Utilizing Recycled Expanded Polystyrene Plastics to Stabilize Metal–Organic Frameworks for Heterogeneous Catalysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of HKUST−1
2.3. Preparation of MIL−53(Al)
2.4. Preparation of MOF−74(Zn)
2.5. Preparation of HKUST−1@EPP
2.6. Preparation of MIL−53(Al)@EPP
2.7. Preparation of MOF−74(Zn)@EPP
2.8. Characterization Method Section
2.9. Stability and Catalytic Experiment
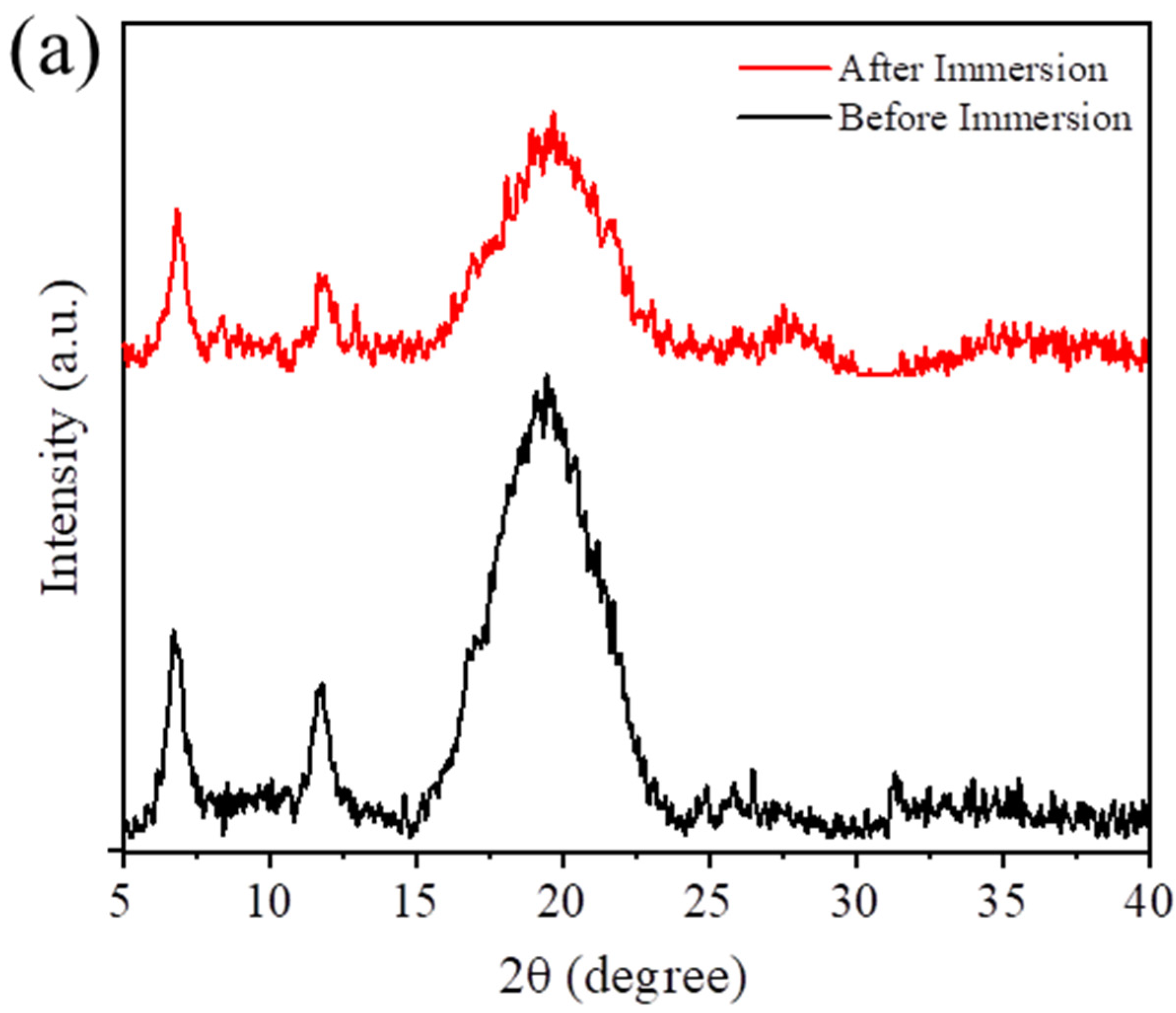
3. Results
3.1. Synthesis and Characterization of Composites
3.2. Catalytic Performance of Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhi, X.; Bairoliya, S.; Cho, Z.; Cao, B. Plastic-microbe interaction in the marine environment: Research methods and opportunities. Environ. Int. 2023, 171, 107716. [Google Scholar]
- Poto, M.; Elvevoll, E.; Sundset, M.; Eilertsen, K.; Morel, M.; Jensen, I. Suggestions for a systematic regulatory approach to ocean plastics. Foods 2021, 10, 2197. [Google Scholar] [CrossRef] [PubMed]
- Vlaanderen, E.; Ghaly, T.; Moore, L.; Focardi, A.; Paulsen, I.; Tetu, S. Plastic leachate exposure drives antibiotic resistance and virulence in marine bacterial communities. Environ. Pollut. 2023, 327, 121558. [Google Scholar] [CrossRef]
- Fonseca, W.; Meng, X.; Deng, D. Trash to treasure: Transforming waste polystyrene cups into negative electrode materials for sodium ion batteries. ACS Sustain. Chem. Eng. 2015, 3, 2153–2159. [Google Scholar] [CrossRef]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef]
- Yansaneh, O.; Zein, S. Latest advances in waste plastic pyrolytic catalysis. Processes 2022, 10, 683. [Google Scholar] [CrossRef]
- Brems, A.; Baeyens, J.; Dewil, R. Recycling and recovery of post-consumer plastic solid waste in a European context. Therm. Sci. 2012, 16, 669–685. [Google Scholar] [CrossRef]
- Thu, H.; Ngoc, M.; Thang, V.; Nguyen, H.; Van-Tien, B. Water transferable, customizable highly ordered honeycomb film from polystyrene foam waste for complex surface patterning in confined space. J. Appl. Polym. Sci. 2024, 141, e55241. [Google Scholar]
- Suellen, S.; Felipe, L.; Esperidian, A.; Hélio, W. Recycling expanded polystyrene with a biodegradable solvent to manufacture 3D printed prototypes and finishing materials for construction. J. Polym. Environ. 2022, 30, 3701–3717. [Google Scholar]
- Luo, X.; Zhan, J.; Mei, Q.; Zhang, S. Selective oxidative upgrade of waste polystyrene plastics by nitric acid to produce benzoic acid. Green Chem. 2023, 25, 6717–6727. [Google Scholar] [CrossRef]
