Sources 2017, 362, 349–357. [Google Scholar] [CrossRef]
- Takamura, T.; Ohara, S.; Uehara, M.; Suzuki, J.; Sekine, K. A vacuum deposited Si film having a Li extraction capacity over 2000 mAh/g with a long cycle life. J. Power Sources 2004, 129, 96–100. [Google Scholar] [CrossRef]
- Phl, N.; Roozeboom, F.; Rah, N.; Baggetto, L. 3-D integrated all-solid-state rechargeable batteries. Adv. Mater. 2007, 24, 19. [Google Scholar]
- Lethien, C.; Zegaoui, M.; Roussel, P.; Tilmant, P.; Rolland, N.; Rolland, P. Micro-patterning of LiPON and lithium iron phosphate material deposited onto silicon nanopillars array for lithium ion solid state 3D micro-battery. Microelectron. Eng. 2011, 88, 3172–3177. [Google Scholar] [CrossRef]
- Huo, H.; Sun, J.; Chen, C.; Meng, X.; He, M.; Zhao, N.; Guo, X. Flexible interfaces between Si anodes and composite electrolytes consisting of poly(propylene carbonates) and garnets for solid-state batteries. J. Power Sources 2018, 383, 150–156. [Google Scholar] [CrossRef]
- Miyazaki, R.; Ohta, N.; Ohnishi, T.; Sakaguchi, I.; Takada, K. An amorphous Si film anode for all-solid-state lithium batteries. J. Power Sources 2014, 272, 541–545. [Google Scholar] [CrossRef]
- Chen, C.; Li, Q.; Li, Y.; Cui, Z.; Guo, X.; Li, H. Sustainable Interfaces between Si Anodes and Garnet Electrolytes for Room-Temperature Solid-State Batteries. ACS Appl. Mater. Interfaces 2018, 10, 2185–2190. [Google Scholar] [CrossRef]
- Xu, K.; Liu, X.; Guan, K.; Yu, Y.; Lei, W.; Zhang, S.; Jia, Q.; Zhang, H. Research Progress on Coating Structure of Silicon Anode Materials for Lithium-ion Battery. ChemSusChem 2021, 14, 5135–5160. [Google Scholar] [CrossRef]
- Mazouzi, D.; Lestriez, B.; Roue, L.; Guyomard, D. Silicon Composite Electrode with High Capacity and Long Cycle Life. J. Electrochem. Solid-State Lett. 2009, 12, A215–A218. [Google Scholar] [CrossRef]
- Poetke, S.; Hippauf, F.; Baasner, A.; Dörfler, S.; Althues, H.; Kaskel, S. Nanostructured Si−C Composites As High-Capacity Anode Material for All-Solid-State Lithium-Ion Batteries. Batter. Supercaps 2021, 4, 1323–1334. [Google Scholar] [CrossRef]
- Gu, L.; Han, J.; Chen, M.; Zhou, W.; Wang, X.; Xu, M.; Lin, H.; Liu, H.; Chen, H.; Chen, J.; et al. Enabling robust structural and interfacial stability of micron-Si anode toward high-performance liquid and solid-state lithium-ion batteries. Energy Storage Mater. 2022, 52, 547–561. [Google Scholar] [CrossRef]
