Enzymatic Synthesis and Structural Modeling of Bio-Based Oligoesters as an Approach for the Fast Screening of Marine Biodegradation and Ecotoxicity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Enzymatic Synthesis of Model Aromatic and Aliphatic Oligoesters for Studies of Biodegradation and Ecotoxicity
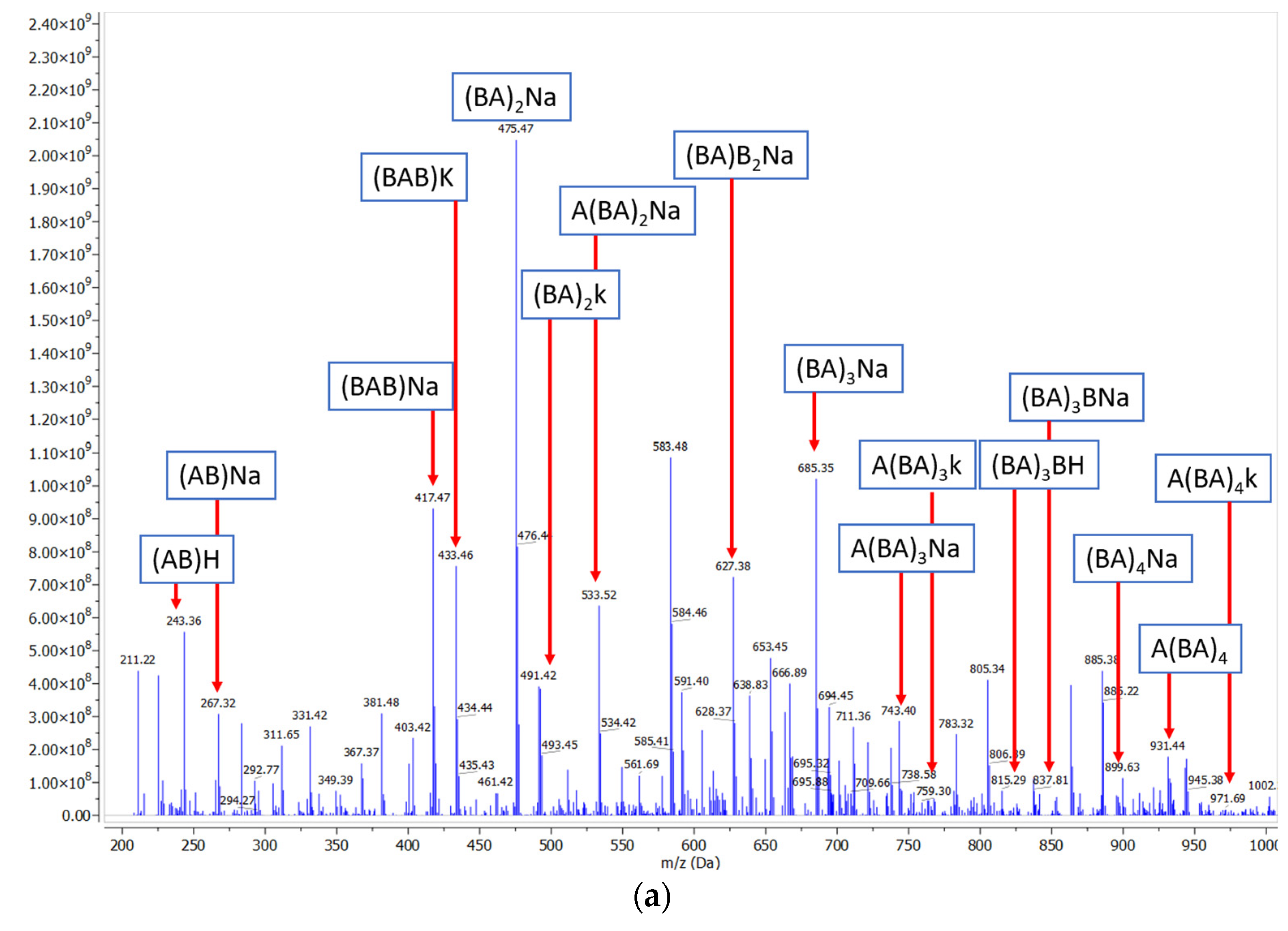
Oligoester Structural Characterization by ESI-MS and NMR
2.2. Biodegradation Studies
2.2.1. Biodegradation of Co-Oligomers
2.2.2. Biodegradation of Ter-Oligomers
2.2.3. Study of the Marine Biodegradation of the Monomers Composing the Oligoester Model
2.2.4. Biodegradation Studies of a Furan-Based Oligoamide
2.3. Computational Analysis of Tetramer Structures Using VolSurf3 Molecular Descriptors
2.4. Ecotoxicity Studies of Model Systems
2.4.1. Aliivibrio fischeri (Luminescent Bacteria Test)
2.4.2. Phaeodactylum tricornutum (Marine Algal Growth Inhibition Test)
2.4.3. Paracentrotus lividus (Embryotoxicity Test)
2.4.4. Daphnia magna (Mobility Test)
2.4.5. Raphidocelis subcapitata (Freshwater Algal Growth Inhibition Test)
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Enzymatic Activity Assay
3.2.2. Enzymatic Synthesis of Oligoesters and Oligoamides in Solvent Less and Organic Solvent Media
3.3. Characterization of Oligoesters and Oligoamides
3.3.1. NMR Analysis
3.3.2. ESI-MS Analysis
3.3.3. Computational Analysis
3.3.4. Thermal Analysis
3.3.5. Biodegradation Studies
3.3.6. Ecotoxicity Studies
- -
- Negative control: Organisms were exposed to the matrix without toxic substances (clean freshwater or seawater).