- Albert, O.; Jerald, Y.; Feng, Z.; Tristan, T.; Jason, Y. Organocatalytic aerobic oxidative degradation of polystyrene to aromatic acids. ACS Sustain. Chem. Eng. 2023, 11, 12514–12522. [Google Scholar]
- Ribeiro, M.; Pinto, R.; Silva, R.; Vieira, C. Sulfonated expanded polystyrene waste promotes the (+)-citronellal cyclization reaction: A sustainable alternative process for biomass valorization. Waste Biomass Valor. 2021, 12, 4695–4702. [Google Scholar] [CrossRef]
- Jin, P.; Tan, W.; Huo, J.; Liu, T.; Liang, Y.; Wang, S.; Bradshaw, D. Hierarchically porous MOF/polymer composites via interfacial nanoassembly and emulsion polymerization. J. Mater. Chem. A 2018, 6, 20473–20479. [Google Scholar] [CrossRef]
- Chen, L.; Ding, X.; Huo, J.; El Hankari, S.; Bradshaw, D. Facile synthesis of magnetic macroporous polymer/MOF composites as separable catalysts. J. Mater. Sci. 2018, 54, 370–3829. [Google Scholar] [CrossRef]
- Huo, J.; Marcello, M.; Garai, A.; Bradshaw, D. MOF-polymer composite microcapsules derived from Pickering emulsions. Adv. Mater. 2013, 25, 2717–2722. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, Y.; Xie, L.; Li, X.; Li, J. Construction and application of base-stable MOFs: A critical review. Chem. Soc. Rev. 2022, 51, 6417–6441. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Hu, C.; Gao, Z.; Yang, B.; Li, Q.; Zhan, X.; Tong, X.; Tian, J. Metal–organic frameworks (MOFs) derived materials used in Zn–air battery. Materials 2022, 15, 5837. [Google Scholar] [CrossRef]
- Jaffar, Z.; Yunus, N.; Shaharun, M.; Allim, M.; Rahim, A. Incorporated metal–organic framework hybrid materials for gas separation, catalysis and wastewater treatment. Processes 2022, 10, 2368. [Google Scholar] [CrossRef]
- Ding, M.; Cai, X.; Jiang, H. Improving MOF stability: Approaches and applications. Chem. Sci. 2019, 10, 10209–10230. [Google Scholar] [CrossRef]
- Xu, T.; Wang, Y.; Xue, Y.; Li, J.; Wang, Y. MXenes@metal-organic framework hybrids for energy storage and electrocatalytic application: Insights into recent advances. Chem. Eng. J. 2023, 470, 144247. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, C. Metal–organic framework thin films: Fabrication, modification, and patterning. Processes 2020, 8, 377. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, W. Metal-organic frameworks for artificial photosynthesis and photocatalysis. Chem. Soc. Rev. 2014, 43, 5982–5993. [Google Scholar] [CrossRef]
- Xia, W.; Mahmood, A.; Zou, R.; Xu, Q. Metal-organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energy Environ. Sci. 2015, 8, 1837–1866. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, Z.; Xiang, S.; Chen, B. Perspective of microporous metal-organic frameworks for CO2 capture and separation. Energy Environ. Sci. 2014, 7, 2868–2899. [Google Scholar] [CrossRef]
