A Naked-Eye Colorimetric Ratio Method for the Selective and Sensitive Detection of L-Cys Based on a Silver Nanoflakes–Chromium (III) Ion System
Abstract
:1. Introduction
2. Results and Discussion
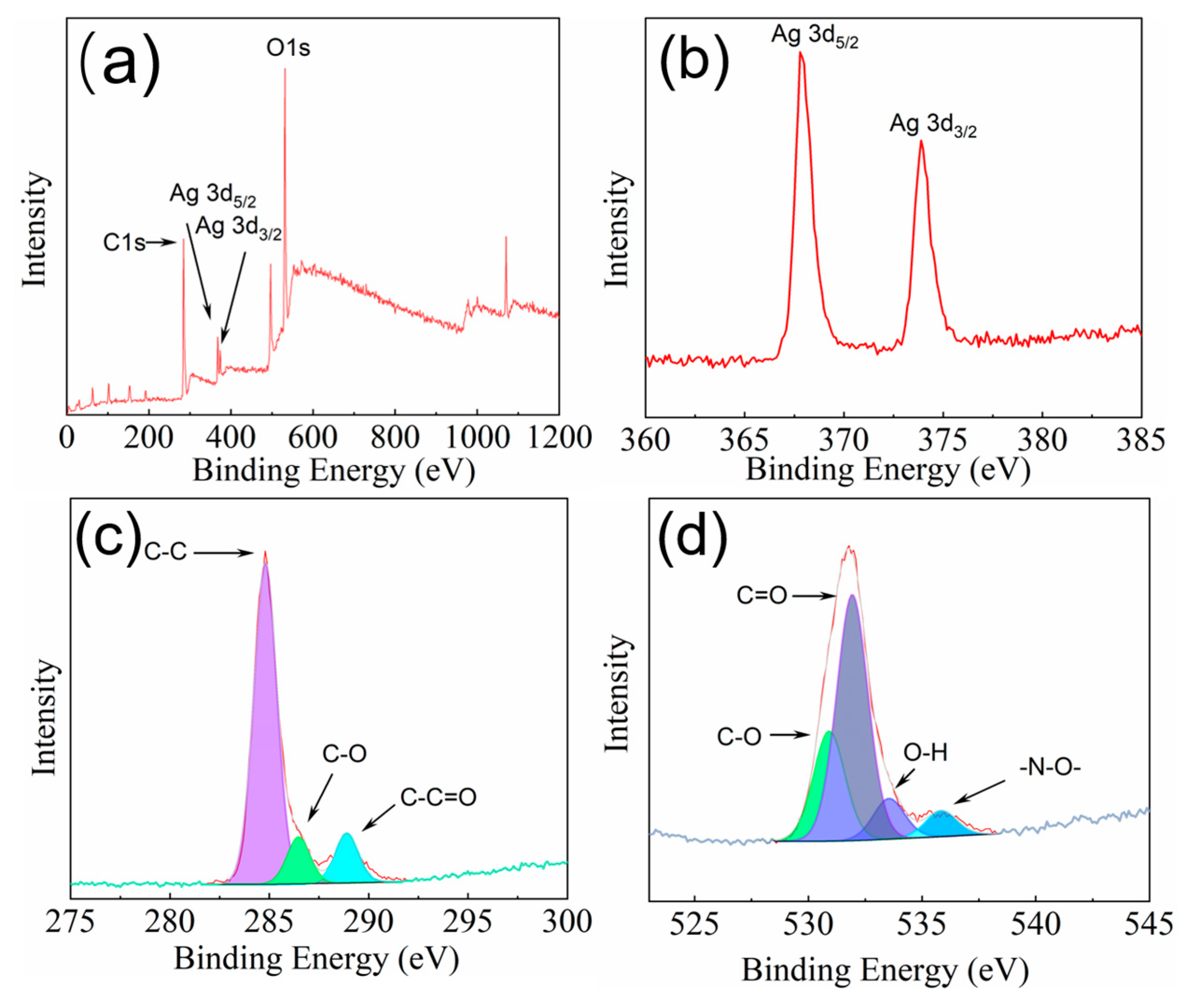
2.1. Preparation and Characterization of Silver Nanoflakes
2.2. The Detection Mechanism of L-Cys
2.3. Optimum Conditions for the Silver Nanoflakes–Chromium (III) Ion System
2.4. Sensitivity of the Novel Sensor for L-Cys
2.5. Selectivity Test for L-Cys
2.6. Practical Application in a Real Sample
3. Experimental Section
3.1. Chemicals and Materials
3.2. Characterization Techniques for Silver Nanoflakes
3.3. Computational Method
3.4. Synthesis of Silver Nanoflakes
3.5. Detection of L-Cys by Silver Nanoflakes–Chromium (III) Ion System
3.6. Determination of L-Cys in Real Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, S.; Zhang, H.; Yue, X.; Wang, J.; Yang, L.; Wang, B.; Song, X. A Ratiometric, Fast-Responsive, and Single-Wavelength Excited Fluorescent Probe for the Discrimination of Cys and Hcy. Anal. Chem. 2021, 93, 10934–10939. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chi, M.; Zhu, Y.; Gao, M.; Wang, C.; Lu, X. A Facile synthesis of superparamagnetic Fe3O4 nanofibers with superior peroxidase-like catalytic activity for sensitive colorimetric detection of L-cysteine. Appl. Surf. Sci. 2018, 440, 237–244. [Google Scholar] [CrossRef]
- Du, Z.; Zhang, R.; Song, B.; Zhang, W.; Wang, Y.L.; Liu, J.; Liu, C.; Xu, Z.P.; Yuan, J. Iridium(III) Complex-Based Activatable Probe for Phosphorescent/Time-Gated Luminescent Sensing and Imaging of Cysteine in Mitochondria of Live Cells and Animals. Chemistry 2019, 25, 1498–1506. [Google Scholar] [CrossRef]
- Maiti, P.; Singha, T.; Chakraborty, U.; Roy, S.D.; Paul, P.K. Selective and sensitive detection of L-Cysteine via fluorometric assay using gold nanoparticles and Rhodamine B in aqueous medium. Mater. Chem. Phys. 2019, 234, 158–167. [Google Scholar] [CrossRef]
- Mohandoss, S.; Palanisamy, S.; Priya, V.V.; Mohan, S.K.; Shim, J.J.; Yelithao, K.; You, S.; Lee, Y.R. Excitation-dependent multiple luminescence emission of nitrogen and sulfur co-doped carbon dots for cysteine sensing, bioimaging, and photoluminescent ink applications. Microchem. J. 2021, 167, 106280. [Google Scholar] [CrossRef]
- Mercy, J.S.I.; Maruthupandi, M.; Mamat, M.H.B.; Vasimalai, N. Facile In-Situ Synthesis of Biopolymer Capped Nano Sized Silver Particles: Smartphone Aided Paper-Based Selective Detection of CYS and TC Drugs in Biological and Drug Samples. J. Cluster Sci. 2021, 33, 1055–1067. [Google Scholar] [CrossRef]
