Arsenic, Cadmium, and Lead Levels in School Meals and Their Risk Assessment in Municipalities in Bahia, Brazil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Planning and Collection Technique
2.3. Microwave-Assisted Mineralization
2.4. Determination of Toxic Elements
2.5. Analytical Quality Assurance
2.6. Risk Assessment of School Meal Consumption
2.7. Statistical Analysis
3. Results and Discussion
3.1. Quality Assurance
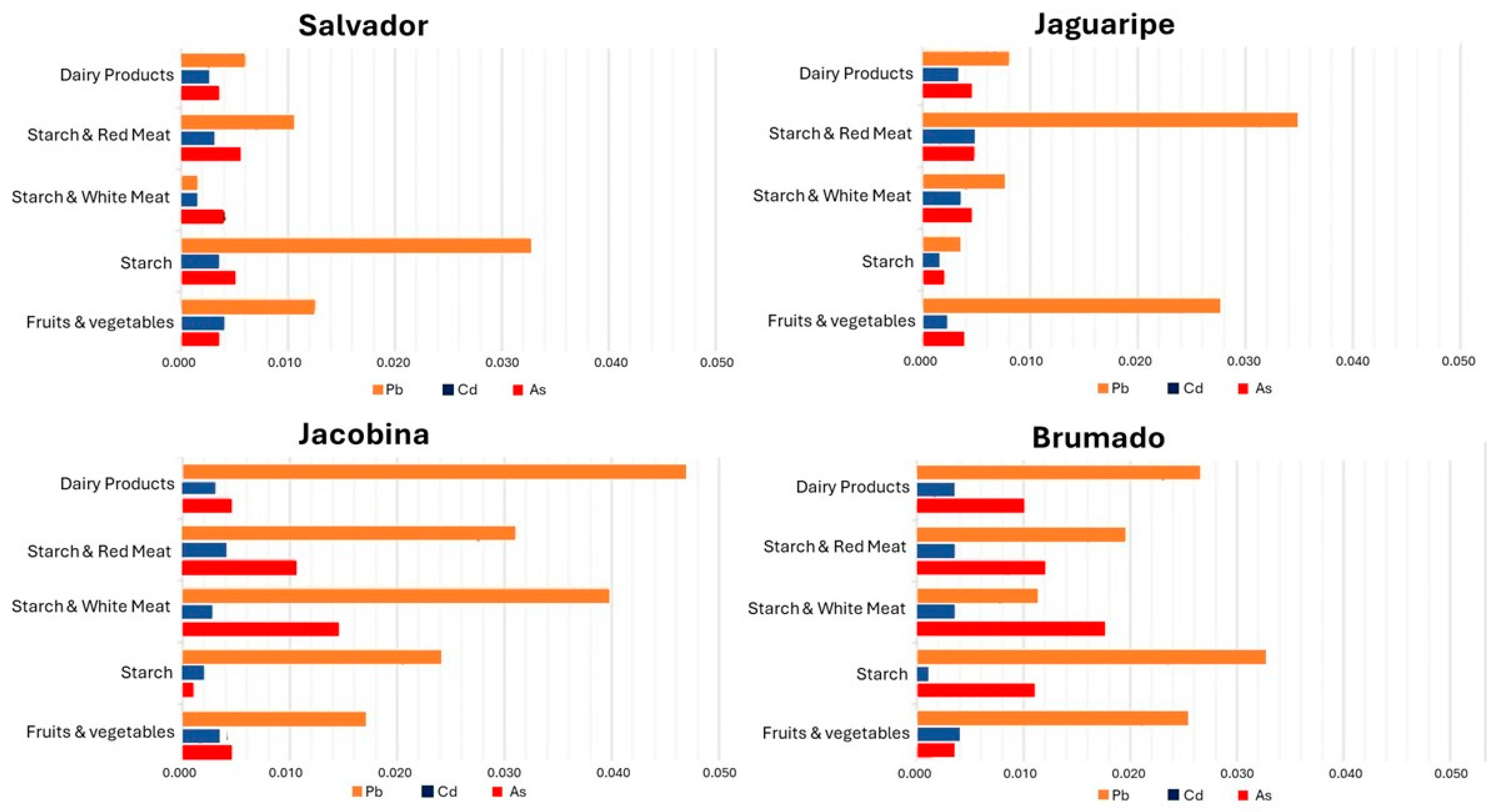
3.2. Levels of Toxic Metals in School Meals
3.2.1. Arsenic
3.2.2. Cadmium
3.2.3. Lead
3.3. Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, C.O. Segurança Alimentar e Nutricional; Rubio: Rio de Janeiro, Brazil, 2015. [Google Scholar]
- Jardim, A.N.O.; Caldas, E.D. Exposição Humana a Substâncias Químicas Potencialmente Tóxicas na Dieta e os Riscos para Saúde. Química Nova 2009, 32, 1898–1909. [Google Scholar] [CrossRef]
- Cesar, J.T.; Valentim, E.A.; Almeida, C.C.B.; Schieferdecker, M.E.M.; Schmidt, S. Alimentação Escolar no Brasil e Estados Unidos: Uma revisão integrativa. Ciência Saúde Coletiva 2016, 23, 991–1007. [Google Scholar] [CrossRef]
- Horta, P.M.; Carmo, A.S.; Junior, E.V.; Santos, L.C. Consuming School Meals Improves Brazilian Children’s Diets According to Their Social Vulnerability Risk. Public Health Nutr. 2019, 22, 2714–2719. [Google Scholar] [CrossRef]
- Sobrinho, J.F. Avaliação da Qualidade dos Cardápios Ofertados para Préescolares de uma Escola Privada no Distrito Federal; Monografia (Especialização)—Universidade Federal de Brasília, Faculdade de Ciências da Saúde: Brasília, DF, Brazil, 2017. [Google Scholar]
