Effects of Four Weeks of In-Season Pre-Workout Supplementation on Performance, Body Composition, Muscle Damage, and Health-Related Markers in Basketball Players: A Randomized Controlled Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Performance Tests and Measurements
2.3.1. Anthropometry and Resting Vital Signs
2.3.2. Jumping Performance
2.3.3. Sprint Performance
2.3.4. Agility T-Test (ATT)
2.3.5. Running-Based Anaerobic Sprint Test (RAST)
2.3.6. Yo-Yo Intermittent Recovery Test Level 1
2.3.7. Supplementation
2.3.8. Blood Sampling and Biochemical Assays
2.4. Statistical Analyses
3. Results
4. Discussion
4.1. Aerobic Performance
4.2. Anaerobic Performance
4.3. Jumping, Sprinting, and Agility Performance
4.4. Body Mass and Body Composition
4.5. Muscle Damage/Health-Related Blood Markers
4.6. Side Effects and Vital Signs
4.7. Study’s Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stojanović, E.; Stojiljković, N.; Scanlan, A.T.; Dalbo, V.J.; Berkelmans, D.M.; Milanović, Z. The Activity Demands and Physiological Responses Encountered During Basketball Match-Play: A Systematic Review. Sports Med. 2018, 48, 111–135. [Google Scholar] [CrossRef]
- Read, P.J.; Hughes, J.; Stewart, P.; Chavda, S.; Bishop, C.; Edwards, M.; Turner, A.N. A needs analysis and field-based testing battery for basketball. Strength Cond. J. 2014, 36, 13–20. [Google Scholar] [CrossRef]
- Abdelkrim, N.B.; El Fazaa, S.; El Ati, J. Time-motion analysis and physiological data of elite under-19-year-old bas-ketball players during competition. Br. J. Sports Med. 2007, 41, 69–75. [Google Scholar] [CrossRef]
- Torres-Ronda, L.; Ric, A.; Llabres-Torres, I.; de Las Heras, B.; Schelling I Del Alcazar, X. Position-Dependent Cardiovascular Response and Time-Motion Analysis during Training Drills and Friendly Matches in Elite Male Basketball Players. J. Strength Cond. Res. 2016, 30, 60–70. [Google Scholar] [CrossRef]
- Ben Abdelkrim, N.; Castagna, C.; Jabri, I.; Battikh, T.; El Fazaa, S.; El Ati, J. Activity profile and physiological requirements of junior elite basketball players in relation to aerobic-anaerobic fitness. J. Strength Cond. Res. 2010, 24, 2330–2342. [Google Scholar] [CrossRef]
- Mancha-Triguero, D.; García-Rubio, J.; Antúnez, A.; Ibáñez, S.J. Physical and Physiological Profiles of Aerobic and Anaerobic Capacities in Young Basketball Players. Int. J. Environ. Res. Public Health 2020, 17, 1409. [Google Scholar] [CrossRef]
- Davis, J.K.; Oikawa, S.Y.; Halson, S.; Stephens, J.; O’riordan, S.; Luhrs, K.; Sopena, B.; Baker, L.B. In-Season Nutrition Strategies and Recovery Modalities to Enhance Recovery for Basketball Players: A Narrative Review. Sports Med. 2022, 52, 971–993. [Google Scholar] [CrossRef]
- Dreher, M.; Ehlert, T.; Simon, P.; Neuberger, E.W.I. Boost me: Prevalence and reasons for the use of stimulant containing pre workout supplements among fitness studio visitors in Mainz (Germany). Front. Psychol. 2018, 9, 1134. [Google Scholar] [CrossRef]
- Shoshan, T.; Post, E. Prevalence of Protein and Pre-Workout Supplement Use among High School Football Players and Potential Product Contamination. Glob. Pediatr. Health 2021, 8. [Google Scholar] [CrossRef]