- Xu, Z.; Qin, T.; Zhou, X.; Wang, L.; Liu, B. Fluorescent probes with multiple channels for simultaneous detection of Cys, Hcy, GSH, and H2S. TrAC Trends Anal. Chem. 2019, 121, 115672. [Google Scholar] [CrossRef]
- Xu, N.; Li, X.; Luan, F.; Tian, C.; Zhang, Z.; Chen, L.; Zhuang, X. Ratiometric fluorescent and electrochemiluminescent dual modal assay for detection of 2, 6-pyridinedicarboxylic acid as an anthrax biomarker. Anal. Chim. Acta 2024, 1288, 342181. [Google Scholar] [CrossRef]
- Xiao, Q.; Gao, H.; Yuan, Q.; Lu, C.; Lin, J.M. High-performance liquid chromatography assay of cysteine and homocysteine using fluorosurfactant-functionalized gold nanoparticles as postcolumn resonance light scattering reagents. J. Chromatogr. A 2013, 1274, 145–150. [Google Scholar] [CrossRef]
- Lin, X.; Wu, H.; Zeng, S. A self-designed device integrated with a fermat spiral microfluidic chip for ratiometric and automated point-of-care testing of anthrax biomarker in real samples. Biosens. Bioelectron. 2023, 230, 115283. [Google Scholar] [CrossRef]
- Zhang, P.P.; Ni, A.Y.; Zhang, J.J.; Zhang, B.L.; Zhou, H.A.; Zhao, H.; Duan, C. Tb-MOF-based luminescent recovery probe for rapid and facile detection of an anthrax biomarker. Sens. Actuators B Chem. 2023, 384, 133624. [Google Scholar] [CrossRef]
- Hai, X.; Lin, X.; Chen, X.; Wang, J. Highly selective and sensitive detection of cysteine with a graphene quantum dots-gold nanoparticles based core-shell nanosensor. Sens. Actuators B Chem. 2018, 257, 228–236. [Google Scholar] [CrossRef]
- Cai, Y.; Fang, J.; Zhu, H.; Qin, W.; Cao, Y.; Yu, H.; Shao, G.; Liu, Y.; Liu, W. A Rapid “off-on” copper-induced AIE active sensor for fluorimetric detection of cysteine. Sens. Actuators B Chem. 2020, 303, 127214. [Google Scholar] [CrossRef]
- Babu, S.; Claville, M.O.; Ghebreyessus, K. Rapid synthesis of highly stable silver nanoparticles and its application for colourimetric sensing of cysteine. J. Exp. Nanosci. 2015, 10, 1242–1255. [Google Scholar] [CrossRef]
- Amirjani, A.; Haghshenas, D.F. Ag nanostructures as the surface plasmon resonance (SPR)˗based sensors: A mechanistic study with an emphasis on heavy metallic ions detection. Sens. Actuators B Chem. 2018, 273, 1768–1779. [Google Scholar] [CrossRef]
- Gokul Eswaran, S.; Ashkar, M.A.; Mamat, M.H.; Sahila, S.; Mahalingam, V.; Koppisetti, H.V.S.R.M.; Vasimalai, N. Preparation of a portable calorimetry kit and one-step spectrophotometric nanomolar level detection of l-Histidine in serum and urine samples using sebacic acid capped silver nanoparticles. J. Sci. Adv. Mater. Dev. 2021, 6, 100–107. [Google Scholar] [CrossRef]
- Chanajaree, R.; Ratanatawanate, C.; Ruangchaithaweesuk, S.; Lee, V.S.; Wittayanarakul, K. Colorimetric detection of Pb2+ ions using curcumin silver nanoparticles. J. Mol. Liq. 2021, 343, 117629. [Google Scholar] [CrossRef]
