Biomarkers of Neurodegeneration and Alzheimer’s Disease Neuropathology in Adolescents and Young Adults with Youth-Onset Type 1 or Type 2 Diabetes: A Proof-of-Concept Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants with Youth-Onset Diabetes (Y-DM)
2.2. Control Participants
2.3. National Institutes of Health Toolbox Cognition Battery (NIHT-CB)
2.4. Plasma-Based Biomarkers of Neurodegeneration and AD Neuropathology
2.5. Positron Emission Tomography (PET) of Amyloid and Tau Density in the Brain
2.6. Statistical Analyses
3. Results
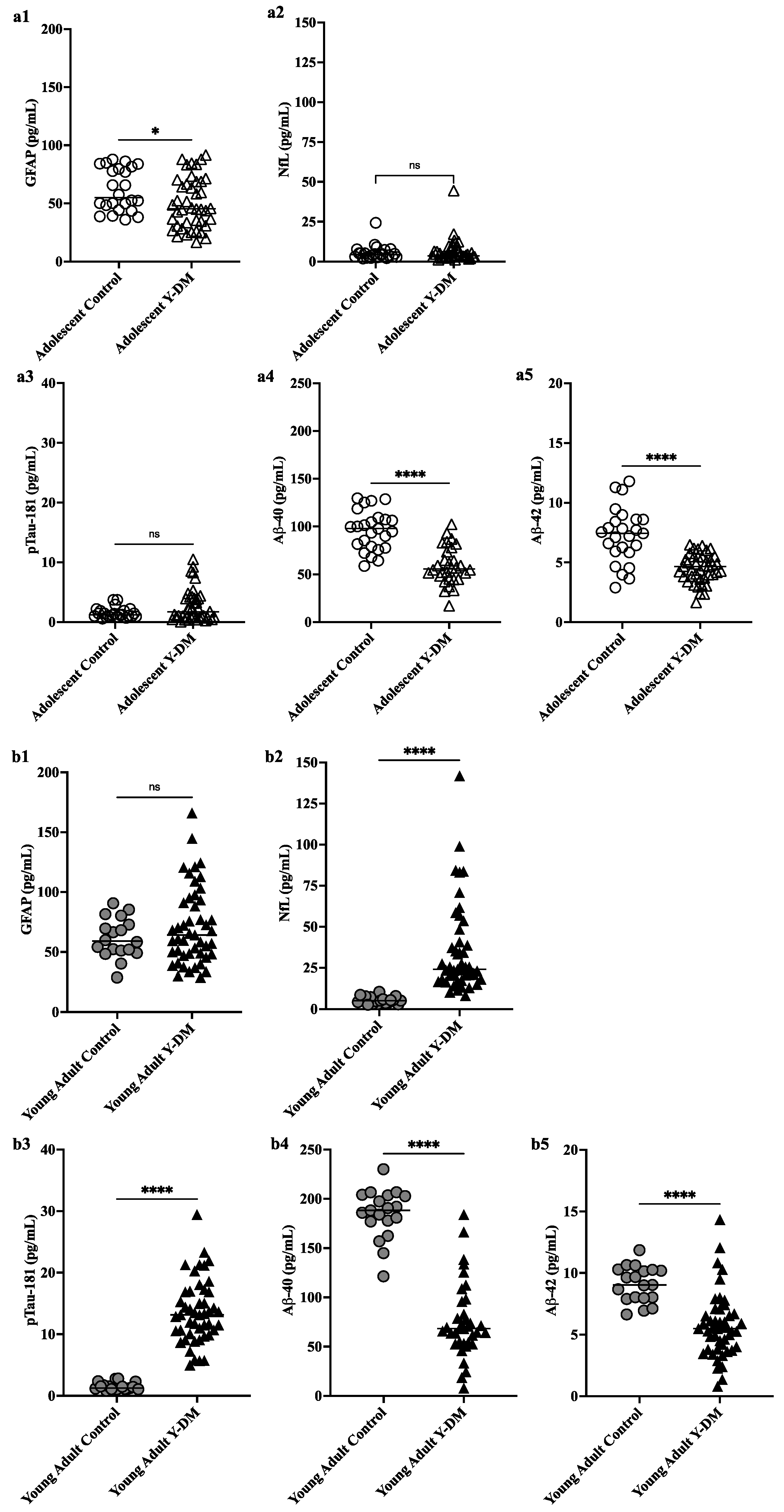
3.1. Plasma Levels of GFAP, NfL, pTau181, Aβ40, and Aβ42 among Adolescents and Young Adults with Y-DM Compared to Adolescents and Young Adults without Diabetes
3.2. Correlations between Change in Plasma Levels of GFAP, NfL, pTau181, Aβ40, and Aβ42 from Adolescence to Young Adulthood and Cognitive Function in Participants with Y-DM
3.3. PET Amyloid and Tau Accumulation in Young Adults with Youth-Onset Diabetes versus Young Adults without Diabetes
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bello-Chavolla, O.Y.; Antonio-Villa, N.E.; Vargas-Vázquez, A.; Ávila-Funes, J.A.; Aguilar-Salinas, C.A. Pathophysiological Mechanisms Linking Type 2 Diabetes and Dementia: Review of Evidence from Clinical, Translational and Epidemiological Research. Curr. Diabetes Rev. 2019, 15, 456–470. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Huang, C.; Deng, H.; Wang, H. Diabetes as a risk factor for dementia and mild cognitive impairment: A meta-analysis of longitudinal studies. Intern. Med. J. 2012, 42, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Cukierman, T.; Gerstein, H.C.; Williamson, J.D. Cognitive decline and dementia in diabetes—Systematic overview of prospective observational studies. Diabetologia 2005, 48, 2460–2469. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Jiang, X.; Liu, S.; Li, M. Changes in Cerebrospinal Fluid Tau and β-Amyloid Levels in Diabetic and Prediabetic Patients: A Meta-Analysis. Front. Aging Neurosci. 2018, 10, 271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, C.; Hua, S.; Liao, H.; Wang, M.; Xiong, Y.; Cao, F. An updated meta-analysis of cohort studies: Diabetes and risk of Alzheimer’s disease. Diabetes Res. Clin. Pract. 2017, 124, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.J.; Mainous, A.G., 3rd; Diaz, V.A.; Geesey, M.E. Changes in age at diagnosis of type 2 diabetes mellitus in the United States, 1988 to 2000. Ann. Fam. Med. 2005, 3, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Barbiellini Amidei, C.; Fayosse, A.; Dumurgier, J.; Machado-Fragua, M.D.; Tabak, A.G.; van Sloten, T.; Kivimäki, M.; Dugravot, A.; Sabia, S.; Singh-Manoux, A. Association between Age at Diabetes Onset and Subsequent Risk of Dementia. JAMA 2021, 325, 1640–1649. [Google Scholar] [CrossRef] [PubMed]