- Douligeris, A.; Methenitis, S.; Lazou, A.; Panayiotou, G.; Feidantsis, K.; Voulgaridou, G.; Manios, Y.; Jamurtas, A.Z.; Giaginis, C.; Papadopoulou, S.K. The Effect of Acute Pre-Workout Supplement Ingestion on Basketball-Specific Performance of Well-Trained Athletes. Nutrients 2023, 15, 2304. [Google Scholar] [CrossRef]
- Harty, P.S.; Zabriskie, H.A.; Erickson, J.L.; Molling, P.E.; Kerksick, C.M.; Jagim, A.R. Multi-ingredient pre-workout supplements, safety implications, and performance outcomes: A brief review. J. Int. Soc. Sports Nutr. 2018, 15, 41. [Google Scholar] [CrossRef]
- Jagim, A.R.; Harty, P.S.; Camic, C.L. Common ingredient profiles of multi-ingredient pre-workout supplements. Nutrients 2019, 11, 254. [Google Scholar] [CrossRef]
- Jagim, A.R.; Camic, C.L.; Harty, P.S. Common Habits, Adverse Events, and Opinions Regarding Pre-Workout Supplement Use Among Regular Consumers. Nutrients 2019, 11, 855. [Google Scholar] [CrossRef]
- Salinero, J.J.; Lara, B.; Del Coso, J. Effects of acute ingestion of caffeine on team sports performance: A systematic review and meta-analysis. Res. Sports Med. 2019, 27, 238–256. [Google Scholar] [CrossRef]
- Huerta Ojeda, A.; Cerda, C.T.; Salvatierra, M.F.P.; Barahona-Fuentes, G.; Aguilera, C.J. Effects of beta-alanine supplementation on physical performance in aerobic–anaerobic transition zones: A systematic review and meta-analysis. Nutrients 2020, 12, 2490. [Google Scholar] [CrossRef]
- Mielgo-Ayuso, J.; Calleja-Gonzalez, J.; Marqués-Jiménez, D.; Caballero-García, A.; Córdova, A.; Fernández-Lázaro, D. Effects of creatine supplementation on athletic performance in soccer players: A systematic review and meta-analysis. Nutrients 2019, 11, 757. [Google Scholar] [CrossRef]
- Trexler, E.T.; Persky, A.M.; Ryan, E.D.; Schwartz, T.A.; Stoner, L.; Smith-Ryan, A.E. Acute Effects of Citrulline Supplementation on High-Intensity Strength and Power Performance: A Systematic Review and Meta-Analysis. Sports Med. 2019, 49, 707–718. [Google Scholar] [CrossRef]
- Hormoznejad, R.; Javid, A.Z.; Mansoori, A. Effect of BCAA supplementation on central fatigue, energy metabolism substrate and muscle damage to the exercise: A systematic review with meta-analysis. Sport Sci. Health 2019, 15, 265–279. [Google Scholar] [CrossRef]
- Jagim, A.R.; Jones, M.T.; Wright, G.A.; Antoine, C.S.; Kovacs, A.; Oliver, J.M. The acute effects of multi-ingredient pre-workout ingestion on strength performance, lower body power, and anaerobic capacity. J. Int. Soc. Sports Nutr. 2016, 13, 11. [Google Scholar] [CrossRef]
- Ellerbroek, A.; Antonio, J. Effects of Pre-Workout Supplements on Strength, Endurance, and Mood. Internet J. Allied Health Sci. Pract. 2019, 17, 7. [Google Scholar] [CrossRef]
- Bergstrom, H.C.; Byrd, M.T.; Wallace, B.J.; Clasey, J.L. Examination of a multi-ingredient preworkout supplement on total volume of resistance exercise and subsequent strength and power performance. J. Strength Cond. Res. 2018, 32, 1479–1490. [Google Scholar] [CrossRef]
