Genomic and Functional Characterization of CTX-M-15-Producing Klebsiella pneumoniae ST307 Isolated from Imported Leopard Tortoises in Germany
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Bacterial Species Identification
2.2. Genomic Analysis
2.3. Phenotypic Analysis
3. Results
3.1. Antimicrobial Resistance Genes and Chromosomal Mutations
3.2. Virulence-Associated Genes
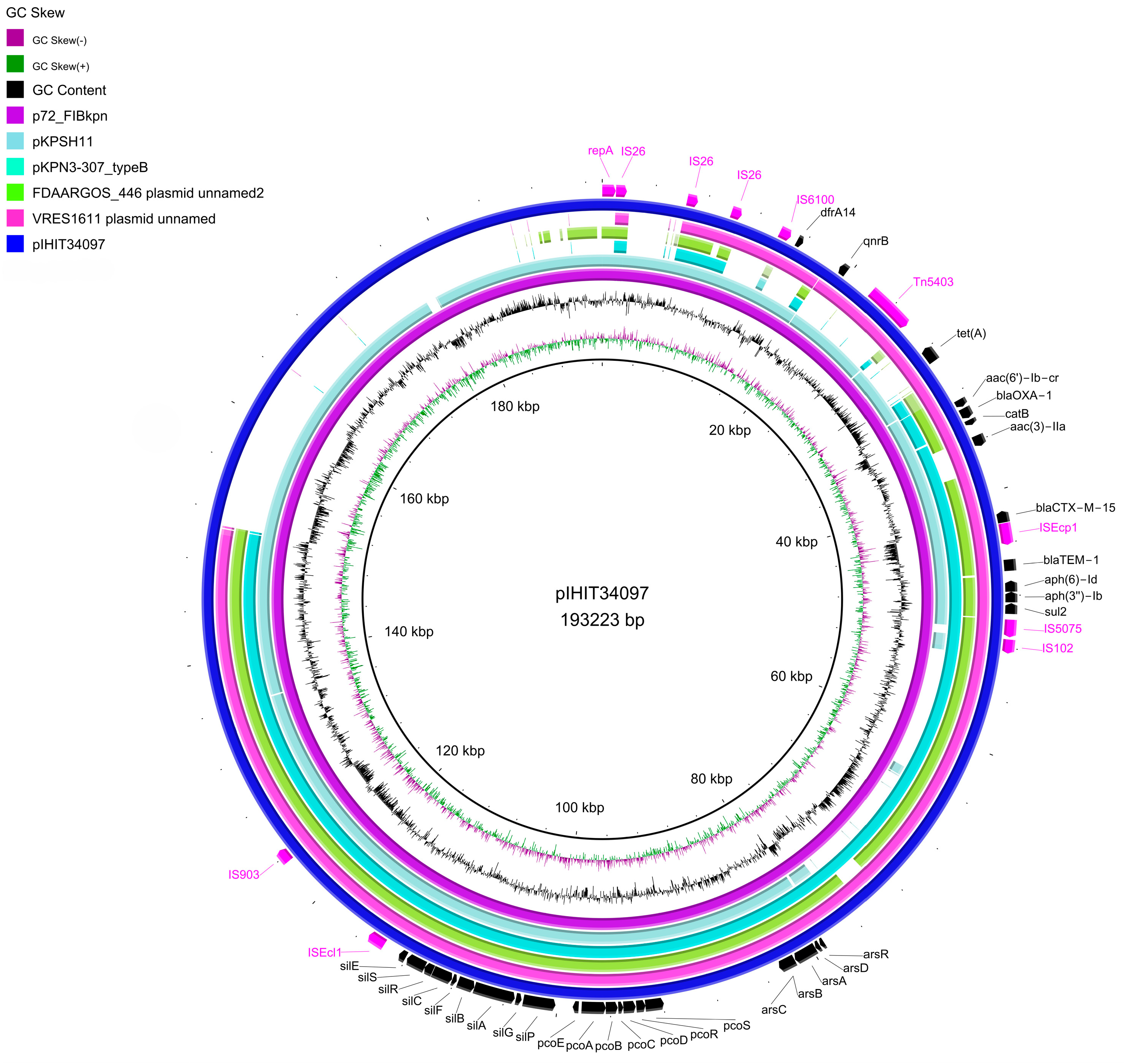
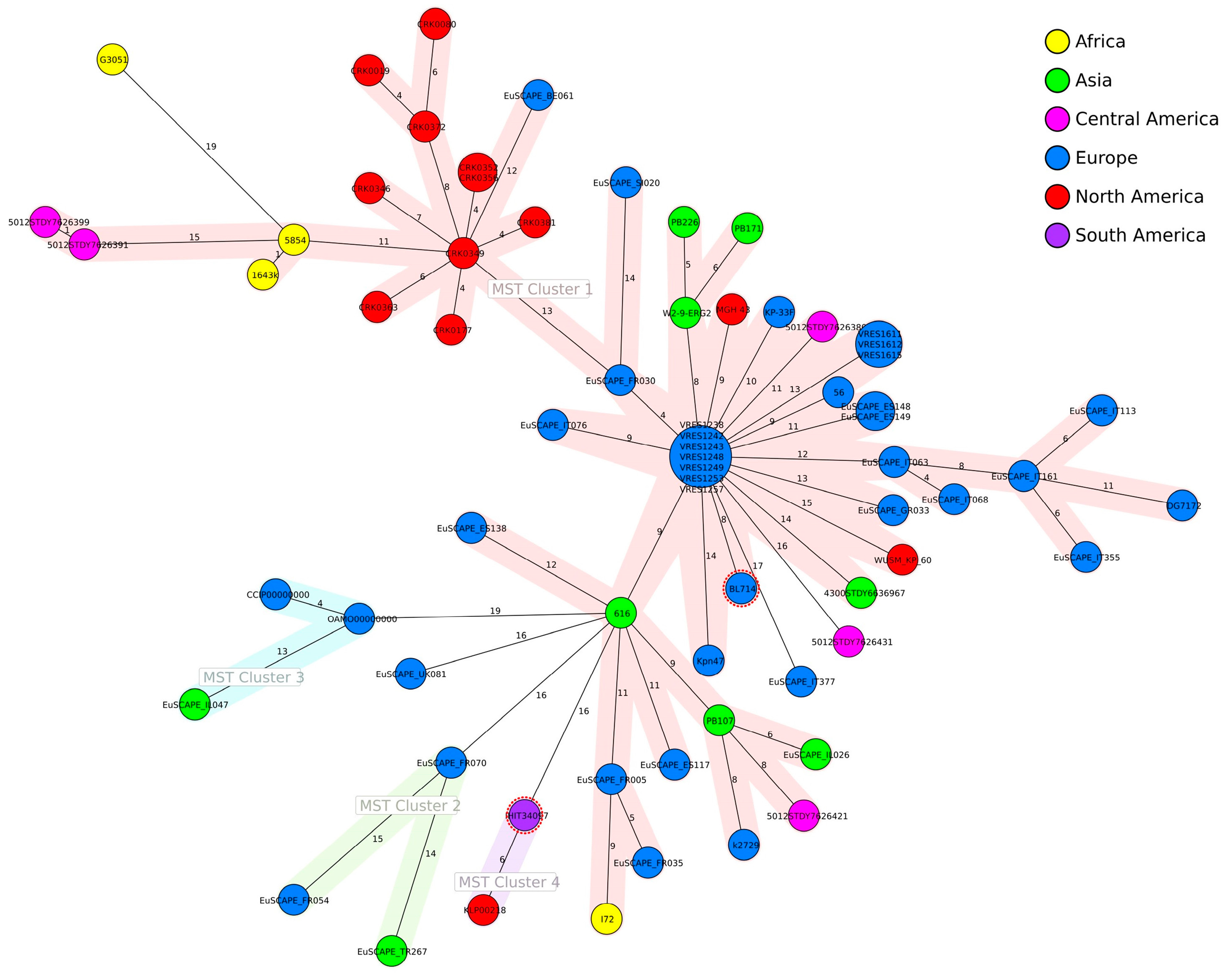
3.3. Genomic Analysis
3.4. Phenotypic Analysis
3.4.1. Antimicrobial Susceptibility Testing
3.4.2. Mucoid Phenotype, Siderophore Production and Serum Resistance
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heiden, S.E.; Hübner, N.-O.; Bohnert, J.A.; Heidecke, C.-D.; Kramer, A.; Balau, V.; Gierer, W.; Schaefer, S.; Eckmanns, T.; Gatermann, S.; et al. A Klebsiella pneumoniae ST307 outbreak clone from Germany demonstrates features of extensive drug resistance, hypermucoviscosity, and enhanced iron acquisition. Genome Med. 2020, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Peirano, G.; Chen, L.; Kreiswirth, B.N.; Pitout, J.D.D. Emerging antimicrobial-resistant high-risk Klebsiella pneumoniae clones ST307 and ST147. Antimicrob. Agents Chemother. 2020, 64, e01148-20. [Google Scholar] [CrossRef]
- Bonnet, R. Growing group of extended-spectrum beta-lactamases: The CTX-M enzymes. Antimicrob. Agents Chemother. 2004, 48, 1–14. [Google Scholar] [CrossRef]
- Munoz-Price, L.S.; Poirel, L.; Bonomo, R.A.; Schwaber, M.J.; Daikos, G.L.; Cormican, M.; Cornaglia, G.; Garau, J.; Gniadkowski, M.; Hayden, M.K.; et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 2013, 13, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Oh, J.Y.; Sum, S.; Park, H.M. Prevalence and antimicrobial resistance of Klebsiella species isolated from clinically ill companion animals. J. Vet. Sci. 2021, 22, e17. [Google Scholar] [CrossRef] [PubMed]