- Li, F.R.; Yang, H.L.; Zhou, R.; Zheng, J.Z.; Chen, G.C.; Wu, X.X.; Zou, M.C.; Wang, J.Y.; Fu, Q.; Wu, X.B. Influence of Diabetes Duration and Glycemic Control on Dementia: A Cohort Study. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 2062–2070. [Google Scholar] [CrossRef]
- Parikh, N.M.; Morgan, R.O.; Kunik, M.E.; Chen, H.; Aparasu, R.R.; Yadav, R.K.; Schulz, P.E.; Johnson, M.L. Risk factors for dementia in patients over 65 with diabetes. Int. J. Geriatr. Psychiatry 2011, 26, 749–757. [Google Scholar] [CrossRef]
- Thomas, K.R.; Bangen, K.J.; Weigand, A.J.; Edmonds, E.C.; Sundermann, E.; Wong, C.G.; Eppig, J.; Werhane, M.L.; Delano-Wood, L.; Bondi, M.W. Type 2 Diabetes Interacts With Alzheimer Disease Risk Factors to Predict Functional Decline. Alzheimer Dis. Assoc. Disord. 2020, 34, 10–17. [Google Scholar] [CrossRef]
- Zilkens, R.R.; Davis, W.A.; Spilsbury, K.; Semmens, J.B.; Bruce, D.G. Earlier Age of Dementia Onset and Shorter Survival Times in Dementia Patients With Diabetes. Am. J. Epidemiol. 2013, 177, 1246–1254. [Google Scholar] [CrossRef]
- Huang, C.C.; Chung, C.M.; Leu, H.B.; Lin, L.Y.; Chiu, C.C.; Hsu, C.Y.; Chiang, C.H.; Huang, P.H.; Chen, T.J.; Lin, S.J.; et al. Diabetes mellitus and the risk of Alzheimer’s disease: A nationwide population-based study. PLoS ONE 2014, 9, e87095. [Google Scholar] [CrossRef]
- Kimura, N. Diabetes Mellitus Induces Alzheimer’s Disease Pathology: Histopathological Evidence from Animal Models. Int. J. Mol. Sci. 2016, 17, 503. [Google Scholar] [CrossRef] [PubMed]
- Leibson, C.L.; Rocca, W.A.; Hanson, V.A.; Cha, R.; Kokmen, E.; O’Brien, P.C.; Palumbo, P.J. Risk of dementia among persons with diabetes mellitus: A population-based cohort study. Am. J. Epidemiol. 1997, 145, 301–308. [Google Scholar] [CrossRef]
- Ma, F.; Wu, T.; Miao, R.; Xiao, Y.Y.; Zhang, W.; Huang, G. Conversion of mild cognitive impairment to dementia among subjects with diabetes: A population-based study of incidence and risk factors with five years of follow-up. J. Alzheimers Dis. 2015, 43, 1441–1449. [Google Scholar] [CrossRef]
- Peila, R.; Rodriguez, B.L.; Launer, L.J. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes 2002, 51, 1256–1262. [Google Scholar] [CrossRef]
- Tolppanen, A.M.; Lavikainen, P.; Solomon, A.; Kivipelto, M.; Uusitupa, M.; Soininen, H.; Hartikainen, S. History of medically treated diabetes and risk of Alzheimer disease in a nationwide case-control study. Diabetes Care 2013, 36, 2015–2019. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.L.; Lu, C.L.; Chang, Y.H.; Li, C.Y. Population-Based Cohort Study on Dementia Risk in Patients with Type 1 Diabetes Mellitus. Neuroepidemiology 2018, 50, 57–62. [Google Scholar] [CrossRef]
- Kuo, S.C.; Lai, S.W.; Hung, H.C.; Muo, C.H.; Hung, S.C.; Liu, L.L.; Chang, C.W.; Hwu, Y.J.; Chen, S.L.; Sung, F.C. Association between comorbidities and dementia in diabetes mellitus patients: Population-based retrospective cohort study. J. Diabetes Complicat. 2015, 29, 1071–1076. [Google Scholar] [CrossRef]