- -
- Positive control: Organisms were exposed to the matrix with a known toxic substance, demonstrating a toxic effect.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhattacharya, P. Bhattacharya Piyal A Review on the Impacts of Microplastic Beads Used in Cosmetics. Acta Biomed. Sci. 2016, 3, 47–52. [Google Scholar]
- Madanhire, I.; Mbhohwa, C. Mitigating Environmental Impact of Petroleum Lubricants; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-319-31357-3. [Google Scholar]
- OECD; WBCSD. Workshop on Microplastics from Tyre Wear: Knowledge, Mitigation Measures, and Policy Options—Summary Note. 2020, 2–4. Available online: https://www.oecd.org/water/Workshop_Tyres_Summary_Note_FINAL.pdf (accessed on 12 December 2023).
- European Commission Microplastics from Textiles: Towards a Circular Economy for Textiles in Europe Microplastics from Textiles: Towards a Circular Economy for Textiles in Europe. Available online: https://www.eea.europa.eu/publications/microplastics-from-textiles-towards-a (accessed on 17 January 2024).
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef]
- Bilal, M.; Mehmood, S.; Iqbal, H.M.N. The Beast of Beauty: Environmental and Health Concerns of Toxic Components in Cosmetics. Cosmetics 2020, 7, 13. [Google Scholar] [CrossRef]
- Materials, H.B.; Applications, M.; Mustansar, C.; Paperback, H.; Isbn, B.; Applications, M. Handbook of Nanomaterials for Manufacturing Applications Purchase Options; Chaudhery Mustansar, Hussain, Ed.; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128213810. [Google Scholar]
- Chen, G.; Li, Y.; Wang, J. Chemosphere Occurrence and Ecological Impact of Microplastics in Aquaculture Ecosystems. Chemosphere 2021, 274, 129989. [Google Scholar] [CrossRef] [PubMed]
- Bansal, M.; Santhiya, D. Behavioural Mechanisms of Microplastic Pollutants in Marine Ecosystem: Challenges and Remediation Measurements. Water Air Soil Pollut. 2021, 232, 372. [Google Scholar] [CrossRef]
- Al Mamun, A.; Agung, T.; Prasetya, E.; Ratna, I.; Ahmad, M. Microplastics in Human Food Chains: Food Becoming a Threat to Health Safety. Sci. Total Environ. 2023, 858, 159834. [Google Scholar] [CrossRef] [PubMed]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of Plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef]
- Thakur, S.; Mathur, S.; Patel, S.; Paital, B. Microplastic Accumulation and Degradation in Environment via Biotechnological Approaches. Water 2022, 14, 4053. [Google Scholar] [CrossRef]
- Plastics-the Facts. 2022. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 7 November 2023).
- Zuiderveen, E.A.R.; Kuipers, K.J.J.; Caldeira, C.; Hanssen, S.V.; van der Hulst, M.K.; de Jonge, M.M.J.; Vlysidis, A.; van Zelm, R.; Sala, S.; Huijbregts, M.A.J. The Potential of Emerging Bio-Based Products to Reduce Environmental Impacts. Nat. Commun. 2023, 14, 8521. [Google Scholar] [CrossRef]
- Ellen Macarthur Foundation; World Economic Forum; McKinsey&Co. The New Plastic Economy: Rethinking the Future of Plastics. In World Economic Forum; 2016; pp. 1–120. Available online: https://www.ellenmacarthurfoundation.org/the-new-plastics-economy-rethinking-the-future-of-plastics (accessed on 17 January 2024).
- Barboza, L.G.A.; Dick Vethaak, A.; Lavorante, B.R.B.O.; Lundebye, A.K.; Guilhermino, L. Marine Microplastic Debris: An Emerging Issue for Food Security, Food Safety and Human Health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef]
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of Bioplastics in Natural Environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Eyheraguibel, B.; Jacquin, J.; Pujo-Pay, M.; Conan, P.; Barbe, V.; Hoypierres, J.; Deligey, G.; Ter Halle, A.; Bruzaud, S.; et al. Biodegradability under Marine Conditions of Bio-Based and Petroleum-Based Polymers as Substitutes of Conventional Microparticles. Polym. Degrad. Stab. 2022, 206, 110159. [Google Scholar] [CrossRef]
- Dilkes-Hoffman, L.S.; Lant, P.A.; Laycock, B.; Pratt, S. The Rate of Biodegradation of PHA Bioplastics in the Marine Environment: A Meta-Study. Mar. Pollut. Bull. 2019, 142, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. The Plastic in Microplastics: A Review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Carson, H.S.; Nerheim, M.S.; Carroll, K.A.; Eriksen, M. The Plastic-Associated Microorganisms of the North Pacific Gyre. Mar. Pollut. Bull. 2013, 75, 126–132. [Google Scholar] [CrossRef]
