Spectroscopic Techniques and Hydrogen-Sensitive Compounds: A New Horizon in Hydrogen Detection
Abstract
:1. Introduction
2. Materials
2.1. Metal Oxide (Metal Attached)
2.2. Metal
2.3. Other Materials and Structures
3. Technical Analysis
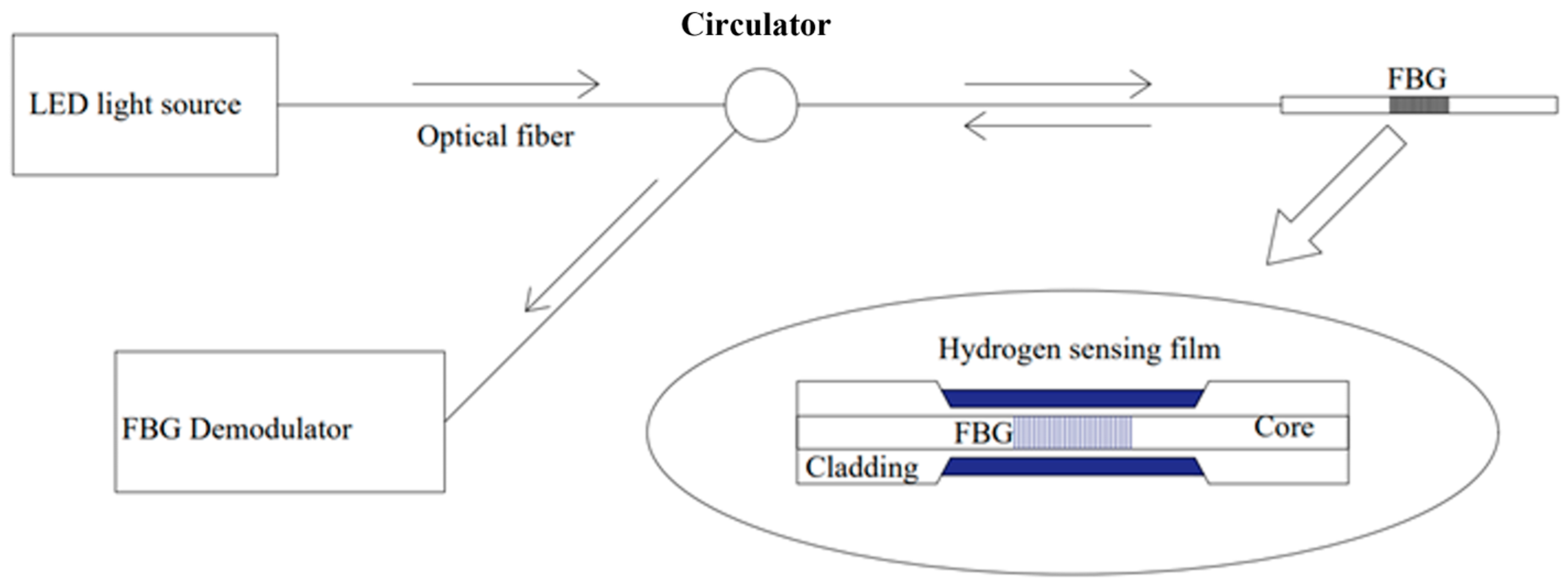
3.1. Fiber Bragg Grating (FBG)
3.2. Interferometric Hydrogen Measurement
3.2.1. Mach–Zehnder Interferometer (MZI)
3.2.2. Fabry–Perot Interferometer (FPI)
3.3. MultiFBG Test System
3.4. FBG with FPI
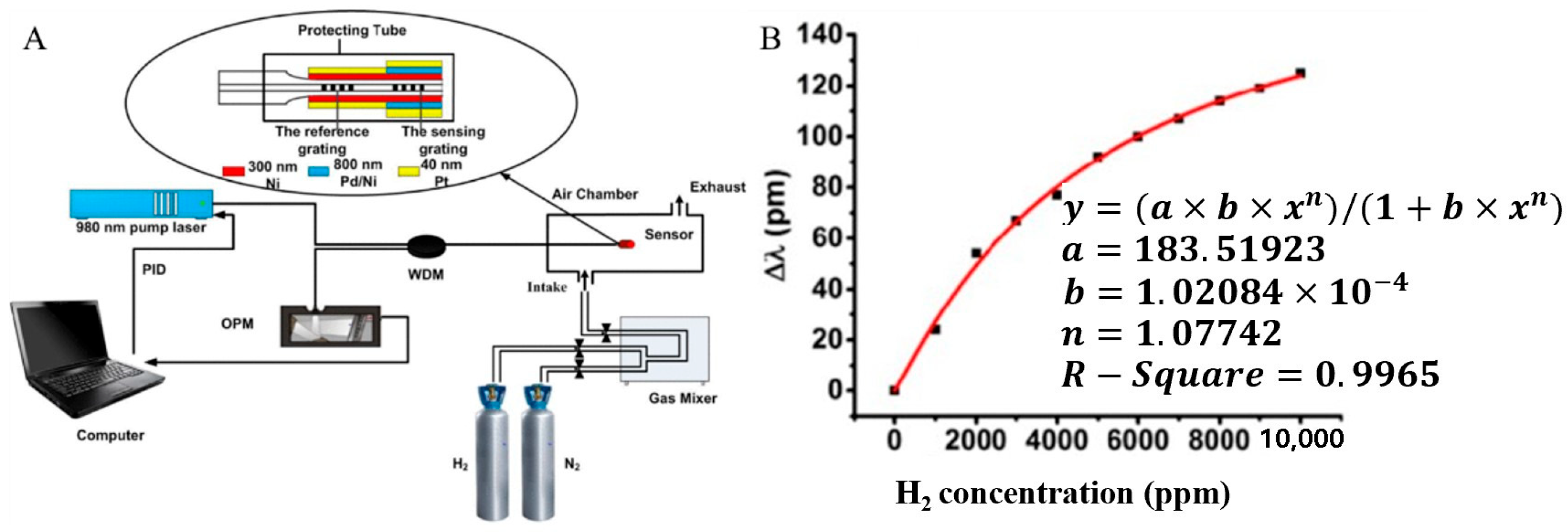
3.5. Mirror H2 Sensor
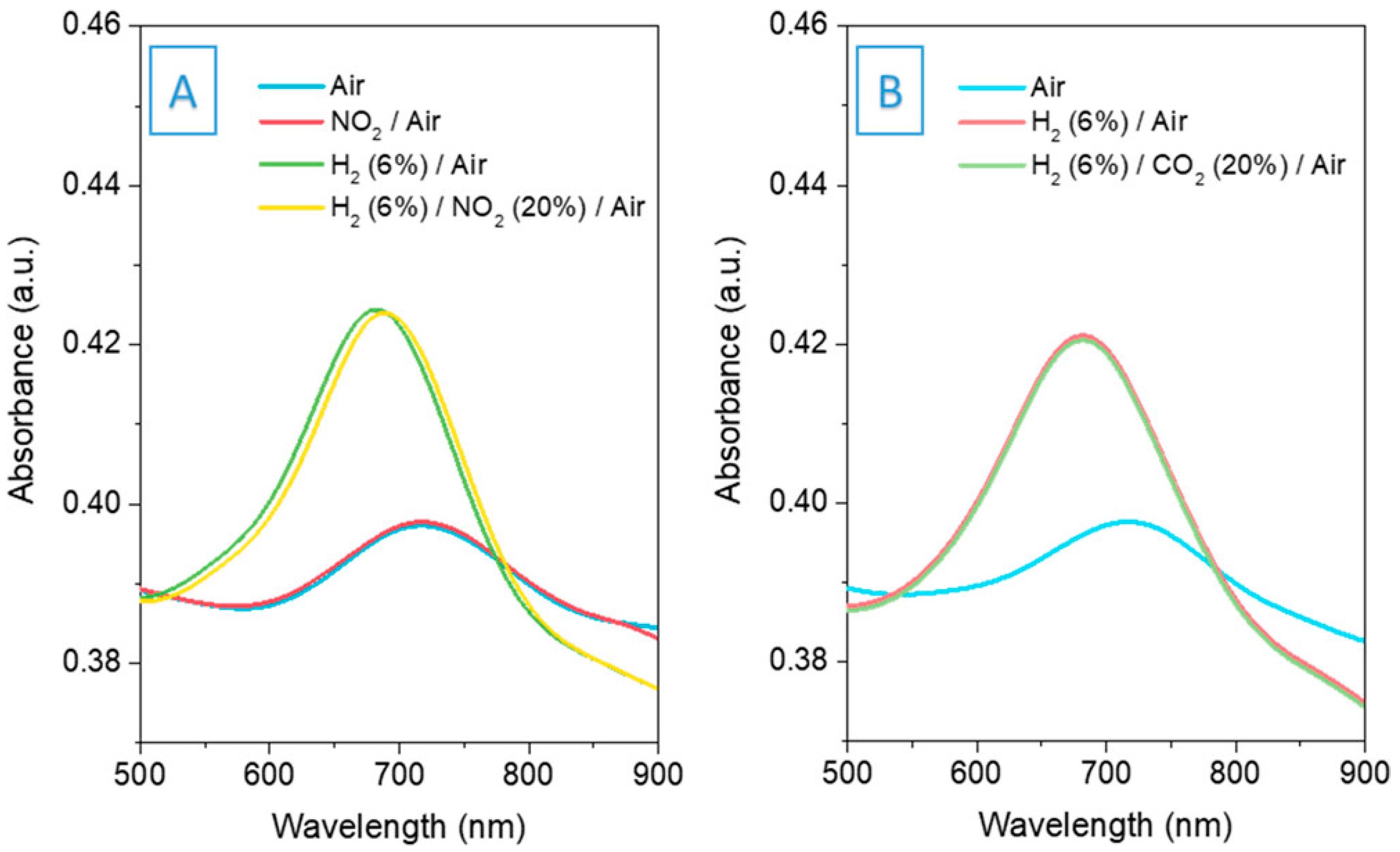
3.6. Surface Plasmon Resonance (SPR) Sensor
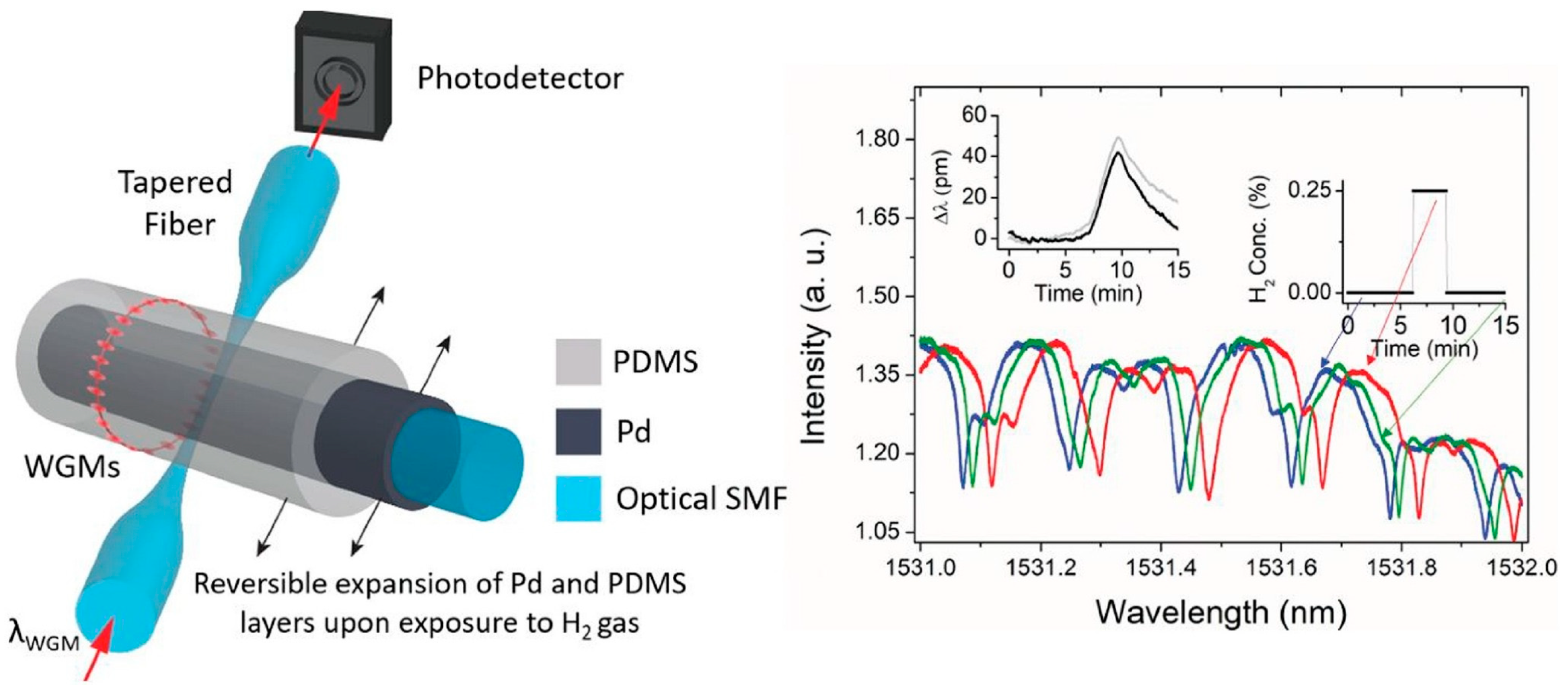
3.7. Optical Fiber Evanescent
3.8. Sensor Structure
3.8.1. Structure of Fiber Sensor
3.8.2. Cantilever Sensor
3.8.3. Multiple-Layer Film Sensor
4. Sensor Fabrication
5. Specific Environmental Hydrogen Measurement
5.1. Temperature Reference
5.2. Special Environment Test
6. Challenges
6.1. Limit of Detection (LOD)
6.2. Adsorption and Resolution Time
7. Conclusions and Outlook
7.1. Conclusions
7.2. Outlook
7.2.1. More Reliable Hydrogen-Sensitive Materials
7.2.2. Telemetry/Multifunction
7.2.3. Concentration Test
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jeon, J.; Kim, S.J. Recent Progress in Hydrogen Flammability Prediction for the Safe Energy Systems. Energies 2020, 13, 6263. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Peng, H.; Qian, X.; Zhang, Y.; An, G.; Zhao, Y. Recent advancements in optical fiber hydrogen sensors. Sens. Actuators B Chem. 2017, 244, 393–416. [Google Scholar] [CrossRef]
- Yan, G.; Zhang, A.P.; Ma, G.; Wang, B.; Kim, B.; Im, J.; He, S.; Chung, Y. Fiber-Optic Acetylene Gas Sensor Based on Microstructured Optical Fiber Bragg Gratings. IEEE Photonics Technol. Lett. 2011, 23, 1588–1590. [Google Scholar] [CrossRef]
- Luo, J.; Liu, S.; Chen, P.; Lu, S.; Zhang, Q.; Chen, Y.; Du, B.; Tang, J.; He, J.; Liao, C.; et al. Fiber optic hydrogen sensor based on a Fabry–Perot interferometer with a fiber Bragg grating and a nanofilm. Lab Chip 2021, 21, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Caucheteur, C.; Debliquy, M.; Lahem, D.; Megret, P. Catalytic Fiber Bragg Grating Sensor for Hydrogen Leak Detection in Air. IEEE Photonics Technol. Lett. 2008, 20, 96–98. [Google Scholar] [CrossRef]