- Lacy, M.E.; Gilsanz, P.; Karter, A.J.; Quesenberry, C.P.; Pletcher, M.J.; Whitmer, R.A. Long-term Glycemic Control and Dementia Risk in Type 1 Diabetes. Diabetes Care 2018, 41, 2339–2345. [Google Scholar] [CrossRef]
- Ouwens, D.M.; van Duinkerken, E.; Schoonenboom, S.N.; Herzfeld de Wiza, D.; Klein, M.; van Golen, L.; Pouwels, P.J.; Barkhof, F.; Moll, A.C.; Snoek, F.J.; et al. Cerebrospinal fluid levels of Alzheimer’s disease biomarkers in middle-aged patients with type 1 diabetes. Diabetologia 2014, 57, 2208–2214. [Google Scholar] [CrossRef]
- Smolina, K.; Wotton, C.J.; Goldacre, M.J. Risk of dementia in patients hospitalised with type 1 and type 2 diabetes in England, 1998–2011: A retrospective national record linkage cohort study. Diabetologia 2015, 58, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, A.M.; Sharrett, A.R.; Albert, M.S.; Coresh, J.; Windham, B.G.; Power, M.C.; Knopman, D.S.; Walker, K.; Burgard, S.; Mosley, T.H.; et al. The Association of Late-Life Diabetes Status and Hyperglycemia with Incident Mild Cognitive Impairment and Dementia: The ARIC Study. Diabetes Care 2019, 42, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, N.; Gonçalves, R.A.; De Felice, F.G.; Fraser, P.E. Impaired peripheral glucose homeostasis and Alzheimer’s disease. Neuropharmacology 2018, 136 Pt B, 172–181. [Google Scholar] [CrossRef]
- van Leijsen EM, C.; Kuiperij, H.B.; Kersten, I.; Bergkamp, M.I.; van Uden IW, M.; Vanderstichele, H.; Stoops, E.; Claassen, J.; van Dijk, E.J.; de Leeuw, F.E.; et al. Plasma Aβ (Amyloid-β) Levels and Severity and Progression of Small Vessel Disease. Stroke 2018, 49, 884–890. [Google Scholar] [CrossRef]
- Karikari, T.K.; Pascoal, T.A.; Ashton, N.J.; Janelidze, S.; Benedet, A.L.; Rodriguez, J.L.; Chamoun, M.; Savard, M.; Kang, M.S.; Therriault, J.; et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: A diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020, 19, 422–433. [Google Scholar] [CrossRef]
- Mattsson, N.; Cullen, N.C.; Andreasson, U.; Zetterberg, H.; Blennow, K. Association between Longitudinal Plasma Neurofilament Light and Neurodegeneration in Patients with Alzheimer Disease. JAMA Neurol. 2019, 76, 791–799. [Google Scholar] [CrossRef]
- Poljak, A.; Crawford, J.D.; Smythe, G.A.; Brodaty, H.; Slavin, M.J.; Kochan, N.A.; Trollor, J.N.; Wen, W.; Mather, K.A.; Assareh, A.A.; et al. The Relationship between Plasma Aβ Levels, Cognitive Function and Brain Volumetrics: Sydney Memory and Ageing Study. Curr. Alzheimer Res. 2016, 13, 243–255. [Google Scholar] [CrossRef]
- Reiman, E.M.; Quiroz, Y.T.; Fleisher, A.S.; Chen, K.; Velez-Pardo, C.; Jimenez-Del-Rio, M.; Fagan, A.M.; Shah, A.R.; Alvarez, S.; Arbelaez, A.; et al. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer’s disease in the presenilin 1 E280A kindred: A case-control study. Lancet Neurol. 2012, 11, 1048–1056. [Google Scholar] [CrossRef]