- Çetin, O.; Yaşar, M.; Demirtaş, B.; Beyleroğlu, M.; Eker, S.; Gürkan, A. Acute effects of pre-workout supplement on aerobic and anaerobic performance in basketball players. Phys. Educ. Stud. 2019, 23, 16–22. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Del Valle Soto, M.; Adams, D.P.; Gutiérrez-Abejón, E.; Seco-Calvo, J. Impact of Optimal Timing of Intake of Multi-Ingredient Performance Supplements on Sports Performance, Muscular Damage, and Hormonal Behavior across a Ten-Week Training Camp in Elite Cyclists: A Randomized Clinical Trial. Nutrients 2021, 13, 3746. [Google Scholar] [CrossRef]
- Köhne, J.L.; Ormsbee, M.J.; McKune, A.J. The effects of a multi-ingredient supplement on markers of muscle damage and inflammation following downhill running in females. J. Int. Soc. Sports Nutr. 2016, 13, 44. [Google Scholar] [CrossRef]
- Ormsbee, M.J.; Ward, E.G.; Bach, C.W.; Arciero, P.J.; McKune, A.J.; Panton, L.B. The impact of a pre-loaded multi-ingredient performance supplement on muscle soreness and performance following downhill running. J. Int. Soc. Sports Nutr. 2015, 12, 2. [Google Scholar] [CrossRef]
- Sansone, P.; Tschan, H.; Foster, C.; Tessitore, A. Monitoring Training Load and Perceived Recovery in Female Basketball: Implications for Training Design. J. Strength Cond. Res. 2020, 34, 2929–2936. [Google Scholar] [CrossRef]
- Calleja-González, J.; Terrados, N.; Mielgo-Ayuso, J.; Delextrat, A.; Jukic, I.; Vaquera, A.; Torres, L.; Schelling, X.; Stojanovic, M.; Ostojic, S.M. Evidence-based post-exercise recovery strategies in basketball. Phys. Sportsmed. 2016, 44, 74–78. [Google Scholar] [CrossRef]
- Lyakh, V.; Mikołajec, K.; Bujas, P.; Witkowski, Z.; Zając, T.; Litkowycz, R.; Banyś, D. Periodization in team sport games—A review of current knowledge and modern trends in competitive sports. J. Hum. Kinet. 2016, 54, 173–180. [Google Scholar] [CrossRef]
- Misra, S. Randomized double blind placebo control studies, the “Gold Standard” in intervention based studies. Indian J. Sex. Transm. Dis. AIDS 2012, 33, 131–134. [Google Scholar] [CrossRef]
- Kontou, E.I.; Berberidou, F.T.; Pilianidis, T.C.; Mantzouranis, N.I.; Methenitis, S.K. Acute effect of upper and lower body postactivation exercises on shot put performance. J. Strength Cond. Res. 2018, 32, 970–982. [Google Scholar] [CrossRef]
- Kostikiadis, I.N.; Methenitis, S.; Tsoukos, A.; Veligekas, P.; Terzis, G.; Bogdanis, G.C. The effect of short-term sport-specific strength and conditioning training on physical fitness of well-trained mixed martial arts athletes. J. Sport. Sci. Med. 2018, 17, 348–358. [Google Scholar]
- Simitzi, V.; Tsoukos, A.; Kostikiadis, I.N.; Parotsidis, C.A.; Paizis, C.; Nassis, G.P.; Methenitis, S.K. The acute effects of different high-intensity conditioning activities on sprint performance differ between sprinters of different strength and power characteristics. Kinesiology 2021, 53, 193–205. [Google Scholar] [CrossRef]