- Ogbolu, D.O.; Alli, O.A.T.; Webber, M.A.; Oluremi, A.S.; Oloyede, O.M. CTX-M-15 is established in most multidrug-resistant uropathogenic Enterobacteriaceae and Pseudomonaceae from hospitals in Nigeria. Eur. J. Microbiol. Immunol. (Bp) 2018, 8, 20–24. [Google Scholar] [CrossRef]
- Karim, A.; Poirel, L.; Nagarajan, S.; Nordmann, P. Plasmid-mediated extended-spectrum beta-lactamase (CTX-M-3 like) from India and gene association with insertion sequence ISEcp1. FEMS Microbiol. Lett. 2001, 201, 237–241. [Google Scholar] [CrossRef]
- Zhou, K.; Lokate, M.; Deurenberg, R.H.; Arends, J.; Lo-Ten Foe, J.; Grundmann, H.; Rossen, J.W.A.; Friedrich, A.W. Characterization of a CTX-M-15 producing Klebsiella pneumoniae outbreak strain assigned to a novel sequence type (1427). Front. Microbiol. 2015, 6, 1250. [Google Scholar] [CrossRef]
- Schaufler, K.; Nowak, K.; Düx, A.; Semmler, T.; Villa, L.; Kourouma, L.; Bangoura, K.; Wieler, L.H.; Leendertz, F.H.; Guenther, S. Clinically relevant ESBL-producing K. pneumoniae ST307 and E. coli ST38 in an urban West African rat population. Front. Microbiol. 2018, 9, 150. [Google Scholar] [CrossRef]
- Davies, Y.M.; Cunha, M.P.V.; Dropa, M.; Lincopan, N.; Gomes, V.T.M.; Moreno, L.Z.; Sato, M.I.Z.; Moreno, A.M.; Knöbl, T. Pandemic clones of CTX-M-15 producing Klebsiella pneumoniae ST15, ST147, and ST307 in companion parrots. Microorganisms 2022, 10, 1412. [Google Scholar] [CrossRef]
- Sartori, L.; Sellera, F.P.; Moura, Q.; Cardoso, B.; Cerdeira, L.; Lincopan, N. Multidrug-resistant CTX-M-15-positive Klebsiella pneumoniae ST307 causing urinary tract infection in a dog in Brazil. J. Glob. Antimicrob. Resist. 2019, 19, 96–97. [Google Scholar] [CrossRef] [PubMed]
- Sghaier, S.; Abbassi, M.S.; Pascual, A.; Serrano, L.; Díaz-De-Alba, P.; Said, M.B.; Hassen, B.; Ibrahim, C.; Hassen, A.; López-Cerero, L. Extended-spectrum β-lactamase-producing Enterobacteriaceae from animal origin and wastewater in Tunisia: First detection of O25b-B23-CTX-M-27-ST131 Escherichia coli and CTX-M-15/OXA-204-producing Citrobacter freundii from wastewater. J. Glob. Antimicrob. Resist. 