- Rochman, C.M. Microplastics Research-from Sink to Source in Freshwater Systems. Science 2018, 360, 28–29. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of Microplastic on Shorelines Woldwide: Sources and Sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef] [PubMed]
- Zappaterra, F.; Renzi, M.; Piccardo, M.; Spennato, M.; Asaro, F.; Di Serio, M.; Vitiello, R.; Turco, R.; Todea, A.; Gardossi, L. Understanding Marine Biodegradation of Bio-Based Oligoesters and Plasticizers. Polymers 2023, 15, 1536. [Google Scholar] [CrossRef]
- Nakayama, A.; Yamano, N.; Kawasaki, N. Biodegradation in Seawater of Aliphatic Polyesters. Polym. Degrad. Stab. 2019, 166, 290–299. [Google Scholar] [CrossRef]
- Dimassi, S.N.; Hahladakis, J.N.; Yahia, M.N.D.; Ahmad, M.I.; Sayadi, S.; Al-Ghouti, M.A. Degradation-Fragmentation of Marine Plastic Waste and Their Environmental Implications: A Critical Review. Arab. J. Chem. 2022, 15, 104262. [Google Scholar] [CrossRef]
- Fritz, I. Ecotoxicological Aspects of the Biodegradation Process of Polymers; Bastioli, C., Ed.; De Gruyter: Berlin, Germany, 2020; ISBN 9781501511967. [Google Scholar]
- Yoshinaga, N.; Tateishi, A.; Kobayashi, Y.; Kubo, T.; Miyakawa, H.; Satoh, K.; Numata, K. Effect of Oligomers Derived from Biodegradable Polyesters on Eco- and Neurotoxicity. Biomacromolecules 2023, 24, 2721–2729. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.; Scherer, C.; Wagner, M. Ecotoxicity Testing of Microplastics: Considering the Heterogeneity of Physicochemical Properties. Integr. Environ. Assess. Manag. 2017, 13, 470–475. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef] [PubMed]
- Derraik, J.G.B. The Pollution of the Marine Environment by Plastic Debris: A Review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef]
- Palsikowski, P.A.; Roberto, M.M.; Sommaggio, L.R.D.; Souza, P.M.S.; Morales, A.R.; Marin-Morales, M.A. Ecotoxicity Evaluation of the Biodegradable Polymers PLA, PBAT and Its Blends Using Allium Cepa as Test Organism. J. Polym. Environ. 2018, 26, 938–945. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green and Sustainable Manufacture of Chemicals from Biomass: State of the Art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Lalanne, L.; Nyanhongo, G.S.; Guebitz, G.M.; Pellis, A. Biotechnological Production and High Potential of Furan-Based Renewable Monomers and Polymers. Biotechnol. Adv. 2021, 48, 107707. [Google Scholar] [CrossRef]
- Cruciani, G.; Pastor, M.; Guba, W. VolSurf: A New Tool for the Pharmacokinetic Optimization of Lead Compounds. Eur. J. Pharm. Sci. 2000, 11, S29–S39. [Google Scholar] [CrossRef]
- Todea, A.; Fortuna, S.; Ebert, C.; Asaro, F.; Tomada, S.; Cespugli, M.; Hollan, F.; Gardossi, L. Rational Guidelines for the Two-Step Scalability of Enzymatic Polycondensation: Experimental and Computational Optimization of the Enzymatic Synthesis of Poly(Glycerolazelate). ChemSusChem 2022, 15, e202102657. [Google Scholar] [CrossRef]
- Pellis, A.; Ferrario, V.; Cespugli, M.; Corici, L.; Guarneri, A.; Zartl, B.; Herrero Acero, E.; Ebert, C.; Guebitz, G.M.; Gardossi, L. Fully Renewable Polyesters: Via Polycondensation Catalyzed by Thermobifida Cellulosilytica Cutinase 1: An Integrated Approach. Green Chem. 2017, 19, 490–502. [Google Scholar] [CrossRef]
- Teixeira, S.; Eblagon, K.M.; Miranda, F.; Pereira, M.F.R.; Figueiredo, J.L. Towards Controlled Degradation of Poly(Lactic) Acid in Technical Applications. J. Carbon Res. 2021, 7, 42. [Google Scholar] [CrossRef]
- Rosato, A.; Romano, A.; Totaro, G.; Celli, A.; Fava, F.; Zanaroli, G.; Sisti, L. Enzymatic Degradation of the Most Common Aliphatic Bio-Polyesters and Evaluation of the Mechanisms Involved: An Extended Study. Polymers 2022, 14, 1850. [Google Scholar] [CrossRef] [PubMed]
- Biodegradation in Water: Screening Tests. Available online: https://echa.europa.eu/it/registration-dossier/-/registered-dossier/15563/5/3/2 (accessed on 3 September 2023).
- Terephthalic Acid Assessment Reports Administrative Data Chemical Safety Report. Available online: https://echa.europa.eu/it/registration-dossier/-/registered-dossier/15563/10 (accessed on 10 September 2023).
- OECD OECD Guidelines for the Testing of Chemicals, Section 3: Degradation and Accumulation. Available online: http://www.oecd-ilibrary.org/ (accessed on 10 September 2023).
- Modaffari, G. Blueing the Coastline. From Heavy Industry to Sport Tourism in the Years Following World War II: The Case of Trieste. Available online: https://rosa.uniroma1.it/rosa03/semestrale_di_geografia/article/view/18302 (accessed on 10 September 2023).