- Soares, T.M.S. O Programa Nacional de Alimentação Escolar (PNAE) no Município de Santo Antônio da Patrulha—RS; Monografia (Trabalho de Conclusão de Curso)—Universidade Federal do Rio Grande do Sul: Tramandaí, RS, Brazil, 2018. [Google Scholar]
- EFSA, European Food Safety Authority. Scientific Opinion on Lead in Food. EFSA Panel on Contaminants in the Food Chain (CONTAM). EFSA J. Parma Italy 2010, 4, 151. Available online: https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2010.1570 (accessed on 7 May 2023).
- Rahmani, J.; Fakhri, Y.; Shahsavani, A.; Bahmani, Z.; Urbina, M.A.; Chirumbolo, S.; Keramati, H.; Moradi, B.; Bay, A.; Bjørklund, G. A systematic review and meta-analysis of metal concentrations in canned tuna fish in Iran and human health risk assessment. Food Chem. Toxicol. 2018, 118, 753–765. [Google Scholar] [CrossRef]
- Gao, Y.; Li, X.; Dong, J.; Cao, Y.; Li, T.; Mielke, H.W. Snack foods and lead ingestion risks for school aged children: A comparative evaluation of potentially toxic metals and children’s exposure response of blood lead, copper and zinc levels. Chemosphere 2020, 261, 127547. [Google Scholar] [CrossRef]
- Amaya, E.; Gil, F.; Freire, C.; Olmedo, P.; Fernández-Rodríguez, M.; Fernández, M.F.; Olea, N. Placental concentrations of heavy metals in a mother–child cohort. Environ. Res. 2013, 120, 63–70. [Google Scholar] [CrossRef]
- IARC. A Review of Human Carcinogens: Part C: Arsenic, Metals, Fibres, and Dusts. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 2012; No. 100C; International Agency for Research on Cancer: Lyon, France, 2012.
