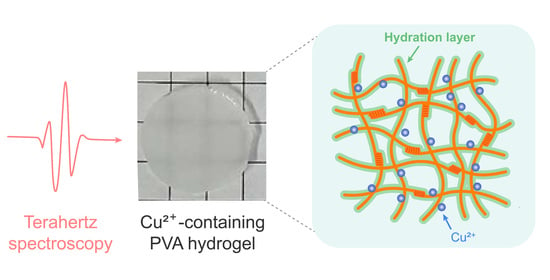
Terahertz Spectroscopic Insight into the Hydrogelation of Copper Ion-Coordinated Poly(vinyl alcohol)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of PVA Solution and PVA Hydrogels
2.2. Tearahetz Spectra of PVA Solution at Different Concentrations
2.3. The Addition of Copper Ions to PVA Solutions Changes the Terahertz Dynamics
2.4. Tearahetz Spectra of PVA Solution at Different Concentrations
2.5. Thermodynamic Properties of Copper Ion–PVA Hydrogel
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of PVA-Cu Hydrogels
4.3. DSC Measurement [30]
4.4. Terahertz Spectroscopy Measurement
4.5. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Bai, J.; Shao, K.; Tang, W.W.; Zhao, X.L.; Lin, D.H.; Huang, S.; Chen, C.; Ding, Z.; Ye, J.Y. Poly(vinyl alcohol) Hydrogels: The Old and New Functional Material. Int. J. Polym. Sci. 2021, 2021, 2225426. [Google Scholar] [CrossRef]
- Li, H.; Yang, P.Y.; Pageni, P.; Tang, C.B. Recent Advances in Metal-Containing Polymer Hydrogels. Macromol. Rapid Commun. 2017, 38, 1700109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Khademhosseini, A. Advances in Engineering Hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Vega, S.L.; Kwon, M.Y.; Burdick, J.A. Recent Advances in Hydrogels for Cartilage Tissue Engineering. Eur. Cell Mater. 2017, 33, 59. [Google Scholar] [CrossRef] [PubMed]
- Whittell, G.R.; Manners, I. Metallopolymers: New Multifunctional Materials. Adv. Mater. 2007, 19, 3439–3468. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, J.; Ren, L.; Ting, C.B. Metal-Containing and Related Polymers for Biomedical Applications. Chem. Soc. Rev. 2016, 45, 5232–5263. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Ding, P.; Wang, Y.; Zhang, Y.; Ossipov, D.; Hilborn, J. Self-Healing Polymeric Hydrogel Formed by Metal–Ligand Coordination Assembly: Design, Fabrication, and Biomedical Applications. Macromol. Rapid Commun. 2019, 40, 1800837. [Google Scholar] [CrossRef]
- Shen, J.F.; Dai, Y.; Xia, F.; Zhang, X.J. Role of Divalent Metal Ions in the Function and Application of Hydrogels. Prog. Polym. Sci. 2022, 2022, 101622. [Google Scholar] [CrossRef]
- Wang, S.; Lv, Y.; Feng, S.; Li, Q.; Zhang, T. Bimetallic ions synergistic cross-linking high-strength rapid self-healing antibacterial hydrogel. Polym. Eng. Sci. 2019, 9, 919–927. [Google Scholar] [CrossRef]
- Gao, L.; Hou, Y.; Wang, H.; Li, M.; Ma, L.; Chu, Z.; Donskyi, I.S.; Haag, R. A Metal-Ion-Incorporated Mussel-Inspired Poly(Vinyl Alcohol)-Based Polymer Coating Offers Improved Antibacterial Activity and Cellular Mechanoresponse Manipulation. Angew. Chem. Int. Ed. 2022, 61, e202201563. [Google Scholar] [CrossRef]
- Wang, J.; Fan, X.; Liu, H.; Tang, K. Self-assembly and Metal Ions-Assisted one Step Fabrication of Recoverable Gelatin Hydrogel with High Mechanical Strength. Polym-Plast. Technol. Mat. 2020, 59, 1899–1909. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Wahid, F.; Zhao, X.J.; Wang, F.P.; Wang, T.F.; Xie, Y.Y.; Jia, S.R.; Zhong, C. Fabrication of Amino Acid-Based SupraMolecular Hydrogel with Silver Ions for Improved Antibacterial Properties. Mater. Lett. 2021, 300, 130161. [Google Scholar] [CrossRef]