- Yin, S.; Tong, C. Europium(III)-Modified Silver Nanoparticles as Ratiometric Colorimetric and Fluorescent Dual-Mode Probes for Selective Detection of Dipicolinic Acid in Bacterial Spores and Lake Waters. ACS Appl. Nano Mater. 2021, 4, 5469–5477. [Google Scholar] [CrossRef]
- UshaVipinachandran, V.; Rajendran, S.; Ali, H.; Ashokan, I.; Bhunia, S.K. Citrate capped silver nanoparticles as an instantaneous colorimetric selective sensor for neomycin and thiamine in wastewater. New J. Chem. 2022, 46, 14081–14090. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Dang, Y.; Liu, Z.; Zhang, Z.; Shan, D.; Zhang, X.; Wang, T.; Lu, X. Ultratrace Naked-eye Colorimetric Ratio Assay of Chromium (III) Ion in Aqueous Solution via Stimuli-Responsive Morphology Transformation of Silver Nano Flakes. Anal. Chem. 2019, 91, 4031–4038. [Google Scholar] [CrossRef]
- Zhang, R.; Yong, J.; Yuan, J.; Ping Xu, Z. Recent advances in the development of responsive probes for selective detection of cysteine. Coord. Chem. Rev. 2020, 408, 213182. [Google Scholar] [CrossRef]
- Gu, T.; Zou, W.; Gong, F.; Xia, J.; Chen, C.; Chen, X. A specific nanoprobe for cysteine based on nitrogen-rich fluorescent quantum dots combined with Cu2+. Biosens. Bioelectron. 2018, 100, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, S.; Xu, H.; Wang, R.; Aldalbahi, A. Nitrogen-Doped Carbon Quantum Dots from Poly(ethyleneimine) for Optical Dual-Mode Determination of Cu2+ and l-Cysteine and Their Logic Gate Operation. ACS Appl. Mater. Interfaces 2020, 12, 47245–47255. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Meng, X.; Hou, B.; Sun, Z.; Zhang, Y.; Li, M. Rapid fluorescent color analysis of copper ions on a smart phone via ratiometric fluorescence sensor. Mikrochim. Acta 2022, 189, 67. [Google Scholar] [CrossRef] [PubMed]
- Monisha; Shrivas, K.; Kant, T.; Patel, S.; Devi, R.; Dahariya, N.S.; Pervez, S.; Deb, M.K.; Rai, M.K.; Rai, J. Inkjet-printed paper-based colorimetric sensor coupled with smartphone for determination of mercury Hg2+. J. Hazard. Mater. 2021, 414, 125440. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, H.; Wang, Y.; Xiong, Z.; Zhao, X.; Xia, Y. Chitosan-derived N-doped carbon dots for fluorescent determination of nitrite and bacteria imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 251, 119468. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, Y.; Gong, J.; Mo, S.; Ren, Y.; Xu, J.; Lu, M. Core-shell structured lignin-stabilized silver nanoprisms for colorimetric detection of sulfur ions. Int. J. Biol. Macromol. 2024, 260, 129626. [Google Scholar] [CrossRef] [PubMed]