- Methenitis, S.K.; Zaras, N.D.; Spengos, K.M.; Stasinaki, A.-N.E.; Karampatsos, G.P.; Georgiadis, G.V.; Terzis, G.D. Role of muscle morphology in jumping, sprinting, and throwing performance in participants with different power training duration experience. J. Strength Cond. Res. 2016, 30, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Markovic, G.; Dizdar, D.; Jukic, I.; Cardinale, M. Reliability and factorial validity of squat and countermovement jump tests. J. Strength Cond. Res. 2004, 18, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdelkrim, N.; Chaouachi, A.; Chamari, K.; Chtara, M.; Castagna, C. Positional role and competitive-level differences in elite-level men’s basketball players. J. Strength Cond. Res. 2010, 24, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Mancha-Triguero, D.; García-Rubio, J.; Calleja-González, J.; Ibáñez, S.J. Physical fitness in basketball players: A systematic review. J. Sports Med. Phys. Fit. 2019, 59, 1513–1525. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.H.; Rebello Mendes, R.; De Almeida, M.B.; Zanetti, M.C.; Leite, G.D.S.; Júnior, A.J.F. Relationship between physical fitness and game-related statistics in elite professional basketball players: Regular season vs. playoffs. Mot. Rev. Educ. Física 2017, 23, e101626. [Google Scholar] [CrossRef]
- Pojskić, H.; Šeparović, V.; Muratović, M.; Uièanin, E. The relationship between physical fitness and shooting accuracy of professional basketball players. Mot. Rev. Educ. Física 2014, 20, 408–417. [Google Scholar] [CrossRef]
- Semenick, D. Tests and measurements: The T-test. Strength Cond. J. 1990, 12, 36–37. [Google Scholar] [CrossRef]
- Gál-Pottyondy, A.; Petró, B.; Czétényi, A.; Négyesi, J.; Nagatomi, R.; Kiss, R.M. Collection and advice on basketball field tests—A literature review. Appl. Sci. 2021, 11, 8855. [Google Scholar] [CrossRef]
- Zagatto, A.M.; Beck, W.R.; Gobatto, C.A. Validity of the running anaerobic sprint test for assessing anaerobic power and predicting short-distance performances. J. Strength Cond. Res. 2009, 23, 1820–1827. [Google Scholar] [CrossRef]
- Camargo, B.F.; de Araújo, G.G.; Gobatto, C.A.; Vieira, N.A.; Messias, L.H.D.; Manchado-Gobatto, F.d.B. Adaptation of invasive and non-invasive protocols to aerobic and anaerobic specific evaluation in female basketball players. Rev. Bras. Med. Esporte 2013, 19, 171–175. [Google Scholar] [CrossRef]
- Grgic, J.; Oppici, L.; Mikulic, P.; Bangsbo, J.; Krustrup, P.; Pedisic, Z. Test–Retest Reliability of the Yo-Yo Test: A Systematic Review. Sports Med. 2019, 49, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Castagna, C.; Impellizzeri, F.M.; Rampinini, E.; D’ottavio, S.; Manzi, V. The Yo–Yo intermittent recovery test in basketball players. J. Sci. Med. Sport 2008, 11, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Guest, N.S.; VanDusseldorp, T.A.; Nelson, M.T.; Grgic, J.; Schoenfeld, B.J.; Jenkins, N.D.M.; Arent, S.M.; Antonio, J.; Stout, J.R.; Trexler, E.T.; et al. International society of sports nutrition position stand: Caffeine and exercise performance. J. Int. Soc. Sports Nutr. 2021, 18, 1. [Google Scholar] [CrossRef]
- Trexler, E.T.; Smith-Ryan, A.E.; Stout, J.R.; Hoffman, J.R.; Wilborn, C.D.; Sale, C.; Kreider, R.B.; Jäger, R.; Earnest, C.P.; Bannock, L.; et al. International society of sports nutrition position stand: Beta-Alanine. J. Int. Soc. Sports Nutr. 2015, 12, 30. [Google Scholar] [CrossRef]