- SEARCH Study Group. SEARCH for Diabetes in Youth: A multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin. Trials 2004, 25, 458–471. [Google Scholar] [CrossRef]
- Dabelea, D.; Bell, R.A.; D’Agostino, R.B.; Jr Imperatore, G.; Johansen, J.M.; Linder, B.; Liu, L.L.; Loots, B.; Marcovina, S.; Mayer-Davis, E.J.; et al. Incidence of diabetes in youth in the United States. JAMA 2007, 297, 2716–2724. [Google Scholar] [PubMed]
- Liese, A.D.; D’Agostino, R.B.; Jr Hamman, R.F.; Kilgo, P.D.; Lawrence, J.M.; Liu, L.L.; Loots, B.; Linder, B.; Marcovina, S.; Rodriguez, B.; et al. The burden of diabetes mellitus among US youth: Prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics 2006, 118, 1510–1518. [Google Scholar]
- O’Bryant, S.E.; Petersen, M.; Hall, J.; Johnson, L.A.; for the HABS-HD Study Team. Medical comorbidities and ethnicity impact plasma Alzheimer’s disease biomarkers: Important considerations for clinical trials and practice. Alzheimer Dement. 2023, 19, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, F.; Stokholm, L.; Pouwer, F.; Rubin, K.; Peto, T.; Frydkjaer-Olsen, U.; Thykjær, A.S.; Simó, R.; Grauslund, J. Diabetic retinopathy independently predicts five-year risk of Alzheimer’s disease. Investig. Ophthalmol. Vis. Sci. 2021, 62, 1071. [Google Scholar]
- Rodill, L.G.; Exalto, L.G.; Gilsanz, P.; Biessels, G.J.; Quesenberry, C.P., Jr.; Whitmer, R.A. Diabetic Retinopathy and Dementia in Type 1 Diabetes. Alzheimer Dis. Assoc. Disord. 2018, 32, 125–130. [Google Scholar] [CrossRef]
- Shapiro, A.L.B.; Bellatorre, A.; Dabelea, D.; Stafford, J.M.; D’Agostino, R.; Shah, A.S.; Urbina, E.M.; Barrett, C.E.; Pihoker, C.; Marcovina, S.; et al. Diabetes Complications and Cognitive Function in Young Adults with Youth-Onset Type 1 or Type 2 Diabetes: The SEARCH for Diabetes in Youth Study. Pediatr. Diabetes 2023, 2023, 4860831. [Google Scholar] [CrossRef]
- Shapiro AL, B.; Wilkening, G.; Aalborg, J.; Ringham, B.M.; Glueck, D.H.; Tregellas, J.R.; Dabelea, D. Childhood Metabolic Biomarkers Are Associated with Performance on Cognitive Tasks in Young Children. J. Pediatr. 2019, 211, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, S.; Dikmen, S.S.; Heaton, R.K.; Tulsky, D.S.; Zelazo, P.D.; Bauer, P.J.; Carlozzi, N.E.; Slotkin, J.; Blitz, D.; Wallner-Allen, K.; et al. Cognition assessment using the NIH Toolbox. Neurology 2013, 80 (Suppl. 3), S54–S64. [Google Scholar] [CrossRef] [PubMed]
- Sattlecker, M.; Kiddle, S.J.; Newhouse, S.; Proitsi, P.; Nelson, S.; Williams, S.; Johnston, C.; Killick, R.; Simmons, A.; Westman, E.; et al. Alzheimer’s disease biomarker discovery using SOMAscan multiplexed protein technology. Alzheimers Dement. 2014, 10, 724–734. [Google Scholar] [CrossRef]
- Lockhart, S.N.; Schöll, M.; Baker, S.L.; Ayakta, N.; Swinnerton, K.N.; Bell, R.K.; Mellinger, T.J.; Shah, V.D.; O’Neil, J.P.; Janabi, M.; et al. Amyloid and tau PET demonstrate region-specific associations in normal older people. Neuroimage 2017, 150, 191–199. [Google Scholar] [CrossRef]