- Solis-Weiss, V.; Aleffi, F.; Bettoso, N.; Rossin, P.; Orel, G.; Fonda-Umani, S. Effects of Industrial and Urban Pollution on the Benthic Macrofauna in the Bay of Muggia (Industrial Port of Trieste, Italy). Sci. Total Environ. 2004, 328, 247–263. [Google Scholar] [CrossRef]
- Negoro, S. Biodegradation of Nylon Oligomers. Appl. Microbiol. Biotechnol. 2000, 54, 461–466. [Google Scholar] [CrossRef]
- Fortuna, S.; Cespugli, M.; Todea, A.; Pellis, A.; Gardossi, L. Criteria for Engineering Cutinases: Bioinformatics Analysis of Catalophores. Catalysts 2021, 11, 784. [Google Scholar] [CrossRef]
- Ferrario, V.; Siragusa, L.; Ebert, C.; Baroni, M.; Foscato, M.; Cruciani, G.; Gardossi, L. BioGPS Descriptors for Rational Engineering of Enzyme Promiscuity and Structure Based Bioinformatic Analysis. PLoS ONE 2014, 9, e109354. [Google Scholar] [CrossRef]
- Crivori, P.; Cruciani, G.; Carrupt, P.A.; Testa, B. Predicting Blood-Brain Barrier Permeation from Three-Dimensional Molecular Structure. J. Med. Chem. 2000, 43, 2204–2216. [Google Scholar] [CrossRef]
- Koukoulitsa, C.; Geromichalos, G.D.; Skaltsa, H. VolSurf Analysis of Pharmacokinetic Properties for Several Antifungal Sesquiterpene Lactones Isolated from Greek Centaurea Sp. J. Comput. Aided. Mol. Des. 2005, 19, 617–623. [Google Scholar] [CrossRef]
- Carosati, E.; Sforna, G.; Pippi, M.; Marverti, G.; Ligabue, A.; Guerrieri, D.; Piras, S.; Guaitoli, G.; Luciani, R.; Costi, M.P.; et al. Ligand-Based Virtual Screening and ADME-Tox Guided Approach to Identify Triazolo-Quinoxalines as Folate Cycle Inhibitors. Bioorganic Med. Chem. 2010, 18, 7773–7785. [Google Scholar] [CrossRef]
- Goodford, P.J. A Computational Procedure for Determining Energetically Favorable Binding Sites on Biologically Important Macromolecules. J. Med. Chem. 1985, 28, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Cruciani, G.; Crivori, P.; Carrupt, P.A.; Testa, B. Molecular Fields in Quantitative Structure-Permeation Relationships: The VolSurf Approach. J. Mol. Struct. THEOCHEM 2000, 503, 17–30. [Google Scholar] [CrossRef]
- Tortorella, S.; Carosati, E.; Sorbi, G.; Bocci, G.; Cross, S.; Cruciani, G.; Storchi, L. Combining Machine Learning and Quantum Mechanics Yields More Chemically Aware Molecular Descriptors for Medicinal Chemistry Applications. J. Comput. Chem. 2021, 42, 2068–2078. [Google Scholar] [CrossRef] [PubMed]
- VolSurf3. Available online: https://www.molhorizon.it/software/volsurf3/ (accessed on 16 March 2024).
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-Learn: Machine Learning in Python Fabian. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-Regression: A Basic Tool of Chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- García, J.I.; Pires, E.; Aldea, L.; Lomba, L.; Perales, E.; Giner, B. Ecotoxicity Studies of Glycerol Ethers in Vibrio Fischeri: Checking the Environmental Impact of Glycerol-Derived Solvents. Green Chem. 2015, 17, 4326–4333. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; De Morais, P.; Coelho, J.A.S.; Sintra, T.; Coutinho, J.A.P.; Afonso, C.A.M. Evaluating the Toxicity of Biomass Derived Platform Chemicals. Green Chem. 2016, 18, 4733–4742. [Google Scholar] [CrossRef]
- UNI EN ISO 10253:2017; Water Quality—Marine Algal Growth Inhibition Test with Skeletonema sp. and Phaeodactylum Tricornutum, Ente Nazionale Italiano di Unificazione (UNI). 2017. Available online: https://www.intertekinform.com/en-us/standards/UNI-EN-ISO-10253-2017-1078918_SAIG_UNI_UNI_2736771/ (accessed on 10 May 2023).
- UNI EN ISO 6341:2013; Water Quality—Determination of the Inhibition of the Mobility of Daphnia Magna Straus (Cladocera, Crustacea)—Acute Toxicity Test, Ente Nazionale Italiano di Unificazione (UNI). 2013. Available online: https://shop.standards.ie/en-ie/standards/uni-en-iso-6341-2013-1100003_saig_uni_uni_2558523/ (accessed on 10 May 2023).
- European Chemicals Agency. Available online: https://echa.europa.eu/it/home (accessed on 10 September 2023).
- ISO 8692:2012; Water quality Fresh water algal growth inhibition test with unicellular green algae, International Standard. 2012. Available online: https://www.iso.org/standard/54150.html (accessed on 10 May 2023).
- Guarneri, A.; Cutifani, V.; Cespugli, M.; Pellis, A.; Vassallo, R.; Asaro, F.; Ebert, C.; Gardossi, L. Functionalization of Enzymatically Synthesized Rigid Poly(Itaconate)s via Post-Polymerization Aza-Michael Addition of Primary Amines. Adv. Synth. Catal. 2019, 361, 2559–2573. [Google Scholar] [CrossRef]
- Hunt, S.M.; Binns, M.R.; Sheil, M.M. Structural Characterization of Polyester Resins by Electrospray Mass Spectrometry. J. Appl. Polym. Sci. 1995, 56, 1589–1597. [Google Scholar] [CrossRef]
- UNI EN ISO 11348-3:2019; Water Quality—Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio Fischeri (Luminescent Bacteria Test), Ente Italiano di Normazione. 2019. Available online: https://store.uni.com/en/uni-en-iso-11348-3-2019, (accessed on 10 May 2023).
- EPA/600/R-95-136/Sezione 16 + ISPRA Quaderni Ricerca Marina 11/2017. Available online: https://www.bsrc.it/analisi-accreditate/ (accessed on 10 May 2023).