- Duffus, J.H. Heavy Metals’ a Meaningless Term? IUPAC Technical Report. Pure Appl. Chem. 2002, 74, 793–807. [Google Scholar] [CrossRef]
- EFSA, European Food Safety Authority. Scientific Opinion of the Panel on Contaminants in the Food-Chain. In Cadmium Dietary Exposure in the European Population; European Food Safety Authority: Parma, Italy, 2012. [Google Scholar]
- Menezes-Filho, J.A.; Carvalho, C.; Rodrigues, J.; Araújo, C.; Santos, N.; Lima, C.; Bandeira, M.; Marques, B.; Anjos, A.; Bah, H. Environmental Co-Exposure to Lead and Manganese and Intellectual Deficit in School-Aged Children. Int. J. Environ. Res. Public Health 2018, 15, 2418. [Google Scholar] [CrossRef]
- Chandravanshi, L.; Shiv, K.; Kumar, S. Developmental toxicity of cadmium in infants and children: A review. Environ. Anal. Health Toxicol. 2021, 36. [Google Scholar] [CrossRef]
- IARC. Inorganic and organic lead compounds. IARC. Monogr. Eval. Carcinog. Risks Hum. 2006, 87.
- Araújo, C.F.S.; Lopes, M.V.; Vasquez, M.R.; Porcino, T.S.; Ribeiro, A.S.V.; Rodrigues, J.L.G.; Oliveira, S.S.P.; Menezes-Filho, J.A. Cadmium and lead in seafood from the Aratu Bay, Brazil and the human health risk assessment. Environ. Monit. Assess. 2016, 188, 259. [Google Scholar] [CrossRef]
- Kato, L.S.; Fernandes, E.A.N.; Raab, A.; Bacchi, M.A.; Feldmann, J. Arsenic and cadmium contents in Brazilian rice from different origins can vary more than two orders of magnitude. Food Chem. 2019, 286, 644–650. [Google Scholar] [CrossRef]
- Silva, L.P.; Campos, N.S.; Lisboa, T.P.; Faria, L.V.; Matos, M.A.C.; Matos, R.C.; Sousa, R.A. Simultaneous determination of cadmium, lead and copper in chocolate samples by square wave anodic stripping voltammetry. Food Addit. Contam. Part A 2021, 38, 418–426. [Google Scholar] [CrossRef]
- Dainez, F.P.A.; Abreu, E.S. Determination of metals in canned foods: Analysis of intact and damaged packaging of peas, corn, pineapple and peach. Arch. Health 2023, 4, 981–994. [Google Scholar] [CrossRef]
- Bastías, J.M.; Bermðdez, M.; Carrasco, J.; Espinoza, O.; Muñoz, M.; Galotto, M.J.; Muñoz, O. Determination of Dietary Intake of Total Arsenic, Inorganic Arsenic and Total Mercury in the Chilean School Meal Program. Food Sci. Technol. Int. 2010, 16, 443–450. [Google Scholar] [CrossRef]
- Maihara, V.A.; Vasconcellos, M.B.A.; Cordeiro, M.B.; Cozzolino, S.M.F. Estimate of toxic element intake in diets of pre-school children and elderly collected by duplicate portion sampling. Food Addit. Contam. 1998, 15, 782–788. [Google Scholar] [CrossRef]
- Leroux, I.; Ferreira, A.; Paniz, F.; Pedron, T.; Salles, F.; Silva, F.; Maltez, H.; Batista, B.; Olympio, K. Lead, Cadmium, and Arsenic Bioaccessibility of 24 h Duplicate Diet Ingested by Preschool Children Attending Day Care Centers in Brazil. Int. J. Environ. Res. Public Health 2018, 15, 1778. [Google Scholar] [CrossRef]
- França, F.C.O.; Andrade, I.S.; Silva, M.V.; Lordêlo, M.S.; Costa, R.G.; Menezes-Filho, J.A. School meals’ centesimal and mineral composition and their nutritional value for Brazilian children. J. Trace Elem. Med. Biol. 2018, 48, 97–104. [Google Scholar] [CrossRef]
- IBGE, Instituto Brasileiro de Geografia e Estatítica. Censo Escolar da Educação Básica, 2016. Brasília. 2017. Available online: http://ces.ibge.gov.br/base-de-dados/metadados/inep/educacao-basica (accessed on 10 July 2020).