- Schöll, M.; Lockhart, S.N.; Schonhaut, D.R.; O’Neil, J.P.; Janabi, M.; Ossenkoppele, R.; Baker, S.L.; Vogel, J.W.; Faria, J.; Schwimmer, H.D.; et al. PET Imaging of Tau Deposition in the Aging Human Brain. Neuron 2016, 89, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.L.; Maass, A.; Jagust, W.J. Considerations and code for partial volume correcting [(18)F]-AV-1451 tau PET data. Data Brief. 2017, 15, 648–657. [Google Scholar] [CrossRef]
- Maass, A.; Lockhart, S.N.; Harrison, T.M.; Bell, R.K.; Mellinger, T.; Swinnerton, K.; Baker, S.L.; Rabinovici, G.D.; Jagust, W.J. Entorhinal Tau Pathology, Episodic Memory Decline, and Neurodegeneration in Aging. J. Neurosci. 2018, 38, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.E.; Davis, W.A.; Taddei, K.; Martins, R.N.; Masters, C.L.; Davis, T.M.; Bruce, D.G. Plasma Amyloid-β Peptides in Type 2 Diabetes: A Matched Case-Control Study. J. Alzheimers Dis. 2017, 56, 1127–1133. [Google Scholar] [CrossRef]
- Hayden, K.M.; Mielke, M.M.; Evans, J.K.; Neiberg, R.; Molina-Henry, D.; Culkin, M.; Marcovina, S.; Johnson, K.C.; Carmichael, O.T.; Rapp, S.R.; et al. Association between Modifiable Risk Factors and Levels of Blood-Based Biomarkers of Alzheimer’s and Related Dementias in the Look AHEAD Cohort. JAR Life 2024, 13, 1–21. [Google Scholar]
- Li, Y.; Schindler, S.E.; Bollinger, J.G.; Ovod, V.; Mawuenyega, K.G.; Weiner, M.W.; Shaw, L.M.; Masters, C.L.; Fowler, C.J.; Trojanowski, J.Q.; et al. Validation of Plasma Amyloid-β 42/40 for Detecting Alzheimer Disease Amyloid Plaques. Neurology 2022, 98, e688–e699. [Google Scholar] [CrossRef]
- Schindler, S.E.; Bollinger, J.G.; Ovod, V.; Mawuenyega, K.G.; Li, Y.; Gordon, B.A.; Holtzman, D.M.; Morris, J.C.; Benzinger TL, S.; Xiong, C.; et al. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 2019, 93, e1647–e1659. [Google Scholar] [CrossRef]
- Ayala-Guerrero, L.; García-delaTorre, P.; Sánchez-García, S.; Guzmán-Ramos, K. Serum Levels of Glial Fibrillary Acidic Protein Association with Cognitive Impairment and Type 2 Diabetes. Arch. Med. Res. 2022, 53, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Ciardullo, S.; Muraca, E.; Bianconi, E.; Cannistraci, R.; Perra, S.; Zerbini, F.; Perseghin, G. Diabetes Mellitus is Associated with Higher Serum Neurofilament Light Chain Levels in the General US Population. J. Clin. Endocrinol. Metab. 2023, 108, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Maalmi, H.; Strom, A.; Petrera, A.; Hauck, S.M.; Strassburger, K.; Kuss, O.; Zaharia, O.P.; Bönhof, G.J.; Rathmann, W.; Trenkamp, S.; et al. Serum neurofilament light chain: A novel biomarker for early diabetic sensorimotor polyneuropathy. Diabetologia 2023, 66, 579–589. [Google Scholar] [CrossRef]