- Rejeeth, C.; Sharma, A.; Nipun Babu, V.; Gautam, R. Label-free colorimetric detection of serum cysteine using Ag-NP probes in the presence of Be2+ ions. New J. Chem. 2020, 44, 9018–9024. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, S.; Li, Y.; Tao, B.; Wu, N.; Wang, H.B.; Fang, L. Etching triangular silver nanoparticles to initiate the fluorescent response of Ru@SiO2 for sensitive detection of glutathione. Colloids Surf. A Physicochem. Eng. Asp. 2023, 671, 131686. [Google Scholar] [CrossRef]
- Tamilselvan, S.; Soniya, R.M.; Vasantharaja, R.; Kannan, M.; Supriya, S.; Dass Batvari, B.P.; Ramesh, T.; Govindaraju, K. Silver nanoparticles based spectroscopic sensing of eight metal ions in aqueous solutions. Environ. Res. 2022, 212, 113585. [Google Scholar] [CrossRef]
- Song, X.; Chen, X.; Liang, Z.; Xu, D.; Liang, Y. A colorimetric sensing probe for chromium (III) ion based on domino like reaction. Colloids Surf. B Biointerfaces 2022, 215, 112494. [Google Scholar] [CrossRef]
- Wei, Y.; Jiang, H.; Deng, P. Direct quantification of cysteine and glutathione by 1H NMR based on β-cyclodextrin modified silver nanoparticles. Microchem. J. 2021, 168, 106471. [Google Scholar] [CrossRef]
- Matsunaga, T.; Kondo, T.; Shitanda, I.; Hoshi, Y.; Itagaki, M.; Tojo, T.; Yuasa, M. Sensitive electrochemical detection of l-Cysteine at a screen-printed diamond electrode. Carbon 2021, 173, 395–402. [Google Scholar] [CrossRef]
- Long, L.; Yuan, F.; Yang, X.; Chen, X.; Li, L.; Wang, K. On-site discrimination of biothiols in biological fluids by a novel fluorescent probe and a portable fluorescence detection device. Sens. Actuators B Chem. 2022, 369, 132211. [Google Scholar] [CrossRef]
- Si, L.; Fu, Q.; Shi, Z.; Zhang, T.; Hou, Q.; Xu, Z.; Ai, S. The fluorescent detection of biothiols and antimicrobial study based on copper (I) iodide coordination polymer. Dyes Pigm. 2023, 215, 111228. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, B.; Liang, M.; Zhang, M.; Hu, Y.; Huang, Z.S.; Ye, X.; Du, B.; Quan, Y.Y.; Jiang, Y. A lysosome-targeted fluorescent probe based on a BODIPY structure for Cys/Hcy detection. Anal. Methods 2024, 16, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Ding, H.; Fan, C.; Liu, G.; Pu, S. Time-dependent NIR fluorescent probe with large Stokes-shift for detecting Cys/Hcy and cell imaging. Dyes Pigm. 2022, 203, 110320. [Google Scholar] [CrossRef]
- Gong, S.; Qin, A.; Zhang, Y.; Li, M.; Chen, X.; Liang, Y.; Xu, X.; Wang, Z.; Wang, S. A new ratiometric AIE fluorescent probe for detecting cysteine in food samples and imaging in the biological system. Food Chem. 2023, 400, 134108. [Google Scholar] [CrossRef]