- Qiu, S.; Xue, M.; Zhu, G. Metal-organic framework membranes: From synthesis to separation application. Chem. Soc. Rev. 2014, 43, 6116–6140. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ou, S.; Wu, C. Porous metal-organic frameworks for heterogeneous biomimetic catalysis. Acc. Chem. Res. 2014, 47, 1199–1207. [Google Scholar] [CrossRef]
- Gascon, J.; Corma, A.; Kapteijn, F.; Llabrés, F. Metal-organic framework catalysis: Quo vadis? ACS Catal. 2013, 4, 361–378. [Google Scholar] [CrossRef]
- Bair, J.; Schramm, Y.; Sergeev, A.; Clot, E.; Eisenstein, O.; Hartwig, J. Linear-selective hydroarylation of unactivated terminal and internal olefins with trifluoromethyl-substituted arenes. J. Am. Chem. Soc. 2014, 136, 13098–13101. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Jhung, S. Composites of metal-organic frameworks: Preparation and application in adsorption. Mater. Today 2014, 17, 136–146. [Google Scholar] [CrossRef]
- Chen, Z.; Kent, O.; Le, O. Rational design of stable functional metal–organic frameworks. Mater. Today 2023, 10, 3257–3268. [Google Scholar] [CrossRef]
- Hung, P.; Lin, P.; Wang, X.; Ho, J.-a.A. Metal–organic frameworks in diagnostics, therapeutics, and other biomedical applications. J. Chin. Chem. Soc. 2023, 70, 1284–1296. [Google Scholar] [CrossRef]
- Yang, S.; Peng, L.; Sun, D.; Asgari, M.; Oveisi, E.; Trukhina, O.; Bulut, S.; Jamali, A.; Queen, W. A new post-synthetic polymerization strategy makes metal-organic frameworks more stable. Chem. Sci. 2019, 10, 4542–4549. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, Z.; Yuan, Z.; Van Horne, C.; Freger, V.; Lin, M.; Cai, R.; Chen, J. A comprehensive review on water stable metal-organic frameworks for large-scale preparation and applications in water quality management based on surveys made since 2015. Crit. Rev. Env. Sci. Technol. 2022, 52, 4038–4071. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Du, Z.; Wang, X.; Yu, S.; Jiang, H. Water-stable metal-organic frameworks with intrinsic peroxidase-like catalytic activity as a colorimetric biosensing platform. Chem. Commun. 2014, 50, 1092–1094. [Google Scholar] [CrossRef] [PubMed]
- Burtch, N.; Jasuja, H.; Walton, K. Water stability and adsorption in metal-organic frameworks. Chem. Rev. 2014, 114, 10575–10612. [Google Scholar] [CrossRef]