- Ma, M.S.; Zhong, Y.L.; Jiang, X.L. Thermosensitive and pH-responsive Tannin-Containing Hydroxypropyl Chitin Hydrogel with Long-Lasting Antibacterial Activity for Wound Healing. Carbohyd. Polym. 2020, 236, 116096. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Su, K.; Yang, H.; Zheng, W.; Zhang, Z.; Ang, S.; Zhang, K.; Wu, P. Novel Natural Glycyrrhetinic Acid-Derived Super Metal Gel and Its Highly Selective Dyes Removal. Gels 2022, 8, 188. [Google Scholar] [CrossRef]
- Li, P.; Feng, Z.; Yu, Z.; Chen, Y.; Li, P.; Yang, Z.; Li, S.; Jin, S. Preparation of Chitosan-Cu2+/NH3 Physical Hydrogel and Its Properties. Int. J. Biol. Macromol. 2019, 133, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.; Wang, H.S.; Zhong, C.; Chu, L.Q. Facile fabrication of moldable antibacterial carboxymethyl chitosan supramolecular hydrogels cross-linked by metal ions complexation. Carbohydr. Polym. 2017, 165, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; He, J.; Wang, X.; Zhang, Y.; Tan, G.; Zhou, Z.; Chen, J.; Chen, D.; Wang, R.; Tian, W.; et al. Tunable Mechanical, Antibacterial, and Cytocompatible Hydrogels Based on a Functionalized Dual Network of Metal Coordination Bonds and Covalent Crosslinking. ACS Appl. Mater. Interfaces 2018, 10, 6190–6198. [Google Scholar] [CrossRef]
- Tian, R.; Qiu, X.; Yuan, P.; Lei, K.; Wang, L.; Bai, Y.; Liu, S.; Chen, X. Fabrication of Self-Healing Hydrogels with On-Demand Antimicrobial Activity and Sustained Biomolecule Release for Infected Skin Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 17018–17027. [Google Scholar] [CrossRef] [PubMed]
- Shuai, F.; Zhang, Y.; Yin, Y.; Zhao, H.; Han, X. Fabrication of an Injectable Iron (III) Crosslinked Alginate-Hyaluronic Acid Hydrogel with Shear-Thinning and Antimicrobial Activities. Carbohydr. Polym. 2021, 260, 117777. [Google Scholar] [CrossRef]
- Tang, S.; Yang, J.; Lin, L.; Peng, K.; Chen, Y.; Jin, S.; Yao, W. Construction of Physically Crosslinked Chitosan/sodium Alginate/calcium Ion Double-Network Hydrogel and Its Application to Heavy Metal Ions Removal. Chem. Eng. J. 2020, 393, 124728. [Google Scholar] [CrossRef]
- Shiraga, K.; Adachi, A.; Nakamura, M.; Tajima, T.; Ajito, K.; Ogawa, Y. Characterization of the Hydrogen Bond Network of Water around Sucrose and Trehalose: Microwave and Terahertz Spectroscopic Study. J. Chem. Phys. 2017, 146, 105102. [Google Scholar] [CrossRef]
- Hoshina, H.; Iwasaki, Y.; Katahira, E.; Okamoto, M.; Otani, C. Structure and Dynamics of Bound Water in Poly (ethylene-vinylalcohol) Copolymers Studied by Terahertz Spectroscopy. Polymer 2018, 148, 49–60. [Google Scholar] [CrossRef]
- Yan, C.; Kramer, P.L.; Yuan, R.; Fayer, M.D. Water Dynamics in Polyacrylamide Hydrogels. J. Am. Chem. Soc. 2018, 140, 9466–9477. [Google Scholar] [CrossRef] [PubMed]
- Pissis, P.; Kyritsis, A. Hydration Studies in Polymer Hydrogels. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 159–175. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Wilson, L.D. A Review on the Design and Hydration Properties of Natural Polymer-Based Hydrogels. Materials 2021, 14, 1095. [Google Scholar] [CrossRef] [PubMed]