- Han, X.; Xu, M.; Gu, L.-H.; Lan, C.-F.; Chen, M.-F.; Lu, J.-J.; Sheng, B.-F.; Wang, P.; Chen, S.-Y.; Chen, J.-Z. Monothetic and conductive network and mechanical stress releasing layer on micron-silicon anode enabling high-energy solid-state battery. Rare Met. 2024, 43, 1017–1029. [Google Scholar] [CrossRef]
- Kim, J.; Kim, C.; Jang, I.; Park, J.; Kim, J.; Paik, U.; Song, T. Si nanoparticles embedded in carbon nanofiber sheathed with Li6PS5Cl as an anode material for all-solid-state batteries. J. Power Sources 2021, 510, 230425. [Google Scholar] [CrossRef]
- Pandey, G.P.; Klankowski, S.A.; Li, Y.; Sun, X.S.; Wu, J.; Rojeski, R.A.; Li, J. Effective Infiltration of Gel Polymer Electrolyte into Silicon-Coated Vertically Aligned Carbon Nanofibers as Anodes for Solid-State Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 20909–20918. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yan, X.; Fu, Z.; Zhang, J.; Xia, Y.; Zhang, W.; Gan, Y.; He, X.; Huang, H. A “Reinforced Concrete” Structure of Silicon Embedded into an In Situ Grown Carbon Nanotube Scaffold as a High-Performance Anode for Sulfide-Based All-Solid-State Batteries. ACS Appl. Energy Mater. 2022, 5, 14353–14360. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, S.; Kang, S.H.; Park, J.; Lee, M.J.; Jin, D.; Shin, D.O.; Lee, Y.G.; Lee, Y.M. Graphite-Silicon Diffusion-Dependent Electrode with Short Effective Diffusion Length for High-Performance All-Solid-State Batteries. Adv. Energy Mater. 2021, 12, 2103108. [Google Scholar] [CrossRef]
- Lee, J.; Jin, D.; Kim, J.Y.; Roh, Y.; Lee, H.; Kang, S.H.; Choi, J.; Jo, T.; Lee, Y.G.; Lee, Y.M. Dry Pre-Lithiation for Graphite-Silicon Diffusion-Dependent Electrode for All-Solid-State Battery. Adv. Energy Mater. 2023, 13, 2300172. [Google Scholar] [CrossRef]
- Yan, W.; Mu, Z.; Wang, Z.; Huang, Y.; Wu, D.; Lu, P.; Lu, J.; Xu, J.; Wu, Y.; Ma, T.; et al. Hard-carbon-stabilized Li–Si anodes for high-performance all-solid-state Li-ion batteries. Nat. Energy 2023, 8, 800–813. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, Z.; Han, X.; Liu, Y.; Pei, S.; Li, Y.; Luo, L.; Su, P.; Lan, C.; Zhang, Z.; et al. An all-electrochem-active silicon anode enabled by spontaneous Li–Si alloying for ultra-high performance solid-state batteries. Energy Environ. Sci. 2024, 17, 1061–1072. [Google Scholar] [CrossRef]