- Gonzalez, A.M.; Trexler, E.T. Effects of Citrulline Supplementation on Exercise Performance in Humans: A Review of the Current Literature. J. Strength Cond. Res. 2020, 34, 1480–1495. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jäger, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN exercise & sports nutrition review update: Research & recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC Consensus Statement: Dietary Supplements and the High-Performance Athlete. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 104–125. [Google Scholar] [CrossRef]
- Habibzadeh, F. Statistical data editing in scientific articles. J. Korean Med. Sci. 2017, 32, 1072–1076. [Google Scholar] [CrossRef]
- Buzdağli, Y.; Tekin, A.; Şiktar, E.; Eskici, G. Effect of Caffeine on Exercise Performance: Current Review. Turk. J. Sport Exerc. 2021, 23, 86–101. [Google Scholar]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.E.; Walter, A.A.; Graef, J.L.; Kendall, K.L.; Moon, J.R.; Lockwood, C.M.; Fukuda, D.H.; Beck, T.W.; Cramer, J.T.; Stout, J.R. Effects of β-alanine supplementation and high-intensity interval training on endurance performance and body composition in men; a double-blind trial. J. Int. Soc. Sports Nutr. 2009, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Ghiasvand, R.; Askari, G.; Malekzadeh, J.; Hajishafiee, M.; Daneshvar, P.; Akbari, F.; Bahreynian, M. Effects of six weeks of β-alanine administration on VO2 max, time to exhaustion and lactate concentrations in physical education students. Int. J. Prev. Med. 2012, 3, 559–563. [Google Scholar] [PubMed]
- Zoeller, R.F.; Stout, J.R.; O’kroy, J.A.; Torok, D.J.; Mielke, M. Effects of 28 days of beta-alanine and creatine monohydrate supplementation on aerobic power, ventilatory and lactate thresholds, and time to exhaustion. Amino Acids 2006, 33, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Nyawose, S.; Naidoo, R.; Naumovski, N.; McKune, A.J. The Effects of Consuming Amino Acids L-Arginine, L-Citrulline (and Their Combination) as a Beverage or Powder, on Athletic and Physical Performance: A Systematic Review. Beverages 2022, 8, 48. [Google Scholar] [CrossRef]
- Figueroa, A.; Wong, A.; Jaime, S.J.; Gonzales, J.U. Influence of L-citrulline and watermelon supplementation on vascular function and exercise performance. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Rhim, H.C.; Kim, S.J.; Park, J.; Jang, K.-M. Effect of citrulline on post-exercise rating of perceived exertion, muscle soreness, and blood lactate levels: A systematic review and meta-analysis. J. Sport Health Sci. 2020, 9, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Bendahan, D.; Mattei, J.P.; Ghattas, B.; Confort-Gouny, S.; Le Guern, M.E.; Cozzone, P.J. Citrulline/malate promotes aerobic energy production in human exercising muscle. Br. J. Sports Med. 2002, 36, 282–289. [Google Scholar] [CrossRef]
- AbuMoh’d, M.F.; Matalqah, L.; Al-Abdulla, Z. Effects of Oral Branched-Chain Amino Acids (BCAAs) Intake on Muscular and Central Fatigue during an Incremental Exercise. J. Hum. Kinet. 2020, 72, 69–78. [Google Scholar] [CrossRef]