| Sample | Solvent | Enzyme | Monomer Conversion (%) * | Mn ** [g/mol] | Mw ** [g/mol] | Đ | ||
|---|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | ||||||
| Co-oligomers | ||||||||
| BDO-FDCA | Toluene | Calbcov | 97 | 84 | - | 635 | 742 | 1.16 |
| BDO-TA | t-BuOH | N435 | 90 | 65 | - | 694 | 838 | 1.20 |
| BDO-AA * | - | Calbcov | 95 | 94 | - | 683 | 729 | 1.06 |
| GLY-FDCA | t-BuOH | Calbcov | 96 | 87 | - | 551 | 936 | 1.69 |
| GLY-TA | t-BuOH | N435 | 98 | 88 | - | 960 | 1169 | 1.21 |
| GLY-AA | - | Calbcov | 92 | 96 | - | 912 | 940 | 1.03 |
| Ter-oligomers | ||||||||
| BDO FDCA AA | Toluene | Calbcov | 81 | 63 | 91 | 844 | 901 | 1.06 |
| BDO TA AA | Toluene | N435 | 72 | 94 | 99 | 615 | 747 | 1.21 |
| GLY FDCA AA | t-BuOH | Calbcov | 86 | 67 | 49 | 481 | 551 | 1.25 |
| GLY TA AA | t-BuOH | N435 | 94 | 97 | 96 | 582 | 675 | 1.15 |
| GLY ERY AA | - | Calbcov | 88 | 81 | 94 | 845 | 1140 | 1.35 |
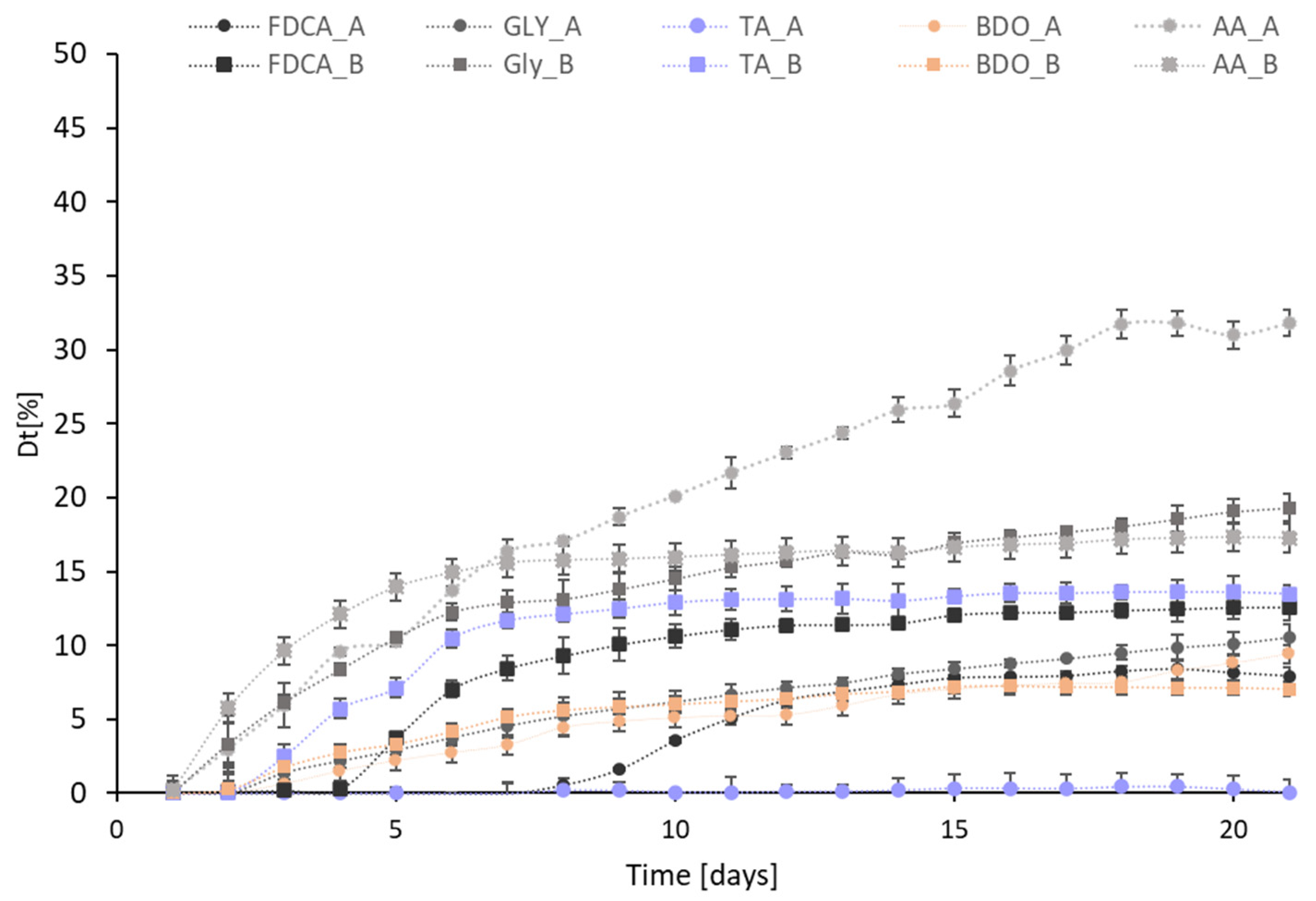
| Sample | ThOD [mg/mg] | BOD5 [mg∙L−1] | BOD10 [mg∙L−1] | BOD21 [mg∙L−1] | Dt5 [%] | Dt10 [%] | Dt21 [%] |
|---|---|---|---|---|---|---|---|
| Co-oligomers | |||||||
| BDO-FDCA | 2.24 | 9.45 | 21.20 | 34.10 | 4.22 | 9.46 | 15.22 |
| BDO-TA | 2.19 | 35.40 | 65.80 | 83.05 | 16.16 | 30.05 | 37.92 |
| BDO-AA * | 1.32 | 25.95 | 45.40 | 66.30 | 19.66 | 34.20 | 49.95 |
| GLY-FDCA | 1.59 | 14.05 | 18.90 | 29.20 | 8.84 | 11.89 | 18.36 |
| GLY-TA | 1.30 | 27.30 | 40.45 | 65.15 | 21.00 | 34.39 | 50.23 |
| GLY-AA | 1.05 | 11.70 | 24.85 | 49.25 | 11.14 | 23.67 | 46.90 |
| Mixtures of ter-oligomers | |||||||
| BDO FDCA AA | 2.40 | 29.90 | 40.35 | 69.60 | 12.46 | 16.81 | 29.00 |
| BDO TA AA | 1.43 | 25.90 | 33.75 | 53.60 | 18.11 | 23.60 | 37.48 |
| GLY FDCA AA | 2.29 | 22.75 | 36.25 | 51.65 | 9.93 | 15.83 | 22.55 |
| GLY TA AA | 2.64 | 23.35 | 35.10 | 51.15 | 8.84 | 13.30 | 19.38 |
| GLY ERY AA | 1.05 | 9.20 | 17.50 | 32.00 | 8.76 | 16.67 | 30.48 |
| Product | Tg [°C] | Tm [°C] | ΔHμ [ϑ/γ] | Tc [°C] | Physical State | Mn [g/mol] | Đ | Dt21 [%] |
|---|---|---|---|---|---|---|---|---|
| Co-oligomers | ||||||||
| GLY-TA | −54.6 | 109.6 | 14.66 | 90 | S | 960 | 1.21 | 50.23 |
| BDO-AA | n.o. | 45.3 | 74.27 | 26.9 | S | 683 | 1.06 | 49.95 |