- Han, X.; Zhou, W.; Chen, M.; Chen, J.; Wang, G.; Liu, B.; Luo, L.; Chen, S.; Zhang, Q.; Shi, S.; et al. Interfacial nitrogen engineering of robust silicon/MXene anode toward high energy solid-state lithium-ion batteries. J. Energy Chem. 2022, 67, 727–735. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, Y.; Peng, X.; Wu, M.; Zhao, T. A High-Capacity Polyethylene Oxide-Based All-Solid-State Battery Using a Metal-Organic Framework Hosted Silicon Anode. ACS Appl. Mater. Interfaces 2022, 14, 24798–24805. [Google Scholar] [CrossRef]
- Ng, S.H.; Wang, J.; Konstantinov, K.; Wexler, D.; Chew, S.Y.; Guo, Z.P.; Liu, H.K. Spray-pyrolyzed silicon/disordered carbon nanocomposites for lithium-ion battery anodes. J. Power Sources 2007, 174, 823–827. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Xia, X.H.; Wang, X.L.; Mai, Y.J.; Shi, S.J.; Tang, Y.Y.; Li, L.; Tu, J.P. Silicon/graphene-sheet hybrid film as anode for lithium ion batteries. Electrochem. Commun. 2012, 23, 17–20. [Google Scholar] [CrossRef]
- Song, T.; Xia, J.; Lee, J.H.; Lee, D.H.; Kwon, M.S.; Choi, J.M.; Wu, J.; Doo, S.K.; Chang, H.; Park, W. Arrays of Sealed Silicon Nanotubes As Anodes for Lithium Ion Batteries. Nano Lett. 2010, 10, 1710–1716. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Lee, D.H.; Kwon, M.S.; Choi, J.M.; Han, H.; Doo, S.G.; Chang, H.; Park, W.I.; Sigmund, W.; Kim, H. Silicon nanowires with a carbon nanofiber branch as lithium-ion anode material. J. Mater. Chem. 2011, 21, 12619–12621. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, J.; Lee, M.J.; Kang, S.H.; Shin, D.O.; Oh, J.; Kim, J.; Kim, K.M.; Lee, Y.-G.; Lee, Y.M. Diffusion-Dependent Graphite Electrode for All-Solid-State Batteries with Extremely High Energy Density. ACS Energy Lett. 2020, 5, 2995–3004. [Google Scholar] [CrossRef]
- Forney, M.W.; Ganter, M.J.; Staub, J.W.; Ridgley, R.D.; Landi, B.J. Prelithiation of silicon-carbon nanotube anodes for lithium ion batteries by stabilized lithium metal powder (SLMP). Nano Lett. 2013, 13, 4158–4163. [Google Scholar] [CrossRef]
- Xu, H.; Li, S.; Zhang, C.; Chen, X.; Liu, W.; Zheng, Y.; Xie, Y.; Huang, Y.; Li, J. Roll-to-roll prelithiation of Sn foil anode suppresses gassing and enables stable full-cell cycling of lithium ion batteries. Energy Environ. Sci. 2019, 12, 2991–3000. [Google Scholar] [CrossRef]
- Chen, J.; Fan, X.; Li, Q.; Yang, H.; Khoshi, M.R.; Xu, Y.; Hwang, S.; Chen, L.; Ji, X.; Yang, C.; et al. Electrolyte design for LiF-rich solid–electrolyte interfaces to enable high-performance microsized alloy anodes for batteries. Nat. Energy 2020, 5, 386–397. [Google Scholar] [CrossRef]