- Gabbett, T.J. Changes in physiological and anthropometric characteristics of rugby league players during a competitive season. J. Strength Cond. Res. 2005, 19, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Mor, A.; Acar, K.; Yılmaz, A.K.; Arslanoglu, E. The effects of BCAA and creatine supplementation on anaerobic capacity and ball kicking speed in male football players. J. Men’s Health 2021, 18, 1–9. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Ratamess, N.A.; Gonzalez, A.; Beller, N.A.; Hoffman, M.W.; Olson, M.; Purpura, M.; Jäger, R. The effects of acute and prolonged CRAM supplementation on reaction time and subjective measures of focus and alertness in healthy college students. J. Int. Soc. Sports Nutr. 2010, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Kern, B.D.; Robinson, T.L. Effects of β-alanine supplementation on performance and body composition in collegiate wrestlers and football players. J. Strength Cond. Res. 2011, 25, 1804–1815. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.E.; Fukuda, D.H.; Kendall, K.L.; Stout, J.R. The effects of a pre-workout supplement containing caffeine, creatine, and amino acids during three weeks of high-intensity exercise on aerobic and anaerobic performance. J. Int. Soc. Sports Nutr. 2010, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Ormsbee, M.J.; Mandler, W.K.; Thomas, D.D.; Ward, E.G.; Kinsey, A.W.; Simonavice, E.; Panton, L.B.; Kim, J.-S. The effects of six weeks of supplementation with multi-ingredient performance supplements and resistance training on anabolic hormones, body composition, strength, and power in resistance-trained men. J. Int. Soc. Sports Nutr. 2012, 9, 49. [Google Scholar] [CrossRef]
- Lowery, R.P.; Joy, J.M.; Dudeck, J.E.; Oliveira De Souza, E.; Mccleary, S.A.; Wells, S.; Wildman, R.; Wilson, J.M. Effects of 8 weeks of Xpand® 2X pre workout supplementation on skeletal muscle hypertrophy, lean body mass, and strength in resistance trained males. J. Int. Soc. Sports Nutr. 2013, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.; Candow, D.G.; Forbes, S.C.; Gualano, B.; Jagim, A.R.; Kreider, R.B.; Rawson, E.S.; Smith-Ryan, A.E.; VanDusseldorp, T.A.; Willoughby, D.S.; et al. Common questions and misconceptions about creatine supplementation: What does the scientific evidence really show? J. Int. Soc. Sports Nutr. 2021, 18, 13. [Google Scholar] [CrossRef]
- de Oliveira, E.P.; Artioli, G.G.; Burini, R.C. Safety of beta-alanine supplementation in humans: A narrative review. Sport Sci. Health 2023, 19, 757–763. [Google Scholar] [CrossRef]
- de Souza, J.G.; Del Coso, J.; Fonseca, F.d.S.; Silva, B.V.C.; de Souza, D.B.; da Silva Gianoni, R.L.; Filip-Stachnik, A.; Serrão, J.C.; Claudino, J.G. Risk or benefit? Side effects of caffeine supplementation in sport: A systematic review. Eur. J. Nutr. 2022, 61, 3823–3834. [Google Scholar] [CrossRef]