- Qiu, H.; Fu, M.; Yang, Y. Fiber optic sensing technologies potentially applicable for hypersonic wind tunnel harsh environments. Adv. Aerodyn. 2020, 2, 10. [Google Scholar] [CrossRef]
- Yang, S.; Wang, G.; Xiang, F.; Qin, Y.; Dai, J.; Yang, M. Pt nanoparticles encapsulated in mesoporous tungsten oxide to enhance the repeatability of a FBG hydrogen sensor. Opt. Mater. Express 2018, 8, 1493. [Google Scholar] [CrossRef]
- Yan, Q.; Tao, S.; Toghiani, H. Optical fiber evanescent wave absorption spectrometry of nanocrystalline tin oxide thin films for selective hydrogen sensing in high temperature gas samples. Talanta 2009, 77, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Dai, J.; Yang, M. Fiber-Optic Hydrogen Sensors: A Review. IEEE Sens. J. 2021, 21, 12706–12718. [Google Scholar] [CrossRef]
- Wang, G.; Qin, Y.; Dai, J.; Yang, S.; Ma, Y.; Zou, T.; Roths, J.; Yang, M. Performance-enhanced optical fiber hydrogen sensors based on WO3-Pd2Pt-Pt composite film with controlled optical heating. Opt. Fiber Technol. 2019, 52, 101979. [Google Scholar] [CrossRef]
- Cao, R.; Wu, J.; Liang, G.; Ohodnicki, P.R.; Chen, K.P. Functionalized PdAu Alloy on Nanocones Fabricated on Optical Fibers for Hydrogen Sensing. IEEE Sens. J. 2020, 20, 1922–1927. [Google Scholar] [CrossRef]
- Yu, X.; Cheng, H.; Zhang, M.; Zhao, Y.; Qu, L.; Shi, G. Graphene-based smart materials. Nat. Rev. Mater. 2017, 2, 17046. [Google Scholar] [CrossRef]
- Alkhabet, M.M.; Yaseen, Z.M.; Eldirderi, M.M.A.; Khedher, K.M.; Jawad, A.H.; Girei, S.H.; Salih, H.K.; Paiman, S.; Arsad, N.; Mahdi, M.A.; et al. Palladium/Graphene Oxide Nanocomposite for Hydrogen Gas Sensing Applications Based on Tapered Optical Fiber. Materials 2022, 15, 8167. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhao, X.; Zhang, C.; Li, Q.-Z.; Cao, J.; Han, D.-F.; Hao, H.; Wang, M. A fast response hydrogen sensor with Pd metallic grating onto a fiber’s end-face. Opt. Commun. 2016, 359, 157–161. [Google Scholar] [CrossRef]