- Kwon, H.J.; Hwang, J.Y.; Shin, H.J.; Jeong, M.G.; Chung, K.Y.; Sun, Y.K.; Jung, H.G. Nano/Microstructured Silicon-Carbon Hybrid Composite Particles Fabricated with Corn Starch Biowaste as Anode Materials for Li-Ion Batteries. Nano Lett. 2020, 20, 625–635. [Google Scholar] [CrossRef]
- Okuno, R.; Yamamoto, M.; Kato, A.; Takahashi, M. Performance improvement of nanoporous Si composite anodes in all-solid-state lithium-ion batteries by using acetylene black as a conductive additive. Electrochem. Commun. 2022, 138, 107288. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, W.; Zhou, J.; Cai, W.; Lu, Y.; Liang, J.; Li, X.; Zhu, S.; Fu, Q.; Qian, Y. Prelithiated Surface Oxide Layer Enabled High-Performance Si Anode for Lithium Storage. ACS Appl. Mater. Interfaces 2019, 11, 18305–18312. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Biesold, G.M.; Sewell, C.D.; Hao, S.M.; Huang, J.; Zhang, W.; Lai, Y.; Lin, Z. Recent Advances in Silicon-Based Electrodes: From Fundamental Research toward Practical Applications. Adv. Mater. 2021, 33, e2004577. [Google Scholar] [CrossRef]
- Ham, S.Y.; Sebti, E.; Cronk, A.; Pennebaker, T.; Deysher, G.; Chen, Y.T.; Oh, J.A.S.; Lee, J.B.; Song, M.S.; Ridley, P.; et al. Overcoming low initial coulombic efficiencies of Si anodes through prelithiation in all-solid-state batteries. Nat. Commun. 2024, 15, 2991. [Google Scholar] [CrossRef]
- Iwamura, S.; Nishihara, H.; Ono, Y.; Morito, H.; Yamane, H.; Nara, H.; Osaka, T.; Kyotani, T. Li-rich Li-Si alloy as a lithium-containing negative electrode material towards high energy lithium-ion batteries. Sci. Rep. 2015, 5, 8085. [Google Scholar] [CrossRef]
- Li, X.; Kersey-Bronec, F.E.; Ke, J.; Cloud, J.E.; Wang, Y.; Ngo, C.; Pylypenko, S.; Yang, Y. Study of Lithium Silicide Nanoparticles as Anode Materials for Advanced Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 16071–16080. [Google Scholar] [CrossRef]
- Sakuma, M.; Suzuki, K.; Hirayama, M.; Kanno, R. Reactions at the electrode/electrolyte interface of all-solid-state lithium batteries incorporating Li-M (M = Sn, Si) alloy electrodes and sulfide-based solid electrolytes. Solid State Ion. 2016, 285, 101–105. [Google Scholar] [CrossRef]
- Zhou, L.; Zuo, T.; Li, C.; Zhang, Q.; Janek, J.; Nazar, L.F. Li3−xZrx(Ho/Lu)1−xCl6 Solid Electrolytes Enable Ultrahigh-Loading Solid-State Batteries with a Prelithiated Si Anode. ACS Energy Lett. 2023, 8, 3102–3111. [Google Scholar] [CrossRef]