- Mariotto, S.; Farinazzo, A.; Magliozzi, R.; Alberti, D.; Monaco, S.; Ferrari, S. Serum and cerebrospinal neurofilament light chain levels in patients with acquired peripheral neuropathies. J. Peripher. Nerv. Syst. 2018, 23, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Brands, A.M.A.; Biessels, G.J.; de Haan, E.H.F.; Kappelle, L.J.; Kessels, R.P.C. The Effects of Type 1 Diabetes on Cognitive Performance: A meta-analysis. Diabetes Care 2005, 28, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Gaudieri, P.A.; Chen, R.; Greer, T.F.; Holmes, C.S. Cognitive Function in Children with Type 1 Diabetes: A meta-analysis. Diabetes Care 2008, 31, 1892–1897. [Google Scholar] [CrossRef]
- Bjørgaas, M.; Gimse, R.; Vik, T.; Sand, T. Cognitive function in type 1 diabetic children with and without episodes of severe hypoglycaemia. Acta Paediatr. 1997, 86, 148–153. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ryder, A.G.; Li, S.; Liu, W.; Zhu, X. Glycemic extremes are related to cognitive dysfunction in children with type 1 diabetes: A meta-analysis. J. Diabetes Investig. 2018, 9, 1342–1353. [Google Scholar] [CrossRef]
- Musen, G.; Tinsley, L.J.; Marcinkowski, K.A.; Pober, D.; Sun, J.K.; Khatri, M.; Huynh, R.; Lu, A.; King, G.L.; Keenan, H.A. Cognitive Function Deficits Associated with Long-Duration Type 1 Diabetes and Vascular Complications. Diabetes Care 2018, 41, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.J.; Hamilton, C.A.; Heslegrave, A.; Barker, S.; Durcan, R.; Lawley, S.; Barnett, N.; Lett, D.; Firbank, M.; Roberts, G.; et al. A Longitudinal Study of Plasma pTau181 in Mild Cognitive Impairment with Lewy Bodies and Alzheimer’s Disease. Mov. Disord. 2022, 37, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Chen, J.; Du, Z.L.; Weng, H.; Zhang, Y.; Li, R.; Jia, Z.; Sun, M.; Jiang, J.; Wang, F.Z.; et al. Plasma p-tau181 Level Predicts Neurodegeneration and Progression to Alzheimer’s Dementia: A Longitudinal Study. Front. Neurol. 2021, 12, 695696. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.D.; Huang, Y.Y.; Shen, X.N.; Guo, Y.; Tan, L.; Dong, Q.; Yu, J.T. Longitudinal plasma phosphorylated tau 181 tracks disease progression in Alzheimer’s disease. Transl. Psychiatry 2021, 11, 356. [Google Scholar] [CrossRef]
- Graff-Radford, N.R.; Crook, J.E.; Lucas, J.; Boeve, B.F.; Knopman, D.S.; Ivnik, R.J.; Smith, G.E.; Younkin, L.H.; Petersen, R.C.; Younkin, S.G. Association of low plasma Abeta42/Abeta40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch. Neurol. 2007, 64, 354–362. [Google Scholar] [CrossRef]
- Lambert, J.C.; Schraen-Maschke, S.; Richard, F.; Fievet, N.; Rouaud, O.; Berr, C.; Dartigues, J.F.; Tzourio, C.; Alpérovitch, A.; Buée, L.; et al. Association of plasma amyloid beta with risk of dementia: The prospective Three-City Study. Neurology 2009, 73, 847–853. [Google Scholar] [CrossRef]