- Du, B.; He, J.; Yang, M.; Wang, Y.; Xu, X.; Wang, J.; Zhang, Z.; Zhang, F.; Guo, K.; Wang, Y. Highly sensitive hydrogen sensor based on an in-fiber Mach-Zehnder interferometer with polymer infiltration and Pt-loaded WO3 coating. Opt. Express 2021, 29, 4147–4158. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xu, B.; Xu, X.; Liao, C.; Wang, Y. Review of Femtosecond-Laser-Inscribed Fiber Bragg Gratings: Fabrication Technologies and Sensing Applications. Photonic Sens. 2021, 11, 203–226. [Google Scholar] [CrossRef]
- Huang, F.; Chen, T.; Si, J.; Pham, X.; Hou, X. Fiber laser based on a fiber Bragg grating and its application in high-temperature sensing. Opt. Commun. 2019, 452, 233–237. [Google Scholar] [CrossRef]
- Sutapun, B.; Tabib-Azar, M.; Kazemi, A. Pd-coated elastooptic fiber optic Bragg grating sensors for multiplexed hydrogen sensing. Sens. Actuators B Chem. 1999, 60, 27–34. [Google Scholar] [CrossRef]
- Caucheteur, C.; Debliquy, M.; Lahem, D.; Mégret, P. Hydrogen Sensor Using Fiber Gratings Covered by a Catalytic Sensitive Layer; SPIE: Bellingham, WA, USA, 2007; Volume 6593. [Google Scholar]
- Zhang, C.; Shen, C.; Liu, X.; Liu, S.; Chen, H.; Huang, Z.; Wang, Z.; Lang, T.; Zhao, C.; Zhang, Y. Pd/Au nanofilms based tilted fiber Bragg grating hydrogen sensor. Opt. Commun. 2022, 502, 127424. [Google Scholar] [CrossRef]
- Shen, C.; Xie, Z.; Huang, Z.; Yan, S.; Sui, W.; Zhou, J.; Wang, Z.; Han, W.; Zeng, X. Review of the Status and Prospects of Fiber Optic Hydrogen Sensing Technology. Chemosensors 2023, 11, 473. [Google Scholar] [CrossRef]
- Butler, M.A. Optical fiber hydrogen sensor. Appl. Phys. Lett. 1984, 45, 1007–1009. [Google Scholar] [CrossRef]
- Gu, F.; Wu, G.; Zeng, H. Hybrid photon–plasmon Mach–Zehnder interferometers for highly sensitive hydrogen sensing. Nanoscale 2015, 7, 924–929. [Google Scholar] [CrossRef]