- Ferrando, A.A.; Wolfe, R.R.; Hirsch, K.R.; Church, D.D.; Kviatkovsky, S.A.; Roberts, M.D.; Stout, J.R.; Gonzalez, D.E.; Sowinski, R.J.; Kreider, R.B.; et al. International society of sports nutrition position stand: Essential amino acid supplementation on skeletal muscle and Performance. J. Int. Soc. Sports Nutr. 2023, 20, 2263409. [Google Scholar] [CrossRef] [PubMed]
- Bassit, R.A.; Pinheiro, C.H.d.J.; Vitzel, K.F.; Sproesser, A.J.; Silveira, L.R.; Curi, R. Effect of short-term creatine supplementation on markers of skeletal muscle damage after strenuous contractile activity. Eur. J. Appl. Physiol. 2010, 108, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Veggi, K.F.T.; Machado, M.; Koch, A.J.; Santana, S.C.; Oliveira, S.S.; Stec, M.J. Oral creatine supplementation augments the repeated bout effect. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 378–387. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Kim, S.; Yoon, D.; Kim, J.; Sung, D.J. Role of creatine supplementation in exercise-induced muscle damage: A mini review. J. Exerc. Rehabil. 2015, 11, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Gholami, F.; Ali, A.; Hasani, A.; Zarei, A. Effect of Beta-Alanine Supplementation on Exercise-Induced Cell Damage and Lactate Accumulation in Female Basketball Players: A Randomized, Double-Blind Study. J. Hum. Kinet. 2022, 83, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Mirenayat, M.S.; Faramarzi, M.; Ghazvini, M.R.; Karimian, J.; Hadi, A.; Heidari, Z.; Rouhani, M.H.; Naeini, A.A.; Doewes, R.I.; Shalaby, M.N. The effects of short term citrulline malate supplementation on oxidative stress and muscle damage in trained soccer players. Hum. Nutr. Metab. 2024, 36, 200242. [Google Scholar] [CrossRef]
- Caldas, L.C.; Salgueiro, R.B.; Clarke, N.D.; Tallis, J.; Barauna, V.G.; Guimaraes-Ferreira, L. Effect of Caffeine Ingestion on Indirect Markers of Exercise-Induced Muscle Damage: A Systematic Review of Human Trials. Nutrients 2022, 14, 1769. [Google Scholar] [CrossRef] [PubMed]
- Kendall, K.L.; Moon, J.R.; Fairman, C.M.; Spradley, B.D.; Tai, C.Y.; Falcone, P.H.; Carson, L.R.; Mosman, M.M.; Joy, J.M.; Kim, M.P.; et al. Ingesting a preworkout supplement containing caffeine, creatine, beta-alanine, amino acids, and B vitamins for 28 days is both safe and efficacious in recreationally active men. Nutr. Res. 2014, 34, 442–449. [Google Scholar] [CrossRef]
- Joy, J.M.; Lowery, R.P.; Falcone, P.H.; Vogel, R.M.; Mosman, M.M.; Tai, C.-Y.; Carson, L.R.; Kimber, D.; Choate, D.; Kim, M.P.; et al. A multi-ingredient, pre-workout supplement is apparently safe in healthy males and females. Food Nutr. Res. 2015, 59, 27470. [Google Scholar] [CrossRef]


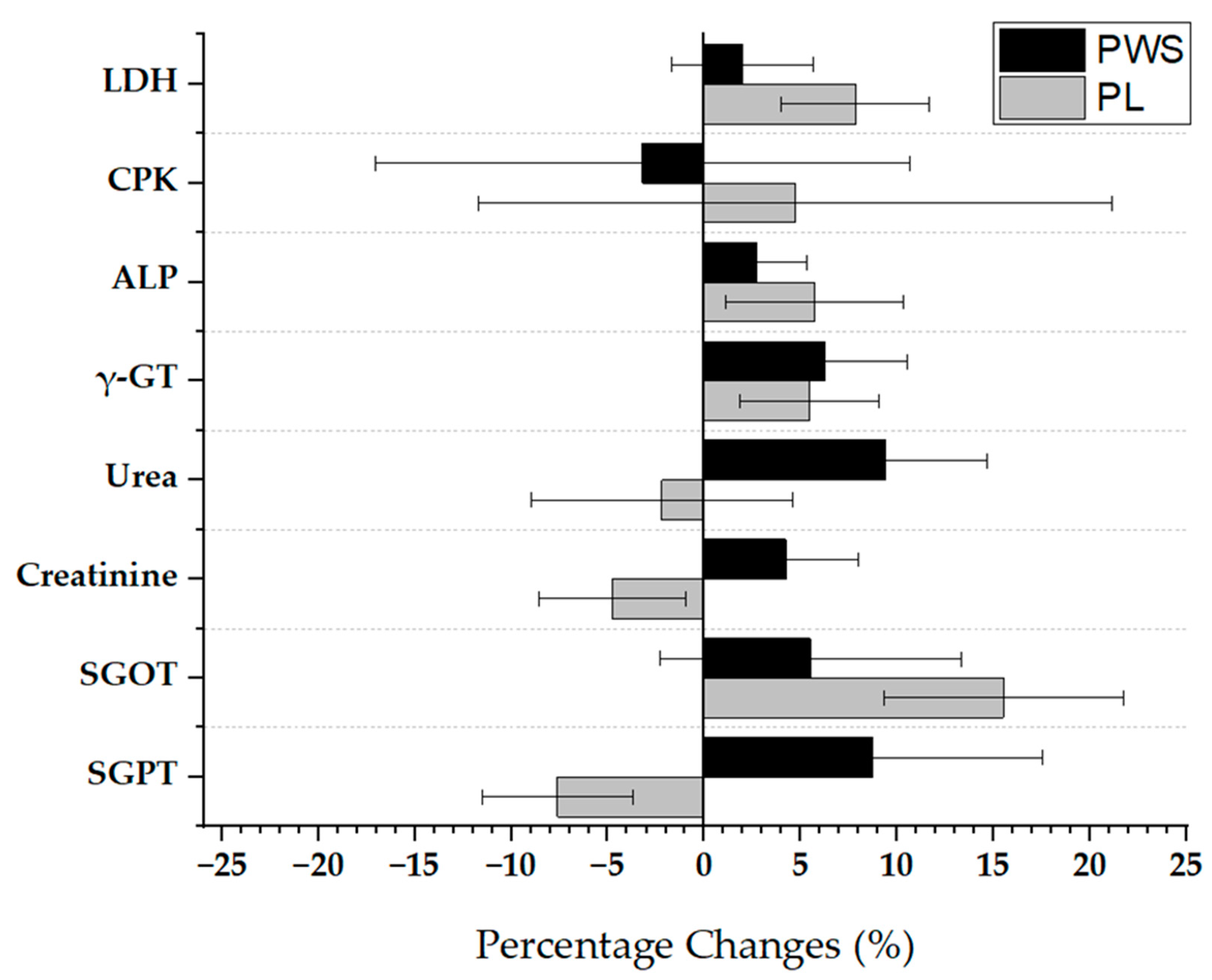

| Pre-Workout Supplement (PWS) (n = 10) | Placebo (PL) (n = 8) | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Height (cm) | 184.2 ± 9.4 | 184.2 ± 9.4 | 187.5 ± 5.9 | 187.5 ± 5.9 |
| Body Weight (kg) | 78.6 (20.8) | 78.7 (20.3) | 84.4 (13.7) | 84.2 (13.4) |
| Body Fat (%) | 15.5 (6.7) | 14.7 (6.1) | 15.9 (4.5) | 15.0 (4.8) |
| Free-Fat Mass (kg) | 70.2 ± 9.6 | 70.8 ± 9.5 | 72.3 ± 4.8 | 72.8 ± 5.1 |
| Fat-Mass (kg) | 12.7 (7.3) | 12.4 (6.4) | 13.1 (5.6) | 13.1 (5.7) |
| Resting Systolic Blood Pressure (mmHg) | 132 (9) | 132 (4) | 136 (10) | 130 (17) |