2019, 17, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Baron, S.A.; Mediannikov, O.; Abdallah, R.; Kuete Yimagou, E.; Medkour, H.; Dubourg, G.; Elamire, Y.; Afouda, P.; Ngom, I.I.; Angelakis, E.; et al. Multidrug-resistant Klebsiella pneumoniae clones from wild chimpanzees and termites in Senegal. Antimicrob. Agents Chemother. 2021, 65, e0255720. [Google Scholar] [CrossRef] [PubMed]
- Mukerji, S.; Sahibzada, S.; Abraham, R.; Stegger, M.; Jordan, D.; Hampson, D.J.; O’Dea, M.; Lee, T.; Abraham, S. Proximity to human settlement is directly related to carriage of critically important antimicrobial-resistant Escherichia coli and Klebsiella pneumoniae in silver gulls. Vet. Microbiol. 2023, 280, 109702. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Castillo, D.; Shiva, C.; Lincopan, N.; Sano, E.; Fontana, H.; Streicker, D.G.; Mahamat, O.O.; Falcon, N.; Godreuil, S.; Benavides, J.A. Global high-risk clone of extended-spectrum β-lactamase (ESBL)-producing Klebsiella pneumoniae ST307 emerging in livestock in Peru. Int. J. Antimicrob. Agents 2021, 58, 106389. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Shimizu, T.; Mukai, Y.; Kuwajima, K.; Sato, T.; Usui, M.; Tamura, Y.; Kimura, Y.; Miyamoto, T.; Tsuyuki, Y.; et al. Phenotypic and molecular characterization of antimicrobial resistance in Klebsiella spp. isolates from companion animals in Japan: Clonal dissemination of multidrug-resistant extended-spectrum β-lactamase-producing Klebsiella pneumoniae. Front. Microbiol. 2016, 7, 1021. [Google Scholar] [CrossRef]
- Carvalho, I.; Alonso, C.A.; Silva, V.; Pimenta, P.; Cunha, R.; Martins, C.; Igrejas, G.; Torres, C.; Poeta, P. Extended-spectrum beta-lactamase-producing Klebsiella pneumoniae isolated from healthy and sick dogs in Portugal. Microb. Drug Resist. 2020, 26, 709–715. [Google Scholar] [CrossRef]
- Loncaric, I.; Cabal Rosel, A.; Szostak, M.P.; Licka, T.; Allerberger, F.; Ruppitsch, W.; Spergser, J. Broad-spectrum cephalosporin-resistant Klebsiella spp. isolated from diseased horses in Austria. Animals 2020, 10, 332. [Google Scholar] [CrossRef]