- Zeakes, J.S.; Murphy, K.A.; Elshabini-Riad, A.; Claus, R.O. Modified extrinsic Fabry-Perot interferometric hydrogen gas sensor. In Proceedings of the LEOS’94, Boston, MA, USA, 31 October–3 November 1994. [Google Scholar]
- Li, Y.; Zhao, C.; Xu, B.; Wang, D.; Yang, M. Optical cascaded Fabry–Perot interferometer hydrogen sensor based on vernier effect. Opt. Commun. 2018, 414, 166–171. [Google Scholar] [CrossRef]
- Li, Y.; Shen, W.; Zhao, C.; Xu, B.; Wang, D.; Yang, M. Optical hydrogen sensor based on PDMS-formed double-C type cavities with embedded Pt-loaded WO3/SiO2. Sens. Actuators B Chem. 2018, 276, 23–30. [Google Scholar] [CrossRef]
- Dai, J.; Peng, W.; Wang, G.; Xiang, F.; Qin, Y.; Wang, M.; Dai, Y.; Yang, M.; Deng, H.; Zhang, P. Ultra-high sensitive optical fiber hydrogen sensor using self-referenced demodulation method and WO3-Pd2Pt-Pt composite film. Opt. Express 2017, 25, 2009–2015. [Google Scholar] [CrossRef] [PubMed]
- Slaman, M.; Dam, B.; Pasturel, M.; Borsa, D.M.; Schreuders, H.; Rector, J.H.; Griessen, R. Fiber optic hydrogen detectors containing Mg-based metal hydrides. Sens. Actuators B Chem. 2007, 123, 538–545. [Google Scholar] [CrossRef]
- Butler, M.A.; Buss, R.J. Kinetics of the micromirror chemical sensor. Sens. Actuators B Chem. 1993, 11, 161–166. [Google Scholar] [CrossRef]
- Dai, J.; Li, Y.; Ruan, H.; Ye, Z.; Chai, N.; Wang, X.; Qiu, S.; Bai, W.; Yang, M. Fiber Optical Hydrogen Sensor Based on WO3-Pd2Pt-Pt Nanocomposite Films. Nanomaterials 2021, 11, 128. [Google Scholar] [CrossRef]
- Bévenot, X.; Trouillet, A.; Veillas, C.; Gagnaire, H.; Clément, M. Hydrogen Leak Detection Using an Optical Fibre Sensor for Aerospace Applications. Sens. Actuators B-Chem. 2000, 67, 57–67. [Google Scholar] [CrossRef]
- Lin, K.; Lu, Y.; Chen, J.; Zheng, R.; Wang, P.; Ming, H. Surface plasmon resonance hydrogen sensor based on metallic grating with high sensitivity. Opt. Express 2008, 16, 18599–18604. [Google Scholar] [CrossRef]
- Nylander, C.; Liedberg, B.; Lind, T. Gas detection by means of surface plasmon resonance. Sens. Actuators 1982, 3, 79–88. [Google Scholar] [CrossRef]