| Resting Diastolic Blood Pressure (mmHg) | 75 ± 11 | 75 ± 9 | 79 ± 6 | 77 ± 8 |
| Resting Heart Rate (beats/min) | 63.8 ± 8.1 | 65.2 ± 6.4 | 62.9 ± 5.8 | 64.5 ± 5.4 |
| PWS (n = 10) | PL (n = 8) | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Counter-Movement Jump Height (cm) | 41.0 (11.0) | 41.5 (9.0) | 37.5 (6.0) | 39.0 (11.0) |
| 20 m Sprint (s) | 3.05 (0.21) | 3.04 (0.19) | 3.25 (0.49) | 3.23 (0.61) |
| Agility T-Test (s) | 10.48 (0.75) | 10.52 (0.77) | 10.62 (0.82) | 10.54 (1.65) |
| RAST Peak Power (watt) | 358.4 ± 48.0 | 331.4 ± 47.9 # | 336.8 ± 49.6 | 278.3 ± 54.5 # |
| RAST Average Power (watt) | 308.4 ± 41.6 | 281.8 ± 34.7 # | 282.3 ± 27.0 | 247.4 ± 52.2 # |
| RAST Minimum Power (watt) | 255.9 ± 46.7 | 241.5 ± 42.2 # | 254.5 ± 37.4 | 212.3 ± 43.2 # |
| RAST Fatigue Index, FI (%) | 28.3 ± 10.1 | 24.2 ± 9.4 | 24.0 ± 8.1 | 25.7 ± 11.5 |
| O2max (mL·kg−1·min−1) | 43.9 ± 3.3 | 47.3 ± 4.1 * | 44.3 ± 3.4 | 43.2 ± 2.1 |
| PWS (n = 10) | PL (n = 8) | Reference Interval | |||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||
| LDH (U/L) | 186 ± 27 | 188 ± 29 | 182 ± 15 | 196 ± 22 | 125–220 |
| CPK (U/L) | 236 ± 86 | 217 ± 89 | 215 ± 63 | 252 ± 32 | 30–180 |
| ALP (U/L) | 73 ± 15 | 75 ± 12 | 70 ± 14 | 72 ± 9 | 38–126 |
| γ- glutamyl transferase (γ-GT) (U/L) | 16 ± 3 | 17 ± 4 | 16 ± 4 | 17 ± 4 | 11–50 |
| Urea (mg/dL) | 33 ± 7 | 35 ± 6 | 36 ± 10 | 35 ± 8 | 10–50 |
| Creatinine (mg/dL) | 0.9 (0.1) | 1.0 (0.2) | 1.1 (0.3) | 1.0 (0.2) | 0.5–1.5 |
| SGOT (U/L) | 27 ± 8 | 28 ± 9 | 25 ± 3 | 27 ± 4 | 0–46 |
| SGPT (U/L) | 24 ± 7 | 25 ± 6 | 23 ± 7 | 24 ± 8 | 0–46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Douligeris, A.; Methenitis, S.; Stavropoulos-Kalinoglou, A.; Panayiotou, G.; Vogazianos, P.; Lazou, A.; Feidantsis, K.; Giaginis, C.; Papanikolaou, K.; Arnaoutis, G.; et al. Effects of Four Weeks of In-Season Pre-Workout Supplementation on Performance, Body Composition, Muscle Damage, and Health-Related Markers in Basketball Players: A Randomized Controlled Study. J. Funct. Morphol. Kinesiol. 2024, 9, 85. https://doi.org/10.3390/jfmk9020085
Douligeris A, Methenitis S, Stavropoulos-Kalinoglou A, Panayiotou G, Vogazianos P, Lazou A, Feidantsis K, Giaginis C, Papanikolaou K, Arnaoutis G, et al. Effects of Four Weeks of In-Season Pre-Workout Supplementation on Performance, Body Composition, Muscle Damage, and Health-Related Markers in Basketball Players: A Randomized Controlled Study. Journal of Functional Morphology and Kinesiology. 2024; 9(2):85. https://doi.org/10.3390/jfmk9020085
Chicago/Turabian StyleDouligeris, Athanasios, Spyridon Methenitis, Antonios Stavropoulos-Kalinoglou, George Panayiotou, Paris Vogazianos, Antonia Lazou, Konstantinos Feidantsis, Constantinos Giaginis, Konstantinos Papanikolaou, Giannis Arnaoutis, and et al. 2024. "Effects of Four Weeks of In-Season Pre-Workout Supplementation on Performance, Body Composition, Muscle Damage, and Health-Related Markers in Basketball Players: A Randomized Controlled Study" Journal of Functional Morphology and Kinesiology 9, no. 2: 85. https://doi.org/10.3390/jfmk9020085