- Garcia-Fierro, R.; Drapeau, A.; Dazas, M.; Saras, E.; Rodrigues, C.; Brisse, S.; Madec, J.-Y.; Haenni, M. Comparative phylogenomics of ESBL-, AmpC- and carbapenemase-producing Klebsiella pneumoniae originating from companion animals and humans. J. Antimicrob. Chemother. 2022, 77, 1263–1271. [Google Scholar] [CrossRef]
- Bonardi, S.; Cabassi, C.S.; Fiaccadori, E.; Cavirani, S.; Parisi, A.; Bacci, C.; Lamperti, L.; Rega, M.; Conter, M.; Marra, F.; et al. Detection of carbapenemase- and ESBL-producing Klebsiella pneumoniae from bovine bulk milk and comparison with clinical human isolates in Italy. Int. J. Food Microbiol. 2023, 387, 110049. [Google Scholar] [CrossRef]
- Pepin-Puget, L.; El Garch, F.; Bertrand, X.; Valot, B.; Hocquet, D. Genome analysis of Enterobacteriaceae with non-wild type susceptibility to third-generation cephalosporins recovered from diseased dogs and cats in Europe. Vet. Microbiol. 2020, 242, 108601. [Google Scholar] [CrossRef]
- Abreu-Salinas, F.; Díaz-Jiménez, D.; García-Meniño, I.; Lumbreras, P.; López-Beceiro, A.M.; Fidalgo, L.E.; Rodicio, M.R.; Mora, A.; Fernández, J. High prevalence and diversity of cephalosporin-resistant Enterobacteriaceae including extraintestinal pathogenic E. coli CC648 lineage in rural and urban dogs in Northwest Spain. Antibiotics 2020, 9, 468. [Google Scholar] [CrossRef] [PubMed]
- Brilhante, M.; Gobeli Brawand, S.; Endimiani, A.; Rohrbach, H.; Kittl, S.; Willi, B.; Schuller, S.; Perreten, V. Two high-risk clones of carbapenemase-producing Klebsiella pneumoniae that cause infections in pets and are present in the environment of a veterinary referral hospital. J. Antimicrob. Chemother. 2021, 76, 1140–1149. [Google Scholar] [CrossRef]
- Foster, G.; AbuOun, M.; Pizzi, R.; Tennant, B.; McCall, M.; Anjum, M.F. Isolation of the human-associated blaCTX-M-15-harbouring Klebsiella pneumoniae ST307 from a tortoise in the UK. Access Microbiol. 2020, 2, acmi000172. [Google Scholar] [CrossRef] [PubMed]