- Ji, W.; Zhang, X.; Liu, M.; Ding, T.; Qu, H.; Qiu, D.; Zheng, D.; Qu, D. High-performance all-solid-state Li-S batteries enabled by an all-electrochem-active prelithiated Si anode. Energy Storage Mater. 2022, 53, 613–620. [Google Scholar] [CrossRef]
- Cloud, J.E.; Wang, Y.; Li, X.; Yoder, T.S.; Yang, Y.; Yang, Y. Lithium silicide nanocrystals: Synthesis, chemical stability, thermal stability, and carbon encapsulation. Inorg. Chem. 2014, 53, 11289–11297. [Google Scholar] [CrossRef]
- Fan, Z.; Ding, B.; Li, Z.; Chang, Z.; Hu, B.; Xu, C.; Zhang, X.; Dou, H.; Zhang, X. In-Situ prelithiation of electrolyte-free silicon anode for sulfide all-solid-state batteries. eTransportation 2023, 18, 100277. [Google Scholar] [CrossRef]
- Kim, M.; Kim, M.J.; Oh, Y.S.; Kang, S.; Shin, T.H.; Lim, H.T. Design Strategies of Li-Si Alloy Anode for Mitigating Chemo-Mechanical Degradation in Sulfide-Based All-Solid-State Batteries. Adv. Sci. 2023, 10, e2301381. [Google Scholar] [CrossRef]
- Oh, Y.S.; Kim, M.; Kang, S.; Park, J.-Y.; Lim, H.-T. Redox activity of Li2S-P2S5 electrolyte inducing chemo-mechanical failure in all-solid-state batteries comprising sulfur composite cathode and Li-Si alloy anode. Chem. Eng. J. 2022, 442, 136229. [Google Scholar] [CrossRef]
- Li, H.; Huang, X.; Chen, L.; Zhou, G.; Zhang, Z.; Yu, D.; Jun Mo, Y.; Pei, N. The crystal structural evolution of nano-Si anode caused by lithium insertion and extraction at room temperature. Solid State Ion. 2000, 135, 181–191. [Google Scholar] [CrossRef]
- Yang, L.; Chen, H.; Jiang, H.; Wei, Y.-J.; Song, W.; Fang, D. Failure mechanisms of 2D silicon film anodes: In Situ observations and simulations on crack evolution. Chem. Commun. 2018, 54, 3997–4000. [Google Scholar] [CrossRef]
- Xie, L.; Tang, C.; Bi, Z.; Song, M.; Fan, Y.; Yan, C.; Li, X.; Su, F.; Zhang, Q.; Chen, C. Hard Carbon Anodes for Next-Generation Li-Ion Batteries: Review and Perspective. Adv. Energy Mater. 2021, 11, 2101650. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Li, C.-H.; Wang, D.-Y.; Chen, C.-C. Chemical doping of a core-shell silicon nanoparticles@polyaniline nanocomposite for the performance enhancement of a lithium ion battery anode. Nanoscale 2016, 8, 1280–1287. [Google Scholar] [CrossRef]