- IAL, Instituto Adolfo Lutz. Physico-Chemical Methods for Food Analysis; Instituto Adolfo Lutz: São Paulo, Brazil, 2008.
- IUPAC. Chemistry Compendium of Chemical Terminology; International Union of Pure and Applied Chemistry: Zurique, Switzerland, 1997; Volume 2. [Google Scholar]
- BRASIL. Agência Nacional de Vigilância Sanitária. Guia para validação de métodos analíticos. RDC Nº 166, 24/07/2017. 2017. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2017/rdc0166_24_07_2017.pdf (accessed on 12 July 2023).
- Rahman, M.M.; Alauddin, M.; Alauddin, S.T.; Siddique, A.B.; Islam, M.R.; Agosta, G.; Mondal, D.; Naidu, R. Bioaccessibility and speciation of arsenic in children’s diets and health risk assessment of an endemic area in Bangladesh. J. Hazard. Mater. 2021, 403, 124064. [Google Scholar] [CrossRef]
- Gomes-Junior, E.A.; Bah, H.A.F.; Rodrigues, Y.J.M.; Bandeira, M.J.; Santos, N.R.; Menezes-Filho, J.A. Lead in soil and vegetables in a glazed ceramic production area: A risk assessment. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100392. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (US EPA). Risk Assessment Information System. 2013. Available online: https://rais.ornl.gov (accessed on 10 July 2020).
- IBGE, Instituto Brasileiro de Geografia e Estatística. Pesquisa de Orçamentos Familiares 2017–2018—POF; IBGE: Rio de Janeiro, Brazil, 2020.
- BRASIL. Lei no. 9.394, de 20 de Dezembro de 1996: Estabelece as Diretrizes e Bases da Educação Nacional. 1996. Available online: http://portal.mec.gov.br/arquivos/pdf/lei%209394.pdf (accessed on 20 June 2023).
- IBGE. Instituto Brasileiro de Geografia e Estatística. Pesquisa de Orçamentos Familiares 2008–2009: Aquisição Alimentar Domiciliar per Capita; IBGE: Rio de Janeiro, Brazil, 2010.
- Brazil National Health Surveillance Agency. Validation of Analytical Methods and Other Provisions; RDC 166, 249/07/2017; Brazil National Health Surveillance Agency: Brasília, Brazil, 2017.
- EC. Commission Decision 657/2002: Implementing Council Directive 96/23/EC Concerning Performance of Analytical Methods and the Interpretation of Result; Official Journal of the European Communities: Bruxelas, Belgium, 2002; pp. 8–36. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32002D0657 (accessed on 2 August 2023).
- Brazil. National Health Surveillance Agency. Mercosur Technical Regulation on Maximum Contaminant Inorganic in Food. RDC 42, 29/08/2013. 2013. Available online: http://portal.anvisa.gov.br/documents/33880/2568070/rdc0042_29_08_2013.pdf/c5a (accessed on 2 August 2023).