- CITES Secretariat. World Wildlife Trade Report 2022 (a Pilot Edition for CoP19). Available online: https://bvearmb.do/handle/123456789/3779 (accessed on 8 April 2024).
- Unger, F.; Eisenberg, T.; Prenger-Berninghoff, E.; Leidner, U.; Semmler, T.; Ewers, C. Imported pet reptiles and their “blind passengers”-in-depth characterization of 80 Acinetobacter species isolates. Microorganisms 2022, 10, 893. [Google Scholar] [CrossRef]
- Unger, F.; Eisenberg, T.; Prenger-Berninghoff, E.; Leidner, U.; Ludwig, M.-L.; Rothe, M.; Semmler, T.; Ewers, C. Imported reptiles as a risk factor for the global distribution of Escherichia coli harbouring the colistin resistance gene mcr-1. Int. J. Antimicrob. Agents 2017, 49, 122–123. [Google Scholar] [CrossRef] [PubMed]
- SPAdes v.3.15.1. Available online: http://cab.spbu.ru/software/spades/ (accessed on 10 September 2021).
- RAST v.2.0. Available online: http://rast.nmpdr.org/ (accessed on 10 September 2021).
- Bacterial Isolate Genome Sequence Database (BIGSdb) Version 1.42.3. Available online: https://bigsdb.pasteur.fr/ (accessed on 20 September 2023).
- Kaptive. Available online: https://kaptive-web.erc.monash.edu/ (accessed on 14 February 2024).
- BacWGSTdb. Available online: http://bacdb.cn/BacWGSTdb/ (accessed on 18 January 2024).
- NCBI Database. Available online: https://www.ncbi.nlm.nih.gov/nucleotide/ (accessed on 18 January 2024).
- Jünemann, S.; Sedlazeck, F.J.; Prior, K.; Albersmeier, A.; John, U.; Kalinowski, J.; Mellmann, A.; Goesmann, A.; von Haeseler, A.; Stoye, J.; et al. Updating benchtop sequencing performance comparison. Nat. Biotechnol. 2013, 31, 294–296. [Google Scholar] [CrossRef] [PubMed]
- PlasmidFinder 2.1. Available online: https://cge.food.dtu.dk/services/PlasmidFinder/ (accessed on 6 February 2024).
- ResFinder 4.4.2. Available online: http://genepi.food.dtu.dk/resfinder (accessed on 6 February 2024).