- Janus, R.; Natkański, P.; Wach, A.; Drozdek, M.; Piwowarska, Z.; Cool, P.; Kuśtrowski, P. Thermal transformation of polyacrylonitrile deposited on SBA-15 type silica. J. Therm. Anal. Calorim. 2012, 110, 119–125. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Krause, L.J. Reversible Cycling of Crystalline Silicon Powder. J. Electrochem. Soc. 2007, 154, A103. [Google Scholar] [CrossRef]
- He, Y.; Jiang, L.; Chen, T.; Xu, Y.; Jia, H.; Yi, R.; Xue, D.; Song, M.; Genc, A.; Bouchet-Marquis, C.; et al. Progressive growth of the solid–electrolyte interphase towards the Si anode interior causes capacity fading. Nat. Nanotechnol. 2021, 16, 1113–1120. [Google Scholar] [CrossRef]
- Cao, D.; Ji, T.; Singh, A.; Bak, S.; Du, Y.; Xiao, X.; Xu, H.; Zhu, J.; Zhu, H. Unveiling the Mechanical and Electrochemical Evolution of Nanosilicon Composite Anodes in Sulfide-Based All-Solid-State Batteries. Adv. Energy Mater. 2023, 13, 2203969. [Google Scholar] [CrossRef]
- Zhao, N.; Khokhar, W.; Bi, Z.; Shi, C.; Guo, X.; Fan, L.-Z.; Nan, C.-W. Solid Garnet Batteries. Joule 2019, 3, 1190–1199. [Google Scholar] [CrossRef]
- Boulineau, S.; Courty, M.; Tarascon, J.-M.; Viallet, V. Mechanochemical synthesis of Li-argyrodite Li6PS5X (X = Cl, Br, I) as sulfur-based solid electrolytes for all solid state batteries application. Solid State Ion. 2012, 221, 1–5. [Google Scholar] [CrossRef]
- Schwietert, T.K.; Arszelewska, V.A.; Wang, C.; Yu, C.; Vasileiadis, A.; de Klerk, N.J.J.; Hageman, J.; Hupfer, T.; Kerkamm, I.; Xu, Y.; et al. Clarifying the relationship between redox activity and electrochemical stability in solid electrolytes. Nat. Mater. 2020, 19, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.H.S.; Wu, E.A.; Nguyen, H.; Chen, Z.; Marple, M.A.T.; Doux, J.-M.; Wang, X.; Yang, H.; Banerjee, A.; Meng, Y.S. Elucidating Reversible Electrochemical Redox of Li6PS5Cl Solid Electrolyte. ACS Energy Lett. 2019, 4, 2418–2427. [Google Scholar] [CrossRef]
- Piper, D.M.; Yersak, T.A.; Lee, S.-H. Effect of Compressive Stress on Electrochemical Performance of Silicon Anodes. J. Electrochem. Soc. 2012, 160, A77–A81. [Google Scholar] [CrossRef]
- Han, S.Y.; Lee, C.; Lewis, J.A.; Yeh, D.; Liu, Y.; Lee, H.-W.; McDowell, M.T. Stress evolution during cycling of alloy-anode solid-state batteries. Joule 2021, 5, 2450–2465. [Google Scholar] [CrossRef]
- Tao, J.; Han, J.; Wu, Y.; Yang, Y.; Chen, Y.; Li, J.; Huang, Z.; Lin, Y. Unraveling the performance decay of micro-sized silicon anodes in sulfide-based solid-state batteries. Energy Storage Mater. 2024, 64, 103082. [Google Scholar] [CrossRef]