| GLY-AA | −41.3 | n.d. | n.d. | amorph | L | 887 | 1.41 | 46.90 |
| BDO-TA | −55.9 | 89.4 | 10.63 | 79.7 | S | 694 | 1.20 | 37.92 |
| GLY-FDCA | n.o. | 105.2 | 93.49 | 86.7 | S | 551 | 1.69 | 18.36 |
| BDO-FDCA | −6.6 | 92.9 | 7.66 | amorph | S | 635 | 1.16 | 15.22 |
| Sample | Inoculum | ThOD [mg/mg] | BOD5 [mg∙L−1] | BOD10 [mg∙L−1] | BOD21 [mg∙L−1] | Dt5 [%] | Dt10 [%] | Dt21 [%] |
|---|---|---|---|---|---|---|---|---|
| FDCA | Trieste | 4.34 | 5.05 | 12.90 | 23.50 | 1.16 | 2.82 | 6.83 |
| FDCA | Zaule | 4.34 | 16.35 | 46.15 | 53.65 | 3.77 | 10.63 | 12.59 |
| TA | Trieste | 7.60 | 0.00 | 1.25 | 2.15 | 0.00 | 0.08 | 0.08 |
| TA | Zaule | 7.60 | 54.25 | 98.15 | 102.6 | 7.14 | 12.91 | 13.50 |
| AA | Trieste | 6.38 | 65.77 | 128.36 | 200.65 | 10.31 | 20.12 | 31.8 |
| AA | Zaule | 6.38 | 89.00 | 101.85 | 110.15 | 13.95 | 15.96 | 17.26 |
| BDO | Trieste | 13.62 | 30.10 | 69.50 | 128.90 | 2.21 | 5.10 | 9.43 |
| BDO | Zaule | 13.62 | 45.65 | 82.05 | 96.10 | 3.35 | 6.02 | 7.06 |
| GLY | Trieste | 5.30 | 15.50 | 32.80 | 55.95 | 2.92 | 6.18 | 10.51 |
| GLY | Zaule | 5.30 | 55.80 | 76.95 | 102.45 | 10.53 | 14.52 | 19.33 |
| ERY | Trieste | 3.98 | 11.95 | 24.65 | 44.85 | 2.99 | 6.55 | 14.22 |
| DMT | Trieste | 7.69 | 1.10 | 18.60 | 48.30 | 0.14 | 2.28 | 6.15 |
| DMF | Trieste | 5.48 | 11.9 | 18.35 | 24.25 | 2.17 | 3.23 | 5.28 |
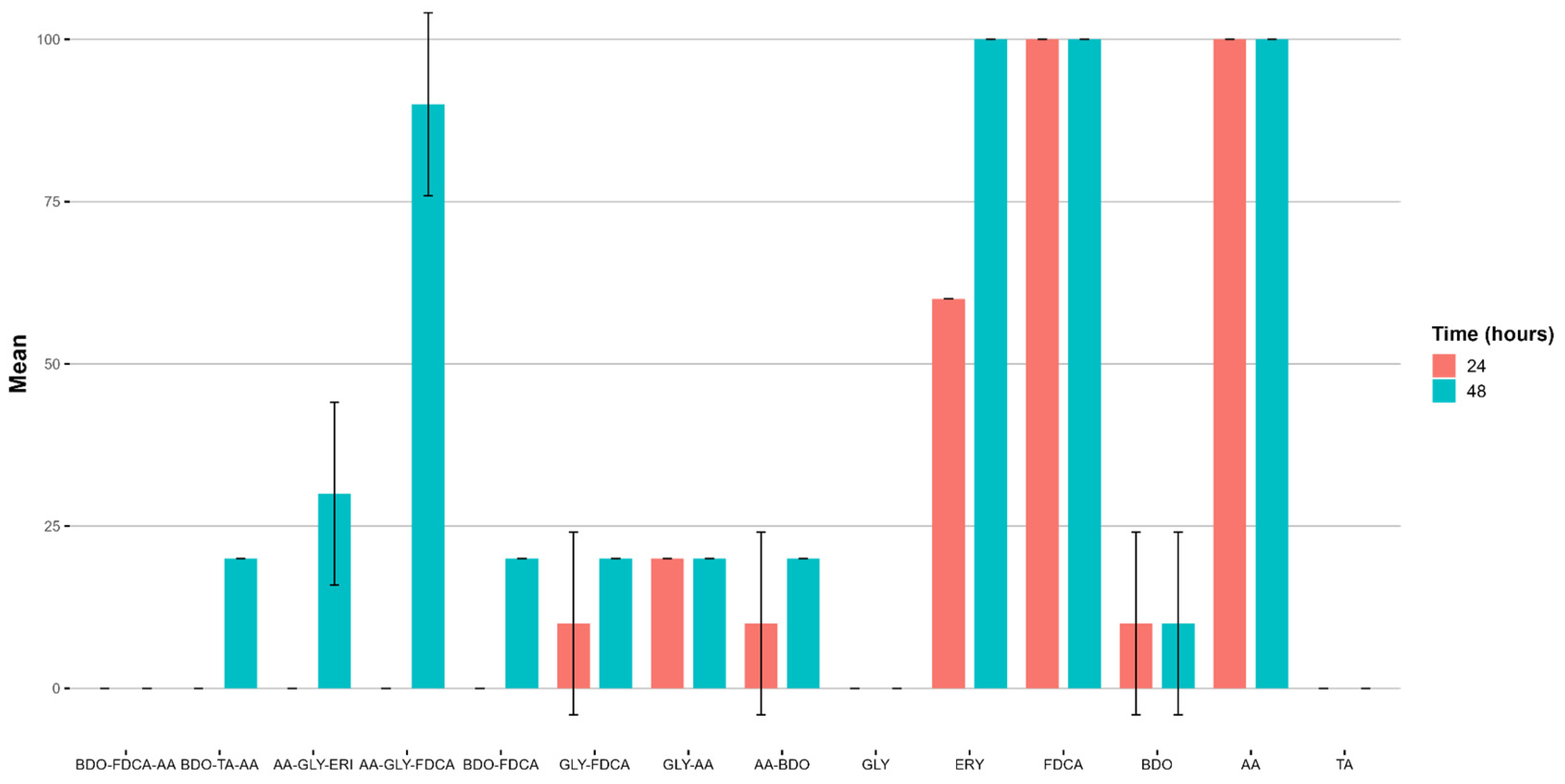
| No | Sample | BOD5 [mg∙L−1] | Dt5 [%] | BOD10 [mg∙L−1] | Dt10 [%] | BOD21 [mg∙L−1] | Dt21 [%] |
|---|---|---|---|---|---|---|---|
| 1 | DAF | 0.01 | 0.10 | 0.05 | 0.36 | 0.05 | 0.37 |
| 2 | DAF-AA | 0.01 | 0.41 | 0.05 | 1.44 | 0.05 | 1.59 |
| Objects | SMILES Codes of the Tetramers | Biodegradation [%] |
|---|---|---|
| AA-BDO-AA-BDO | C(CCCO)OC(=O)CCCCC(=O)OCCCCOC(=O)CCCCC(=O)O | 50.23 |
| AA-DAF-AA-DAF | NCc1ccc(o1)CNC(=O)CCCCC(=O)NCc1ccc(o1)CNC(=O)CCCCC(=O)N | 1.59 |
| AA-GLY-AA-GLY | C(C(O)CO)OC(=O)CCCCC(=O)OCC(O)COC(=O)CCCCC(=O)O | 46.9 |
| AA-GLY-AA-ERY | OCC(O)C(O)COC(=O)CCCCC(=O)OCC(O)COC(=O)CCCCC(=O)O | 30.9 |
| BDO-FDCA-BDO-AA | O=C(CCCCC(=O)O)OCCCCOC(=O)c1ccc(o1)C(=O)OCCCCO | 26.81 |
| BDO-FDCA-BDO-FDCA | OCCCCOC(=O)c1ccc(o1)C(=O)OCCCCOC(=O)c1ccc(o1)C(=O)O | 15.22 |
| BDO-TA-BDO-TA | OCCCCOC(=O)c1ccc(cc1)C(=O)OCCCCOC(=O)c1ccc(cc1)C(=O)O | 37.92 |