- Chen, H.L.; Lee, C.C.; Huang, W.J.; Huang, H.T.; Wu, Y.C.; Hsu, Y.C.; Kao, Y.T. Arsenic speciation in rice and risk assessment of inorganic arsenic in Taiwan population. Environ. Sci. Pollut. Res. 2015, 23, 4481–4488. [Google Scholar] [CrossRef]
- Shibata, T.; Meng, C.; Umoren, J.; West, H. Risk Assessment of Arsenic in Rice Cereal and Other Dietary Sources for Infants and Toddlers in the U.S. Int. J. Environ. Res. Public Health 2016, 13, 361. [Google Scholar] [CrossRef]
- Roma, A.; Esposito, M.; Chiaravalle, E.; Miedico, O.; Filippis, S.P.; Brambilla, G. Occurrence of cadmium, lead, mercury, and arsenic in prepared meals in Italy: Potential relevance for intake assessment. J. Food Compos. Anal. 2017, 63, 28–33. [Google Scholar] [CrossRef]
- Kim, K.; Melough, M.; Vance, T.; Noh, H.; Koo, S.; Chun, O. Dietary Cadmium Intake and Sources in the US. Nutrients 2018, 11, 2. [Google Scholar] [CrossRef]
- Kippler, M.; Tofail, F.; Hamadani, J.D.; Gardner, R.M.; Grantham-Mcgregor, S.M.; Bottai, M.; Vahter, M. Early-Life Cadmium Exposure and Child Development in 5-Year-Old Girls and Boys: A cohort study in rural bangladesh. Environ. Health Perspect. 2012, 120, 1462–1468. [Google Scholar] [CrossRef]
- Ciesielski, T.; Weuve, J.; Bellinger, D.C.; Schwartz, J.; Lanphear, B.; Wright, R.O. Cadmium Exposure and Neurodevelopmental Outcomes in U.S. Children. Environ. Health Perspect. 2012, 120, 758–763. [Google Scholar] [CrossRef]
- Jeong, K.S.; Park, H.; Ha, E.; Hong, Y.C.; Ha, M.; Park, H.; Kim, B.N.; Lee, B.E.; Lee, S.J.; Lee, K.Y. Performance IQ in children is associated with blood cadmium concentration in early pregnancy. J. Trace Elem. Med. Biol. 2015, 30, 107–111. [Google Scholar] [CrossRef]
- EU. Commission Regulation (EC) no 1881/2006: Setting Maximum Levels for Certain Contaminants in Foodstuffs; Official Journal of the European Union: Bruxelas, Belgium, 2006; Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:364:0005:0024:EN:PDF (accessed on 12 August 2023).
- Nacano, L.R.; Freitas, R.; Barbosa, F. Evaluation of Seasonal Dietary Exposure to Arsenic, Cadmium and Lead in Schoolchildren Through the Analysis of Meals Served by Public Schools of Ribeirão Preto, Brazil. J. Toxicol. Environ. Health Part A 2014, 77, 367–374. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, J.Á. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef]
- Charkiewicz, A.E.; Backstrand, J.R. Lead Toxicity and Pollution in Poland. Int. J. Environ. Res. Public Health 2020, 17, 4385. [Google Scholar] [CrossRef]
- Vasconcelos, M.; Quintal, A.P.N.; Pôrto, L.B.G.; Souza, S.V.C. Lead in Brazilian food: Exposure assessment and risk characterization. Food Addit. Contam. Part A 2021, 38, 315–325. [Google Scholar] [CrossRef]
- Storelli, M.M. Potential human health risks from metals (Hg, Cd, and Pb) and polychlorinated biphenyls (PCBs) via seafood consumption: Estimation of target hazard quotients (thqs) and toxic equivalents (teqs). Food Chem. Toxicol. 2008, 46, 2782–2788. [Google Scholar] [CrossRef]

| Parameters | S1 (Elementary School) | S2 (Middle School) | S3 (Overall Average) |
|---|---|---|---|