- Benson, D.; Tracy, C.E.; Hishmeh, G.; Ciszek, P.; Lee, S.-H.; Haberman, D. Low-Cost Fiber Optic Hydrogen Gas Detector Using Guided-Wave Surface-Plasmon Resonance in Chemochromic Thin Films; Photonics East (ISAM, VVDC, IEMB); SPIE: Bellingham, WA, USA, 1999; Volume 3535. [Google Scholar]
- Ohodnicki, P.R.; Baltrus, J.P.; Brown, T.D. Pd/SiO2 and AuPd/SiO2 nanocomposite-based optical fiber sensors for H2 sensing applications. Sens. Actuators B Chem. 2015, 214, 159–168. [Google Scholar] [CrossRef]
- Kim, H.-M.; Kim, H.-J.; Yang, S.-C.; Park, J.-H.; Lee, S.-K. Fiber optic localized surface plasmon resonance hydrogen sensor based on gold nanoparticles capped with palladium. J. Ind. Eng. Chem. 2022, 111, 281–288. [Google Scholar] [CrossRef]
- Tabib-Azar, M.; Sutapun, B.; Glawe, D. Fiber optic MEMS pressure sensors based on evanescent field interaction. In Optoelectronics and High-Power Lasers and Applications; SPIE: Bellingham, WA, USA, 1998; Volume 3276. [Google Scholar]
- Tabib-Azar, M.; Sutapun, B.; Petrick, R.; Kazemi, A. Highly sensitive hydrogen sensors using palladium-coated fiber optics with exposed cores and evanescent field interactions. In Micromachining and Microfabrication; SPIE: Bellingham, WA, USA, 1998; Volume 3513. [Google Scholar]
- Zhou, X.; Dai, Y.; Karanja, J.M.; Liu, F.; Yang, M. Microstructured FBG hydrogen sensor based on Pt-loaded WO3. Opt. Express 2017, 25, 8777–8786. [Google Scholar] [CrossRef]
- Karanja, J.M.; Dai, Y.; Zhou, X.; Liu, B.; Yang, M. Micro-structured femtosecond laser assisted FBG hydrogen sensor. Opt. Express 2015, 23, 31034–31042. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Tao, S. Sol-Gel Synthesis of Palladium-Doped Silica Nanocomposite Fiber Using Triton X-100 Micelle Template and the Application for Hydrogen Gas Sensing. IEEE Sens. J. 2007, 7, 323–328. [Google Scholar] [CrossRef]
- Liu, R.X.; Zhang, X.; Zhu, X.S.; Shi, Y.W. Optical Fiber Hydrogen Sensor Based on the EVA/Pd Coated Hollow Fiber. IEEE Photonics J. 2022, 14, 6825806. [Google Scholar] [CrossRef]