| FDCA-GLY-AA-GLY | OC(COC(=O)c1ccc(o1)C(=O)O)COC(=O)CCCCC(=O)OCC(O)CO | 22.55 |
| FDCA-GLY-FDCA-GLY | OCC(O)COC(=O)c1ccc(o1)C(=O)OCC(O)COC(=O)c1ccc(o1)C(=O)O | 18.36 |
| GLY-TA-GLY-TA | OCC(COC(=O)c1ccc(cc1)C(=O)OCC(COC(=O)c1ccc(cc1)C(=O)O)O)O | 50.12 |
| TA-BDO-AA-BDO | O=C(CCCCC(=O)O)OCCCCOC(=O)c1ccc(cc1)C(=O)OCCCCO | 37.48 |
| TA-GLY-AA-GLY | OC(COC(=O)c1ccc(cc1)C(=O)O)COC(=O)CCCCC(=O)OCC(O)CO | 19.38 |
| Aliivibrio fischeri | Phaeodactylum tricornutum | Paracentrotus lividus | ||||
|---|---|---|---|---|---|---|
| Samples | EC50 | EC20 | EC50 | EC20 | EC50 | EC20 |
| BDO-FDCA-AA | >100 | 95 (15 min); 98 (30 min) | >100 | >100 | 74.0 | 46.3 |
| BDO-TA-AA | 71 (15 min); 75 (30 min) | 32 (15 min); 40 (30 min) | >100 | >100 | 64.5 | 26.4 |
| AA-GLY-ERY | >100 | >100 | >100 | >100 | >100 | 82.7 |
| AA-GLY-FDCA | >100 | >100 | >100 | >100 | >100 | >100 |
| BDO-FDCA | >100 | >100 | >100 | >100 | 66.6 | 32.3 |
| GLY-FDCA | >100 | >100 | >100 | >100 | 74.3 | 35.3 |
| GLY-AA | >100 | >100 | >100 | >100 | 87.3 | 73.4 |
| AA-BDO | >100 | >100 | >100 | >100 | 87.3 | 73.4 |
| GLY | >100 | >100 | >100 | >100 | 100.0 | 83.0 |
| ERY | >100 | >100 | >100 | >100 | >100 | >100 |
| FDCA | >100 | >100 | >100 | >100 | >100 | 95.9 |
| BDO | >100 | >100 | >100 | >100 | 92.9 | 78.2 |
| AA | >100 | >100 | >100 | >100 | >100 | 90.9 |
| TA | >100 | >100 | >100 | >100 | >100 | >100 |
| Sample | Daphnia magna | Raphidocelis subcapitata | ||
|---|---|---|---|---|
| EC50 | EC20 | EC50 | EC20 | |
| BDO-FDCA-AA | >100 | >100 | >100 | >100 |
| BDO-TA-AA | >100 | 100 (48 h) | >100 | >100 |
| AA-GLY-ERY | >100 | 40.9 (48 h) | >100 | >100 |
| AA-GLY-FDCA | 56.5 (48 h) | 27.9 (48 h) | >100 | >100 |
| BDO-FDCA | >100 | 100 (48 h) | >100 | >100 |
| GLY-FDCA | >100 | 100 (48 h) | >100 | >100 |
| GLY-AA | >100 | 100 (24–48 h) | >100 | >100 |
| AA-BDO | >100 | 100 (48 h) | >100 | 95.6 |
| GLY | >100 | >100 | >100 | >100 |
| ERY | 72.7 (24 h) | <100 | >100 | >100 |
| FDCA | >100 | <100 | >100 | 53.0 |
| BDO | >100 | >100 | >100 | >100 |
| AA | 32.5 (48 h) | 17.9 (48 h) | >100 | 97.3 |
| TA | >100 | >100 | >100 | >100 |
| Organism | Biological Community | Endpoint | Analytical Method |