- Canivet, J.; Fateeva, A.; Guo, Y.; Coasne, B.; Farrusseng, D. Water adsorption in MOFs: Fundamentals and applications. Chem. Soc. Rev. 2014, 43, 5594–5617. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kansara, A.; Saha, D.; Gonnade, R.; Mullangi, D.; Manna, B.; Desai, A.; Thorat, S.; Singh, P.; Mukherjee, A.; et al. An ultrahydrophobic fluorous metal-organic framework derived recyclable composite as a promising platform to tackle marine oil spills. Chem. Eur. J. 2016, 22, 10937–10943. [Google Scholar] [CrossRef]
- Nguyen, J.; Cohen, S. Moisture-resistant and superhydrophobic metal-organic frameworks obtained via postsynthetic modification. J. Am. Chem. Soc. 2010, 132, 4560–4561. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; He, H.; Gao, W.; Aguila, B.; Wojtas, L.; Dai, Z.; Li, J.; Chen, Y.; Xiao, F.; Ma, S. Imparting amphiphobicity on single-crystalline porous materials. Nat. Commun. 2016, 7, 13300. [Google Scholar] [CrossRef]
- Deng, X.; Zheng, S.; Zhong, Y.; Hu, J.; Chung, L.; He, J. Conductive MOFs based on thiol-functionalized linkers: Challenges, opportunities, and recent advances. Coord. Chem. Rev. 2022, 450, 214235. [Google Scholar] [CrossRef]
- Spiegel, S.; Wagner, I.; Begum, S.; Schwotzer, M.; Wessely, I.; Braese, S.; Tsotsalas, M. Dynamic surface modification of metal-organic framework nanoparticles via alkoxyamine functional groups. Langmuir 2022, 10, 6531–6538. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Yang, Q.; Xu, Q.; Yu, S.; Jiang, H. Polydimethylsiloxane coating for a palladium/MOF composite: Highly improved catalytic performance by surface hydrophobization. Angew. Chem. Int. Ed. 2016, 55, 7379–7383. [Google Scholar] [CrossRef] [PubMed]
- Wee, L.; Lohe, M.; Janssens, N.; Kaskel, S.; Martens, J. Fine tuning of the metal-organic framework Cu3(BTC)2 HKUST−1 crystal size in the 100 nm to 5 micron range. J. Mater. Chem. 2012, 22, 13742–13746. [Google Scholar] [CrossRef]
- Vinu, A.; Ariga, K.; Mori, T.; Nakanishi, T.; Hishita, S.; Golberg, D.; Bando, Y. Preparation and characterization of well-ordered hexagonal mesoporous carbon nitride. Adv. Mater. 2005, 17, 1648–1652. [Google Scholar] [CrossRef]
- Ploychompoo, S.; Liang, Q.; Zhou, X.; Wei, C.; Luo, H. Fabrication of Zn-MOF-74/polyacrylamide coated with reduced graphene oxide (Zn-MOF−74/rGO/PAM) for As(iii) removal. Phys. E Low-Dimens. Syst. Nanostruct. 2021, 125, 114377. [Google Scholar] [CrossRef]
- Rowsell, J.; Yaghi, O. Effects of functionalization, catenation, and variation of the metal oxide and organic linking units on the low-pressure hydrogen adsorption properties of metal-organic frameworks. J. Am. Chem. Soc. 2006, 128, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Q. Conversion of 5-hydroxymethylfurfural into 5-ethoxymethylfurfural and ethyl levulinate catalyzed by MOF-based heteropolyacid materials. Green Chem. 2016, 18, 5884–5889. [Google Scholar] [CrossRef]