- Xiong, C.; Zhou, J.; Liao, C.; Zhu, M.; Wang, Y.; Liu, S.; Li, C.; Zhang, Y.; Zhao, Y.; Gan, Z.; et al. Fiber-Tip Polymer Microcantilever for Fast and Highly Sensitive Hydrogen Measurement. ACS Appl. Mater. Interfaces 2020, 12, 33163–33172. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y. Enhanced sensitivity of transmission based optical fiber hydrogen sensor with multi-layer Pd–Y alloy thin film. Sens. Actuators B Chem. 2016, 227, 178–184. [Google Scholar] [CrossRef]
- Xiang, F.; Wang, G.; Qin, Y.; Yang, S.; Zhong, X.; Dai, J.; Yang, M. Improved Performance of Fiber Bragg Hydrogen Sensors Assisted by Controllable Optical Heating System. IEEE Photonics Technol. Lett. 2017, 29, 1233–1236. [Google Scholar] [CrossRef]
- Fisser, M.; Badcock, R.A.; Teal, P.D.; Janssens, S.; Hunze, A. Palladium-Based Hydrogen Sensors Using Fiber Bragg Gratings. J. Light. Technol. 2018, 36, 850–856. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, Y.; Bai, X.; Chen, K.; Guan, B.O. High-sensitivity and fast-response fiber-tip Fabry-Perot hydrogen sensor with suspended palladium-decorated graphene. Nanoscale 2019, 11, 15821–15827. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, F.A.A.; Eklund, R.; Nilsson, S.; Langhammer, C. A fiber-optic nanoplasmonic hydrogen sensor via pattern-transfer of nanofabricated PdAu alloy nanostructures. Nanoscale 2018, 10, 20533–20539. [Google Scholar] [CrossRef] [PubMed]
- Darmadi, I.; Östergren, I.; Lerch, S.; Lund, A.; Moth-Poulsen, K.; Müller, C.; Langhammer, C. Bulk-Processed Plasmonic Plastic Nanocomposite Materials for Optical Hydrogen Detection. Acc. Chem. Res. 2023, 56, 1850–1861. [Google Scholar] [CrossRef]
- Dai, J.; Yang, M.; Chen, Y.; Cao, K.; Liao, H.; Zhang, P. Side-polished fiber Bragg grating hydrogen sensor with WO3-Pd composite film as sensing materials. Opt. Express 2011, 19, 6141–6148. [Google Scholar] [CrossRef] [PubMed]
- Miliutina, E.; Guselnikova, O.; Chufistova, S.; Kolska, Z.; Elashnikov, R.; Burtsev, V.; Postnikov, P.; Svorcik, V.; Lyutakov, O. Fast and All-Optical Hydrogen Sensor Based on Gold-Coated Optical Fiber Functionalized with Metal–Organic Framework Layer. ACS Sens. 2019, 4, 3133–3140. [Google Scholar] [CrossRef]