|---|---|---|---|
| Freshwater | |||
| Daphnia magna | Crustacean | Motility | UNI EN ISO 6341:2012 Water quality—Determination of the inhibition of the mobility of Daphnia magna Straus (Cladocera, Crustacea) [61]. |
| Raphidocelis subcapitata | Algae | Growth | UNI EN ISO 8692 (2012). Water quality—Freshwater algal growth inhibition test with unicellular green algae [62]. |
| Seawater | |||
| Aliivibrio fischeri | Bacteria | Light Emission | UNI EN ISO 11348-3 (2019). Water quality—Determination of the inhibitory effect of water samples on the light emission of Vibrio fischeri (Luminescent bacteria test)—Part 3: Method using freeze-dried bacteria [66]. |
| Phaeodactilum tricornutum | Algae | Growth | UNI UN ISO 10253 (2016) Water quality—Marine algal growth inhibition test with Skeletonema costatum and Phaeodactylum tricorinutum [60] |
| Paracentrotus lividus | Echinoidea | Embryos | EPA/600/R-95-136/Sezione 16 + ISPRA Quaderni Ricerca Marina 11/2017 [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todea, A.; Bîtcan, I.; Giannetto, M.; Rădoi, I.I.; Bruschi, R.; Renzi, M.; Anselmi, S.; Provenza, F.; Bentivoglio, T.; Asaro, F.; et al. Enzymatic Synthesis and Structural Modeling of Bio-Based Oligoesters as an Approach for the Fast Screening of Marine Biodegradation and Ecotoxicity. Int. J. Mol. Sci. 2024, 25, 5433. https://doi.org/10.3390/ijms25105433
Todea A, Bîtcan I, Giannetto M, Rădoi II, Bruschi R, Renzi M, Anselmi S, Provenza F, Bentivoglio T, Asaro F, et al. Enzymatic Synthesis and Structural Modeling of Bio-Based Oligoesters as an Approach for the Fast Screening of Marine Biodegradation and Ecotoxicity. International Journal of Molecular Sciences. 2024; 25(10):5433. https://doi.org/10.3390/ijms25105433
Chicago/Turabian StyleTodea, Anamaria, Ioan Bîtcan, Marco Giannetto, Iulia Ioana Rădoi, Raffaele Bruschi, Monia Renzi, Serena Anselmi, Francesca Provenza, Tecla Bentivoglio, Fioretta Asaro, and et al. 2024. "Enzymatic Synthesis and Structural Modeling of Bio-Based Oligoesters as an Approach for the Fast Screening of Marine Biodegradation and Ecotoxicity" International Journal of Molecular Sciences 25, no. 10: 5433. https://doi.org/10.3390/ijms25105433