- Fang, C.-T.; Chuang, Y.-P.; Shun, C.-T.; Chang, S.-C.; Wang, J.-T. A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J. Exp. Med. 2004, 199, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Himpsl, S.D.; Mobley, H.L.T. Siderophore detection using chrome azurol S and cross-feeding assays. Methods Mol. Biol. 2019, 2021, 97–108. [Google Scholar] [CrossRef]
- Taylor, P.W.; Kroll, H.P. Killing of an encapsulated strain of Escherichia coli by human serum. Infect. Immun. 1983, 39, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Eger, E.; Heiden, S.E.; Becker, K.; Rau, A.; Geisenhainer, K.; Idelevich, E.A.; Schaufler, K. Hypervirulent Klebsiella pneumoniae sequence type 420 with a chromosomally inserted virulence plasmid. Int. J. Mol. Sci. 2021, 22, 9196. [Google Scholar] [CrossRef]
- Lowe, M.; Kock, M.M.; Coetzee, J.; Hoosien, E.; Peirano, G.; Strydom, K.-A.; Ehlers, M.M.; Mbelle, N.M.; Shashkina, E.; Haslam, D.B.; et al. Klebsiella pneumoniae ST307 with blaOXA-181, South Africa, 2014-2016. Emerg. Infect. Dis. 2019, 25, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Alikhan, N.-F.; Petty, N.K.; Ben Zakour, N.L.; Beatson, S.A. BLAST Ring Image Generator (BRIG): Simple prokaryote genome comparisons. BMC Genom. 2011, 12, 402. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Russo, T.A.; Olson, R.; Fang, C.-T.; Stoesser, N.; Miller, M.; MacDonald, U.; Hutson, A.; Barker, J.H.; La Hoz, R.M.; Johnson, J.R. Identification of biomarkers for differentiation of hypervirulent Klebsiella pneumoniae from classical K. pneumoniae. J. Clin. Microbiol. 2018, 56, e00776-18. [Google Scholar] [CrossRef] [PubMed]
- Trotta, A.; Cirilli, M.; Marinaro, M.; Bosak, S.; Diakoudi, G.; Ciccarelli, S.; Paci, S.; Buonavoglia, D.; Corrente, M. Detection of multi-drug resistance and AmpC β-lactamase/extended-spectrum β-lactamase genes in bacterial isolates of loggerhead sea turtles (Caretta caretta) from the Mediterranean Sea. Mar. Pollut. Bull. 2021, 164, 112015. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.W.; Fernandes, M.R.; Sellera, F.P.; Costa, D.G.C.; Loureiro Bracarense, A.P.; Lincopan, N. Genetic background of CTX-M-15-producing Enterobacter hormaechei ST114 and Citrobacter freundii ST265 co-infecting a free-living green turtle (Chelonia mydas). Zoonoses Public Health 2019, 66, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Cortés, G.; Lozano-Zarain, P.; Torres, C.; Castañeda, M.; Sánchez, G.M.; Alonso, C.A.; López-Pliego, L.; Mayen, M.G.G.; Martínez-Laguna, Y.; Del Rocha-Gracia, R.C. Detection and molecular characterization of Escherichia coli strains producers of extended-spectrum and CMY-2 type beta-lactamases, isolated from turtles in Mexico. Vector Borne Zoonotic Dis. 2016, 16, 595–603. [Google Scholar] [CrossRef]
- Hossain, S.; de Silva, B.C.J.; Dahanayake, P.S.; Heo, G.-J. Phylogenetic relationships, virulence and antimicrobial resistance properties of Klebsiella sp. isolated from pet turtles in Korea. Lett. Appl. Microbiol. 2020, 70, 71–78. [Google Scholar] [CrossRef]
- Morrison, B.J.; Rubin, J.E. Detection of multidrug-resistant Gram-negative bacteria from imported reptile and amphibian meats. J. Appl. Microbiol. 2020, 129, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Colon, V.A.; Lugsomya, K.; Lam, H.K.; Wahl, L.C.; Parkes, R.S.V.; Cormack, C.A.; Horlbog, J.A.; Stevens, M.; Stephan, R.; Magouras, I. Serotype diversity and antimicrobial resistance profile of Salmonella enterica isolates from freshwater turtles sold for human consumption in wet markets in Hong Kong. Front. Vet. Sci. 2022, 9, 912693. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Scientific opinion of the panel on biological hazards on a request from the European Commission on public health risks involved in the human consumption of reptile meat. EFSA J. 2007, 578, 1–55. [Google Scholar] [CrossRef]
- EU Considers Limit for Salmonella Levels in Reptile Meat. Available online: https://www.foodsafetynews.com/2019/01/eu-considers-limit-for-salmonella-levels-in-reptile-meat/ (accessed on 21 March 2024).