- Sun, C.; Ohodnicki, P.R.; Yu, Y. Double-Layer Zeolite Nano-Blocks and Palladium-Based Nanocomposite Fiber Optic Sensors for Selective Hydrogen Sensing at Room Temperature. IEEE Sens. Lett. 2017, 1, 1500504. [Google Scholar] [CrossRef]
- Westerwaal, R.J.; Rooijmans, J.S.A.; Leclercq, L.; Gheorghe, D.G.; Radeva, T.; Mooij, L.; Mak, T.; Polak, L.; Slaman, M.; Dam, B.; et al. Nanostructured Pd–Au based fiber optic sensors for probing hydrogen concentrations in gas mixtures. Int. J. Hydrogen Energy 2013, 38, 4201–4212. [Google Scholar] [CrossRef]
- Perrotton, C.; Westerwaal, R.J.; Javahiraly, N.; Slaman, M.; Schreuders, H.; Dam, B.; Meyrueis, P. A reliable, sensitive and fast optical fiber hydrogen sensor based on surface plasmon resonance. Opt. Express 2013, 21, 382–390. [Google Scholar] [CrossRef]
- Buric, M.; Chen, K.P.; Bhattarai, M.; Swinehart, P.R.; Maklad, M. Active Fiber Bragg Grating Hydrogen Sensors for All-Temperature Operation. IEEE Photonics Technol. Lett. 2007, 19, 255–257. [Google Scholar] [CrossRef]
- Monzón-Hernández, D.; Luna-Moreno, D.; Martínez-Escobar, D. Fast response fiber optic hydrogen sensor based on palladium and gold nano-layers. Sens. Actuators B Chem. 2009, 136, 562–566. [Google Scholar] [CrossRef]
- Alkhabet, M.M.; Girei, S.H.; Paiman, S.; Arsad, N.; Mahdi, M.A.; Yaacob, M.H. Tapered Optical Fiber for Hydrogen Sensing Application Based on Molybdenum Trioxide (MoO3). Eng. Proc. 2021, 10, 75. [Google Scholar] [CrossRef]
- Xu, B.; Li, P.; Wang, D.N.; Zhao, C.L.; Dai, J.; Yang, M. Hydrogen sensor based on polymer-filled hollow core fiber with Pt-loaded WO3/SiO2 coating. Sens. Actuators B Chem. 2017, 245, 516–523. [Google Scholar] [CrossRef]
- Barone, F.; Signorini, A.; Ntibarikure, L.; Fiore, T.; Di Pasquale, F.; Oton, C.J. Fiber-Optic Liquid Level Sensing by Temperature Profiling with an FBG Array. Sensors 2018, 18, 2422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Li, C. Dynamic optical arbitrary waveform generation with amplitude controlled by interference of two FBG arrays. Opt. Express 2012, 20, 23074–23081. [Google Scholar] [CrossRef] [PubMed]