- Chen, X.G.; Mei, Y.; Song, Q.H. A 3-(2′-nitro vinyl)-4-phenylselenyl coumarin as a fluorescent probe for distinguishing detection of Cys/Hcy and GSH. Dyes Pigm. 2022, 203, 110312. [Google Scholar] [CrossRef]
- Lee, J.-T.; Mathur, S.; Shen, S.; Wu, J.M.; Chen, J. MoSe2 Nanoflowers for Highly Efficient Industrial Wastewater Treatment with Zero Discharge. Adv. Sci. 2021, 8, 2102857. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Z.; Zhang, S.; Yang, Q.; Li, Z.; Zang, X.; Zhao, X.; Shang, N.; Khaorapapong, N.; Xu, X.; et al. Inorganic-Organic Nanoarchitectonics: MXene/Covalent Organic Framework Heterostructure for Superior Microextraction. Small 2023, 91, 2305730. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Xie, S.; Cao, Z.; Wang, L.; Xu, D.; Zhang, H.; Matz, J.; Dong, P.; Fang, H.; Shen, J.; et al. High-rate aqueous zinc-organic battery achieved by lowering HOMO/LUMO of organic cathode. Energy Storage Mater. 2021, 37, 378–386. [Google Scholar] [CrossRef]
- Li, H.; Fan, J.; Wang, J.; Tian, M.; Du, J.; Sun, S.; Peng, X. A fluorescent chemodosimeter specific for cysteine: Effective discrimination of cysteine from homocysteine. Chem. Commun. 2009, 39, 5904–5906. [Google Scholar] [CrossRef] [PubMed]





| Strategy | Sample | LOD | Recovery (%) | Ref. |
|---|---|---|---|---|
| β-CD AgNPs | Human urine | 40 μM | 95.0–110.0 | [32] |
| Screen-printed diamond electrode | Bovine plasma | 0.62 nM | 86.0–104.0 | [33] |
| YF | Human urine | 59 nM | - | [34] |
| CP | Human urine | 1.10 μM | 94.3–102.2 | [35] |
| NBD-BT | Cell | 97.6 nM | - | [36] |
| DTRN | Cell | 0.09 μM | - | [37] |
| NFA | Food | 0.21 μM | 96.7–101.6 | [38] |
| Silver nanoflakes-Cr3+ | Beer | 41.2 nM | 93.8–104.0 | This work |
| Silver nanoflakes-Cr3+ | Human urine | 41.2 nM | 93.3–107.1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, Y.; Gu, Y.; Zhou, J.; Li, M.; Qi, J. A Naked-Eye Colorimetric Ratio Method for the Selective and Sensitive Detection of L-Cys Based on a Silver Nanoflakes–Chromium (III) Ion System. Chemosensors 2024, 12, 80. https://doi.org/10.3390/chemosensors12050080
Zhang X, Zhang Y, Gu Y, Zhou J, Li M, Qi J. A Naked-Eye Colorimetric Ratio Method for the Selective and Sensitive Detection of L-Cys Based on a Silver Nanoflakes–Chromium (III) Ion System. Chemosensors. 2024; 12(5):80. https://doi.org/10.3390/chemosensors12050080
Chicago/Turabian StyleZhang, Xi, Yunyi Zhang, Yuwei Gu, Junyu Zhou, Ming Li, and Jian Qi. 2024. "A Naked-Eye Colorimetric Ratio Method for the Selective and Sensitive Detection of L-Cys Based on a Silver Nanoflakes–Chromium (III) Ion System" Chemosensors 12, no. 5: 80. https://doi.org/10.3390/chemosensors12050080