- CITES Appendices I, II and III. Available online: https://cites.org/eng/app/appendices.php (accessed on 27 March 2024).
- Cayot, L.J. The restoration of giant tortoise and land iguana populations in Galapagos. Galapagos Res. 2008, 65, 39–43. [Google Scholar]
- Pees, M.; Brockmann, M.; Steiner, N.; Marschang, R.E. Salmonella in reptiles: A review of occurrence, interactions, shedding and risk factors for human infections. Front. Cell Dev. Biol. 2023, 11, 1251036. [Google Scholar] [CrossRef] [PubMed]
- Zając, M.; Skarżyńska, M.; Lalak, A.; Kwit, R.; Śmiałowska-Węglińska, A.; Pasim, P.; Szulowski, K.; Wasyl, D. Salmonella in captive reptiles and their environment-can we tame the dragon? Microorganisms 2021, 9, 1012. [Google Scholar] [CrossRef]
- Hedley, J.; Whitehead, M.L.; Munns, C.; Pellett, S.; Abou-Zahr, T.; Calvo Carrasco, D.; Wissink-Argilaga, N. Antibiotic stewardship for reptiles. J. Small Anim. Pract. 2021, 62, 829–839. [Google Scholar] [CrossRef]
- Nieto-Claudin, A.; Deem, S.L.; Rodríguez, C.; Cano, S.; Moity, N.; Cabrera, F.; Esperón, F. Antimicrobial resistance in Galapagos tortoises as an indicator of the growing human footprint. Environ. Pollut. 2021, 284, 117453. [Google Scholar] [CrossRef]
- Amancha, G.; Celis, Y.; Irazabal, J.; Falconi, M.; Villacis, K.; Thekkur, P.; Nair, D.; Perez, F.; Verdonck, K. High levels of antimicrobial resistance in Escherichia coli and Salmonella from poultry in Ecuador. Rev. Panam. Salud Publica 2023, 47, e15. [Google Scholar] [CrossRef]
- Ramey, A.M.; Ahlstrom, C.A. Antibiotic resistant bacteria in wildlife: Perspectives on trends, acquisition and dissemination, data gaps, and future directions. J. Wildl. Dis. 2020, 56, 1–15. [Google Scholar] [CrossRef]
- Carroll, D.; Wang, J.; Fanning, S.; McMahon, B.J. Antimicrobial resistance in wildlife: Implications for public health. Zoonoses Public Health 2015, 62, 534–542. [Google Scholar] [CrossRef]
- Nieto-Claudin, A.; Esperón, F.; Blake, S.; Deem, S.L. Antimicrobial resistance genes present in the faecal microbiota of free-living Galapagos tortoises (Chelonoidis porteri). Zoonoses Public Health 2019, 66, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, E.; Hong, P.-Y.; Bedon, L.C.; Mackie, R.I. Carriage of antibiotic-resistant enteric bacteria varies among sites in Galapagos reptiles. J. Wildl. Dis. 2012, 48, 56–67. [Google Scholar] [CrossRef]
- Kocsis, B. Hypervirulent Klebsiella pneumoniae: An update on epidemiology, detection and antibiotic resistance. Acta Microbiol. Et Immunol. Hung. 2023, 70, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Y.; Liu, P.; Jian, Z.; Yan, Q.; Tang, B.; Yang, A.; Liu, W. Genomic and clinical characterization of Klebsiella pneumoniae carrying the pks island. Front. Microbiol. 2023, 14, 1189120. [Google Scholar] [CrossRef]
- Russo, T.A.; Marr, C.M. Hypervirulent Klebsiella pneumoniae. Clin. Microbiol. Rev. 2019, 32, e00001-19. [Google Scholar] [CrossRef] [PubMed]
- Mathers, A.J.; Peirano, G.; Pitout, J.D.D. The role of epidemic resistance plasmids and international high-risk clones in the spread of multidrug-resistant Enterobacteriaceae. Clin. Microbiol. Rev. 2015, 28, 565–591. [Google Scholar] [CrossRef]
- Wyres, K.L.; Hawkey, J.; Hetland, M.A.K.; Fostervold, A.; Wick, R.R.; Judd, L.M.; Hamidian, M.; Howden, B.P.; Löhr, I.H.; Holt, K.E. Emergence and rapid global dissemination of CTX-M-15-associated Klebsiella pneumoniae strain ST307. J. Antimicrob. Chemother. 2019, 74, 577–581. [Google Scholar] [CrossRef]
- Carattoli, A. Resistance plasmid families in Enterobacteriaceae. Antimicrob. Agents Chemother. 2009, 53, 2227–2238. [Google Scholar] [CrossRef]
- WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/ (accessed on 1 February 2024).