- Li, J.; Sun, X.; Subhan, S.; Gong, W.; Li, W.; Sun, W.; Zhang, Y.; Lu, M.; Ji, H.; Zhao, Z.; et al. Construction of novel Cu-based bimetal polycrystal@carbon catalyst prepared from bimetal HKUST−1 type MOFs (MOF−199s) for ultrafast reduction of 4-nitrophenol via interfacial synergistic catalysis. Chem. Eng. J. 2022, 446, 137314. [Google Scholar] [CrossRef]
- Al-Janabi, N.; Alfutimie, A.; Siperstein, F.; Fan, X. Underlying mechanism of the hydrothermal instability of Cu3(BTC)2 metal-organic framework. Front. Chem. Sci. Eng. 2016, 10, 103–107. [Google Scholar] [CrossRef]
- Majano, G.; Martin, O.; Hammes, M.; Smeets, S.; Baerlocher, C.; Perez-Ramirez, J. Solvent-mediated reconstruction of the metal-organic framework HKUST−1 (Cu3(BTC)2). Adv. Funct. Mater. 2014, 24, 3855–3865. [Google Scholar] [CrossRef]
- Carotenuto, G.; Nicolais, F. Reversible thermochromic nanocomposites based on thiolate-capped silver nanoparticles embedded in amorphous polystyrene. Materials 2009, 2, 1323–1340. [Google Scholar] [CrossRef]
- Van Beurden, K.; Koning, S.; Molendijk, D.; Schijndel, J. The knoevenagel reaction: A review of the unfinished treasure map to forming carbon-carbon bonds. Green Chem. Lett. Rev. 2020, 13, 85–100. [Google Scholar] [CrossRef]
- Johari, S.; Johan, M.; Khaligh, N. An overview of metal-free sustainable nitrogen-based catalytic knoevenagel condensation reaction. Org. Biomol. Chem. 2022, 20, 2164–2186. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Zhang, J.; Chang, S.; Hou, Y.; Zhu, Q. 2D hybrid architectures constructed from two kinds of polyoxovanadates as efficient heterogeneous catalysts for cyanosilylation and knoevenagel condensation. Inorg. Chem. 2020, 59, 10578–10590. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, R.; Shen, E.; Liang, C.; Song, D.; El Hankari, S.; Huo, J. Utilizing Recycled Expanded Polystyrene Plastics to Stabilize Metal–Organic Frameworks for Heterogeneous Catalysis. Processes 2024, 12, 961. https://doi.org/10.3390/pr12050961
Yin R, Shen E, Liang C, Song D, El Hankari S, Huo J. Utilizing Recycled Expanded Polystyrene Plastics to Stabilize Metal–Organic Frameworks for Heterogeneous Catalysis. Processes. 2024; 12(5):961. https://doi.org/10.3390/pr12050961
Chicago/Turabian StyleYin, Ruizhi, Enxi Shen, Chenjia Liang, Dezhong Song, Samir El Hankari, and Jia Huo. 2024. "Utilizing Recycled Expanded Polystyrene Plastics to Stabilize Metal–Organic Frameworks for Heterogeneous Catalysis" Processes 12, no. 5: 961. https://doi.org/10.3390/pr12050961