- Chatterjee, P.; Pedrini, S.; Ashton, N.J.; Tegg, M.; Goozee, K.; Singh, A.K.; Karikari, T.K.; Simrén, J.; Vanmechelen, E.; Armstrong, N.J.; et al. Diagnostic and prognostic plasma biomarkers for preclinical Alzheimer’s disease. Alzheimers Dement. 2022, 18, 1141–1154. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, D.S.; Ashton, N.J.; Blennow, K.; Zetterberg, H.; Simrén, J.; Lantero-Rodriguez, J.; Karikari, T.K.; Hiniker, A.; Rissman, R.A.; Salmon, D.P.; et al. Plasma biomarkers for Alzheimer’s Disease in relation to neuropathology and cognitive change. Acta Neuropathol. 2022, 143, 487–503. [Google Scholar] [CrossRef]
- Chatterjee, P.; Pedrini, S.; Doecke, J.D.; Thota, R.; Villemagne, V.L.; Doré, V.; Singh, A.K.; Wang, P.; Rainey-Smith, S.; Fowler, C.; et al. Plasma Aβ42/40 ratio, p-tau181, GFAP, and NfL across the Alzheimer’s disease continuum: A cross-sectional and longitudinal study in the AIBL cohort. Alzheimers Dement. 2023, 19, 1117–1134. [Google Scholar] [CrossRef] [PubMed]
- Ennis, G.E.; Betthauser, T.J.; Koscik, R.L.; Chin, N.A.; Christian, B.T.; Asthana, S.; Johnson, S.C.; Bendlin, B.B. The relationship of insulin resistance and diabetes to tau PET SUVR in middle-aged to older adults. Alzheimers Res. Ther. 2023, 15, 55. [Google Scholar] [CrossRef]
- van Arendonk, J.; Neitzel, J.; Steketee RM, E.; van Assema DM, E.; Vrooman, H.A.; Segbers, M.; Ikram, M.A.; Vernooij, M.W. Diabetes and hypertension are related to amyloid-beta burden in the population-based Rotterdam Study. Brain 2023, 146, 337–348. [Google Scholar] [CrossRef]
- Fukasawa, R.; Hanyu, H.; Shimizu, S.; Kanetaka, H.; Sakurai, H.; Ishii, K. Identification of diabetes-related dementia: Longitudinal perfusion SPECT and amyloid PET studies. J. Neurol. Sci. 2015, 349, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Takenoshita, N.; Fukasawa, R.; Ogawa, Y.; Shimizu, S.; Umahara, T.; Ishii, K.; Shimada, H.; Higuchi, M.; Suhara, T.; Hanyu, H. Amyloid and Tau Positron Emission Tomography in Suggested Diabetes-related Dementia. Curr. Alzheimer Res. 2018, 15, 1062–1069. [Google Scholar] [CrossRef]
- Takenoshita, N.; Shimizu, S.; Kanetaka, H.; Sakurai, H.; Suzuki, R.; Miwa, T.; Odawara, M.; Ishii, K.; Shimada, H.; Higuchi, M.; et al. Classification of Clinically Diagnosed Alzheimer’s Disease Associated with Diabetes Based on Amyloid and Tau PET Results. J. Alzheimers Dis. 2019, 71, 261–271. [Google Scholar] [CrossRef]
- Lesman-Segev, O.H.; Golan Shekhtman, S.; Springer, R.R.; Livny, A.; Lin, H.-M.; Yuxia, O.; Zadok, M.; Ganmore, I.; Heymann, A.; Hoffmann, C.; et al. Amyloid deposition and small vessel disease are associated with cognitive function in older adults with type 2 diabetes. Sci. Rep. 2024, 14, 2741. [Google Scholar] [CrossRef]