- Zapanta, M.J.; Van De Walle, D.; Postelmans, A.; Dewettinck, K.; Saeys, W. Differentiation of the Microstructures of Agarose Hydrogels Using Terahertz Time Domain Spectroscopy (THz-TDS). In Proceedings of the 2023 48th International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz), Montreal, QC, Canada, 17–22 September 2023; pp. 1–2. [Google Scholar]
- Rossi, B.; Venuti, V.; Mele, A.; Punta, C.; Melone, L.; Crupi, V.; Majolino, D.; Trotta, F.; D’Amico, F.; Gessini, A.; et al. Probing the Molecular Connectivity of Water Confined in Polymer Hydrogels. J. Chem. Phys. 2015, 142, 014901. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, X.; Wang, Y.; Huang, G.; Yang, X.; Zhang, Y.; Xiong, Y.; Liu, L.; Zhao, X.; Fu, W. A Novel THz Molecule-Selective Sensing Strategy in Aqueous Environments: THz-ATR Spectroscopy Integrated with a Smart Hydrogel. Talanta 2021, 228, 122213. [Google Scholar] [CrossRef]
- Charkhesht, A.; Lou, D.; Sindle, B.; Wen, C.Y.; Cheng, S.F.; Vinh, N.Q. Insights into Hydration Dynamics and Cooperative Interactions in Glycerol–Water Mixtures by Terahertz Dielectric Spectroscopy. J. Phys. Chem. B 2019, 123, 8791–8799. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Zohuriaan-Mehr, M.J. DSC Studies on Synthesis of Superabsorbent Hydrogels. Polymer 2002, 43, 269–277. [Google Scholar] [CrossRef]
- Edikresnha, D.; Suciati, T.; Khairurrijal, K. Freeze-thawed Hydrogel Loaded by Piper Crocatum Extract with in-vitro Antibacterial and release tests. J. Mater. Res. Technol. 2021, 15, 17–36. [Google Scholar] [CrossRef]





| ε∞ | ε1 | ε2 | ε1 + ε2 | ε1/(ε1 + ε2) | ε2/(ε1 + ε2) | τ1 | τ2 | |
|---|---|---|---|---|---|---|---|---|
| 2% PVA | 2.279 | 21.271 | 2.337 | 23.608 | 90.10% | 9.90% | 2.461 | 0.0816 |
| 6% PVA | 2.416 | 19.798 | 2.213 | 22.011 | 89.95% | 10.05% | 2.423 | 0.0845 |
| 12% PVA | 2.603 | 17.178 | 2.016 | 19.194 | 89.50% | 10.50% | 2.289 | 0.0944 |
| 12% PVA | ε∞ | ε1 | ε2 | ε1 + ε2 | ε1 /(ε1 + ε2) | ε2 /(ε1 + ε2) | τ1 | τ2 |
|---|---|---|---|---|---|---|---|---|
| +0 mM Cu2+ | 2.668 | 18.687 | 1.991 | 20.678 | 90.37% | 9.63% | 2.441 | 0.099 |
| +0.6 mM Cu2+ | 2.663 | 18.170 | 2.059 | 20.229 | 89.82% | 10.18% | 2.394 | 0.097 |
| +3.0 mM Cu2+ | 2.622 | 17.738 | 2.090 | 19.828 | 89.46% | 10.54% | 2.377 | 0.095 |
| 12% PVA | ε∞ | ε1 | ε2 | ε1 + ε2 | ε1 /(ε1 + ε2) | ε2 /(ε1 + ε2) | τ1 | τ2 |
|---|---|---|---|---|---|---|---|---|
| pH 4.0 | 2.622 | 17.738 | 2.090 | 19.828 | 89.46% | 10.54% | 2.377 | 0.095 |
| pH 7.5 | 2.683 | 18.162 | 2.055 | 20.217 | 89.84% | 10.16% | 2.437 | 0.102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Wang, Y.; Lü, J.; Li, X. Terahertz Spectroscopic Insight into the Hydrogelation of Copper Ion-Coordinated Poly(vinyl alcohol). Gels 2024, 10, 324. https://doi.org/10.3390/gels10050324
Wang W, Wang Y, Lü J, Li X. Terahertz Spectroscopic Insight into the Hydrogelation of Copper Ion-Coordinated Poly(vinyl alcohol). Gels. 2024; 10(5):324. https://doi.org/10.3390/gels10050324
Chicago/Turabian StyleWang, Wenjing, Yadi Wang, Junhong Lü, and Xueling Li. 2024. "Terahertz Spectroscopic Insight into the Hydrogelation of Copper Ion-Coordinated Poly(vinyl alcohol)" Gels 10, no. 5: 324. https://doi.org/10.3390/gels10050324