| Antimicrobial 1 | Interpretation | MIC (mg/L) |
| Amoxicillin/Clavulanic acid | I | =16/8 |
| Cephalexin * | R | >16 |
| Cefovecin * | R | >4 |
| Chloramphenicol | S | =4 |
| Doxycycline * | R | >1 |
| Enrofloxacin * | R | >2 |
| Florfenicol * | I | =8 |
| Gentamicin | R | >4 |
| Pradofloxacin * | R | >1 |
| Trimethoprim/Sulfamethoxazole | R | >2/38 |
| Tetracycline | NS | >4 |
| Antimicrobial 2 | Interpretation | MIC (mg/L) |
| ESBL | Positive | |
| Ampicillin/Sulbactam | R | ≥32 |
| Piperacillin/Tazobactam | NS | =16 |
| Cefuroxim | R | ≥64 |
| Cefuroxim-Axetil | R | ≥64 |
| Cefpodoxim | R | ≥8 |
| Cefotaxim | R | ≥64 |
| Ceftazidim | R | =32 |
| Imipenem | S | ≤0.25 |
| Meropenem | S | ≤0.25 |
| Gentamicin | R | ≥16 |
| Ciprofloxacin | R | ≥4 |
| Tigecycline | S | ≤0.5 |
| Fosfomycin | S | ≤16 |
| Nitrofurantoin | S | =32 |
| Trimethoprim/Sulfamethoxazole | R | ≥320 |
| Antimicrobial 3 | Interpretation | MIC (mg/L) |
| Temocillin | NS | ≤4 |
| Ceftazidim/Avibactam | S | =0.25 |
| Ceftolozan/Tazobactam | S | ≤0.25 |
| Imipenem/Relebactam | S | ≤0.25 |
| Meropenem/Vaborbactam | S | ≤0.5 |
| Eravacyclin | S | 0.25 |
| Colistin | I | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, T.J.; Aurich, S.; Unger, F.; Eisenberg, T.; Ewers, C. Genomic and Functional Characterization of CTX-M-15-Producing Klebsiella pneumoniae ST307 Isolated from Imported Leopard Tortoises in Germany. Appl. Microbiol. 2024, 4, 782-793. https://doi.org/10.3390/applmicrobiol4020054
Schmidt TJ, Aurich S, Unger F, Eisenberg T, Ewers C. Genomic and Functional Characterization of CTX-M-15-Producing Klebsiella pneumoniae ST307 Isolated from Imported Leopard Tortoises in Germany. Applied Microbiology. 2024; 4(2):782-793. https://doi.org/10.3390/applmicrobiol4020054
Chicago/Turabian StyleSchmidt, Tammy J., Sophie Aurich, Franziska Unger, Tobias Eisenberg, and Christa Ewers. 2024. "Genomic and Functional Characterization of CTX-M-15-Producing Klebsiella pneumoniae ST307 Isolated from Imported Leopard Tortoises in Germany" Applied Microbiology 4, no. 2: 782-793. https://doi.org/10.3390/applmicrobiol4020054