- Zimmermann, R.; Huber, E.; Schamber, C.; Lelental, N.; Mroczko, B.; Brandner, S.; Maler, J.M.; Oberstein, T.; Szmitkowski, M.; Rauh, M.; et al. Plasma concentrations of the amyloid-β peptides in young volunteers: The influence of the APOE genotype. J. Alzheimers Dis. 2014, 40, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Verberk IM, W.; Misdorp, E.O.; Koelewijn, J.; Ball, A.J.; Blennow, K.; Dage, J.L.; Fandos, N.; Hansson, O.; Hirtz, C.; Janelidze, S.; et al. Characterization of pre-analytical sample handling effects on a panel of Alzheimer’s disease-related blood-based biomarkers: Results from the Standardization of Alzheimer’s Blood Biomarkers (SABB) working group. Alzheimers Dement. 2022, 18, 1484–1497. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef] [PubMed]


| (a) | ||||
| Adolescent Controls (n = 25) | Adolescent Y-DM (n = 42) | Young Adult Controls (n = 21) | Young Adult Y-DM (n = 47) | |
| Age (years), mean (SD) | 14.8 (2.7) | 15.0 (2.6) | 24.9 (2.8) | 27.4 (2.2) |
| Sex (female), n (%) | 15 (60) | 24 (57) | 10 (48) | 28 (59) |
| Race and ethnicity, n (%) Hispanic Non-Hispanic Black Non-Hispanic White Other or multiple race and ethnicity | 10 (40) 4 (16) 10 (40) 1 (4) | 9 (21) 20 (48) 12 (28) 1 (3) | 3 (14) 1 (5) 16 (76) 1 (5) | 9 (19) 22 (47) 15 (32) 1 (2) |
| Y-DM status, n (%) T1D T2D | -- -- | 20 (48) 22 (52) | -- -- | 25 (53) 22 (47) |
| Diabetes duration (years), mean (SD) | -- | 1.0 (0.6) | -- | 13.6 (2.4) |
| Retinopathy (yes), n (%) | -- | -- | -- | 44 (94) |
| Microalbuminuria (yes), n (%) | -- | -- | -- | 23 (49) |
| (b) | ||||
| Young Adult Controls (n = 6) | Young Adult Y-DM (n = 7) | |||
| Age (years), mean (SD) | 25.1 (4.5) | 27.5 (5.7) | ||
| Sex (female), n (%) | 4 (67) | 5 (71) | ||
| Race and ethnicity, n (%) Hispanic Non-Hispanic Black Non-Hispanic White Other or multiple race and ethnicity | 0 2 (33) 2 (33) 2 (33) | 2 (28) 1 (14) 2 (28) 2 (28) | ||
| Y-DM status, n (%) T1D T2D | -- -- | 6 (86) 1 (14) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shapiro, A.L.B.; Coughlan, C.; Bettcher, B.M.; Pauley, M.E.; Kim, J.; Bjornstad, P.; Rajic, B.; Truong, J.; Bell, C.; Choi, Y.J.; et al. Biomarkers of Neurodegeneration and Alzheimer’s Disease Neuropathology in Adolescents and Young Adults with Youth-Onset Type 1 or Type 2 Diabetes: A Proof-of-Concept Study. Endocrines 2024, 5, 197-213. https://doi.org/10.3390/endocrines5020014
Shapiro ALB, Coughlan C, Bettcher BM, Pauley ME, Kim J, Bjornstad P, Rajic B, Truong J, Bell C, Choi YJ, et al. Biomarkers of Neurodegeneration and Alzheimer’s Disease Neuropathology in Adolescents and Young Adults with Youth-Onset Type 1 or Type 2 Diabetes: A Proof-of-Concept Study. Endocrines. 2024; 5(2):197-213. https://doi.org/10.3390/endocrines5020014
Chicago/Turabian StyleShapiro, Allison L. B., Christina Coughlan, Brianne M. Bettcher, Meghan E. Pauley, Jeongchul Kim, Petter Bjornstad, Benjamin Rajic, Jennifer Truong, Christopher Bell, Ye Ji Choi, and et al. 2024. "Biomarkers of Neurodegeneration and Alzheimer’s Disease Neuropathology in Adolescents and Young Adults with Youth-Onset Type 1 or Type 2 Diabetes: A Proof-of-Concept Study" Endocrines 5, no. 2: 197-213. https://doi.org/10.3390/endocrines5020014