| Duration of exposure (years) | 9 | 3 | 12 |
| Body weight (Kg) | 25.4 | 51.8 | 41.2 |
| Exposure frequency (days/year) | 202 | 202 | 202 |
| CRM | Certified Value (mg·kg−1) | Measured Value (mg·kg−1) | LOD (mg·kg−1) | LOQ (mg·kg−1) | Precision (rSR %) | Accuracy (RE %) | |
|---|---|---|---|---|---|---|---|
| As | Spinach leaves | 0.068 ± 0.012 | 0.067 | 0.008 | 0.012 | 7.73 | 1.47 |
| Cd | Apple leaves | 0.013 ± 0.002 | 0.012 | 0.009 | 0.024 | 6.80 | 9.09 |
| Pb | Apple leaves | 0.470 ± 0.024 | 0.471 | 0.005 | 0.013 | 1.61 | 0.15 |
| Municipalities | Semester | Median | IQR 1 | Min | Max |
|---|---|---|---|---|---|
| Brumado | 1st Sem | 2 | 1 | <LD | 8 |
| 2nd Sem | 3 | 4 | 2 | 8 | |
| Jacobina | 1st Sem | 1 | <LOD | 1 | 3 |
| 2nd Sem | 5 | 3 | 3 | 11 | |
| Jaguaripe | 1st Sem | 2 | 1 | 1 | 6 |
| 2nd Sem | 4 | 3 | 2 | 10 | |
| Salvador | 1st Sem | 4 | 5 | <LOD | 1 |
| 2nd Sem | 4 | 3 | 2 | 10 |
| Municipalities | Semester | Median | IQR 1 | Min | Max |
|---|---|---|---|---|---|
| Brumado | 1st Sem | 2 | 2 | 1 | 4 |
| 2nd Sem | 3 | 4 | 2 | 8 | |
| Jacobina | 1st Sem | 1 | <LOD | 1 | 3 |
| 2nd Sem | 5 | 3 | 3 | 11 | |
| Jaguaripe | 1st Sem | 2 | 1 | 1 | 6 |
| 2nd Sem | 4 | 3 | 2 | 10 | |
| Salvador | 1st Sem | 2 | 1 | 1 | 6 |
| 2nd Sem | 4 | 3 | 2 | 10 |
| Municipalities | Semester | Median | IQR 1 | Min | Max |
|---|---|---|---|---|---|
| Brumado | 1st Sem | 7 | 9 | 1 | 43 |
| 2nd Sem | 42 | 40 | 11 | 96 | |
| Jacobina | 1st Sem | 25 | 22 | 1 | 58 |
| 2nd Sem | 38 | 55 | 11 | 101 | |
| Jaguaripe | 1st Sem | 3 | 8 | 2 | 157 |
| 2nd Sem | 12 | 74 | 4 | 137 | |
| Salvador | 1st Sem | 3 | 8 | 2 | 39 |
| 2nd Sem | 12 | 74 | 4 | 137 |
| Municipalities | Brumado | Jacobina | Jaguaripe | Salvador | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scenarios Metals | S1 | S2 | S3 | S1 | S2 | S3 | S1 | S2 | S3 | S1 | S2 | S3 |
| As–HQ | 0.05 | 0.02 | 0.05 | 0.06 | 0.03 | 0.04 | 0.05 | 0.03 | 0.03 | 0.08 | 0.04 | 0.05 |
| Cd–HQ | 0.02 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Pb–HQ | 0.02 | 0.01 | 0.01 | 0.03 | 0.01 | 0.02 | 0.01 | 0.0 | 0.0 | 0.06 | 0.03 | 0.04 |
| THQ | 0.09 | 0.04 | 0.07 | 0.11 | 0.05 | 0.07 | 0.08 | 0.04 | 0.04 | 0.15 | 0.08 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, L.d.S.; Chagas, F.F.; Martinho, M.G.D.; Gomes-Júnior, E.A.; Silva, M.V.L.; Menezes-Filho, J.A. Arsenic, Cadmium, and Lead Levels in School Meals and Their Risk Assessment in Municipalities in Bahia, Brazil. Foods 2024, 13, 1500. https://doi.org/10.3390/foods13101500
Santos LdS, Chagas FF, Martinho MGD, Gomes-Júnior EA, Silva MVL, Menezes-Filho JA. Arsenic, Cadmium, and Lead Levels in School Meals and Their Risk Assessment in Municipalities in Bahia, Brazil. Foods. 2024; 13(10):1500. https://doi.org/10.3390/foods13101500
Chicago/Turabian StyleSantos, Larissa da S., Fabiana F. Chagas, Martinho G. Dinis Martinho, Erival A. Gomes-Júnior, Mariângela V. Lopes Silva, and José A. Menezes-Filho. 2024. "Arsenic, Cadmium, and Lead Levels in School Meals and Their Risk Assessment in Municipalities in Bahia, Brazil" Foods 13, no. 10: 1500. https://doi.org/10.3390/foods13101500