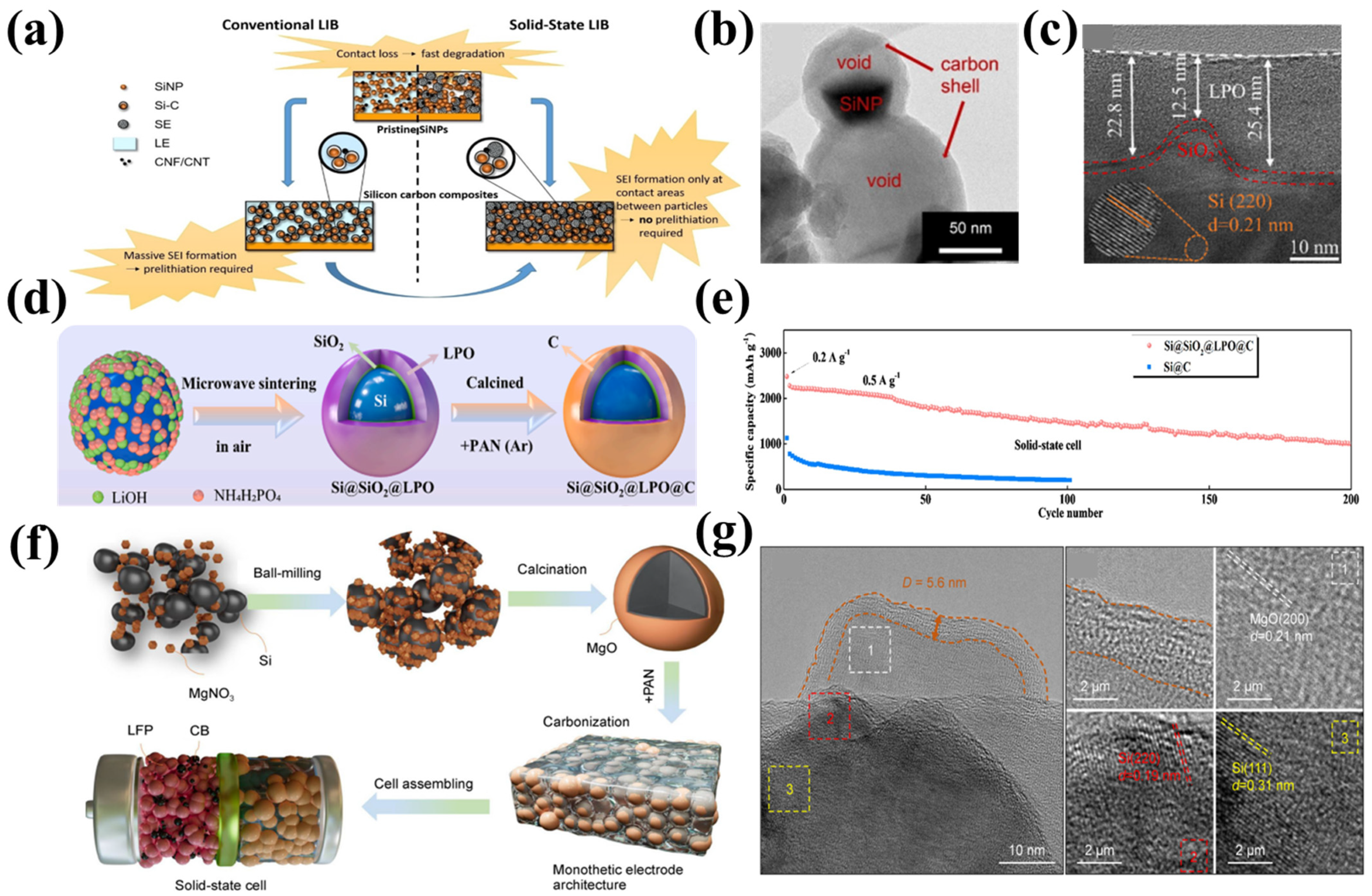





| Si-Based Anode | Solid-State Electrolytes | Ionic Conductivity | Counter Electrode and Voltage Range | ICE | Current | Cycling Performance | Refs |
|---|---|---|---|---|---|---|---|
| Si-C | Li6PS5Cl | / | Li 0.01–2.00 V | 73.2% | 0.2 mA cm−2 | 53rd: >1000 mAh g−1Si-C | [61] |
| Si@SiO2@LPO@C | PEO@LATP | 2.86 × 10−5 S cm−1 (50 °C) | Li 0.005–1.5 V | 88.7% | 0.5 A g−1 (50 °C) | 200th: 1001.9 mAh g−1 | [62] |
| Si@MgO@C | PEO@LATP@ NCF | 1.68 × 10−4 S cm−1 (50 °C) | Li 0.005–1.5 V | 81.4% | 0.3 A g−1 | 100th: 1658.9 mAh g−1 | [63] |
| Si/CNF@LPSCl | Li6PS5Cl | / | Li-In 0.05–1.5 V | / | 0.5 C (25 °C) | Capacity retention: 83.4% (50 cycles) | [64] |
| Si-VACNF | Gel Polymer | ~2.2 × 10−3 S cm−1 | Li 0.05–1.5 V | 76% | 2.6 A g−1 | 100th: ~1050 mAh g−1 | [65] |
| Si/CNTs/C | Li6PS5Cl | 2.13 × 10−3 S cm−1 (30 °C) | Li | 59.7%, | 50 mA g−1 | 50th: 1226 mAh g−1 | [66] |
| Si/Graphite | Li6PS5Cl | / | Li | / | 1.77 mA cm−2 (50 °C) | Capacity retention: 88.8% (100 cycles) | [67] |
| PL-Si/Graphite | Li6PS5Cl | / | Li 0.01–2.0 V | ~108.9% | 0.5 C (60 °C) | Capacity retention: 79.5% (150 cycles) | [68] |
| LiSH46 | Li6PS5Cl | / | NCM811 2.5–4.2 V | / | 1 C (55 °C) | Capacity retention: 61.5% (5000 cycles) | [69] |
| Si/Li21Si5 | Li6PS5Cl | / | LCO/Li3InCl6 2–4.2 V | 97.8% | 0.5 C (25 °C) | Capacity retention: 80% (175 cycles) | [70] |
| Si-PAN | 77.5Li2S-22.5P2S5 | / | Li-Ln 100 mV–1 V | ~84% | 0.1 C (60 °C) | 200th: 1606 mAh g−1 | [41] |
| Si-N-MXene | PEO@LATP | 3.4 × 10−4 S cm−1 (50 °C) | Li 0.005–1.5 V | 82.02% | 0.4 A g−1 (50 °C) | 90th: 881 mAh g−1 | [71] |
| Si@MOF | PVDF/PEO/garnet | 81 × 10−4 S cm−1 (25 °C) | Li 0.01–1.5 V | 72.0 | 0.2 A g−1 (60 °C) | 50th: 1442 mAh g−1 | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Rong, Y.; Duan, Y.; Wu, Y.; He, D.; Qi, X.; Wang, J. Development of Si-Based Anodes for All-Solid-State Li-Ion Batteries. Coatings 2024, 14, 608. https://doi.org/10.3390/coatings14050608
Zhao X, Rong Y, Duan Y, Wu Y, He D, Qi X, Wang J. Development of Si-Based Anodes for All-Solid-State Li-Ion Batteries. Coatings. 2024; 14(5):608. https://doi.org/10.3390/coatings14050608
Chicago/Turabian StyleZhao, Xuyang, Yunpeng Rong, Yi Duan, Yanlong Wu, Deyu He, Xiaopeng Qi, and Jiantao Wang. 2024. "Development of Si-Based Anodes for All-Solid-State Li-Ion Batteries" Coatings 14, no. 5: 608. https://doi.org/10.3390/coatings14050608