- Avetisov, V.; Bjoroey, O.; Wang, J.; Geiser, P.; Paulsen, K.G. Hydrogen Sensor Based on Tunable Diode Laser Absorption Spectroscopy. Sensors 2019, 19, 5313. [Google Scholar] [CrossRef]
- Ma, Y.; Liang, T.; Qiao, S.; Liu, X.; Lang, Z. Highly Sensitive and Fast Hydrogen Detection Based on Light-Induced Thermoelastic Spectroscopy. Ultrafast Sci. 2023, 3, 0024. [Google Scholar] [CrossRef]
















| Type | Sensitive Material | Concentration Range | Response Time | Reference |
|---|---|---|---|---|
| FBG | Pt–WO3 | 0.04–4% | 10~20 s | [39] |
| FBG | Pd | 1–5% | 0.4 h | [46] |
| FBG | Pd/Ag | 1–4% | 300 s | [40] |
| FBG | (K/S/C)-Pt/WO3 | 0.04–1.5% | 50 s | [14] |
| FBG | Pd | 0.5–10% | 10 s | [55] |
| Evanescent | SO2 | 0.02–10% | 10 s | [8] |
| Evanescent | Pd/Au | 0.8–4.6% | 4.5 s | [56] |
| Evanescent | MoO3 | 0.125–2% | 220 s | [57] |
| SPR | Pd/SiO2/Au | 0.5–4% | 15 s | [54] |
| SPR | Pd | 0.08–4% | 116 s | [36] |
| MZI | Pd–Au alloy | 0.5–20% | 200 s | [23] |
| MZI | Pt–WO3 | 0.1–0.8% | 35 s | [15] |
| FPI | Pt–WO3/SO2 | 0.3–2.4% | 50 s | [25] |
| FPI | Pt–WO3/SO2 | 0.2–0.8% | 23 s | [26] |
| Micromirror | WO3–Pd2Pt–Pt | 0.01–0.5% | 20 s | [31] |
| Micromirror | WO3–Pd2Pt–Pt | 0.000228–0.2% | 200 s | [10] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, B.; Hu, Y.; Yao, L.; Jin, Q.; Zheng, C.; Wu, Y.; Wu, X.; Gao, X. Spectroscopic Techniques and Hydrogen-Sensitive Compounds: A New Horizon in Hydrogen Detection. Sensors 2024, 24, 3146. https://doi.org/10.3390/s24103146
Si B, Hu Y, Yao L, Jin Q, Zheng C, Wu Y, Wu X, Gao X. Spectroscopic Techniques and Hydrogen-Sensitive Compounds: A New Horizon in Hydrogen Detection. Sensors. 2024; 24(10):3146. https://doi.org/10.3390/s24103146
Chicago/Turabian StyleSi, Bu, Yan Hu, Longchao Yao, Qiwen Jin, Chenghang Zheng, Yingchun Wu, Xuecheng Wu, and Xiang Gao. 2024. "Spectroscopic Techniques and Hydrogen-Sensitive Compounds: A New Horizon in Hydrogen Detection" Sensors 24, no. 10: 3146. https://doi.org/10.3390/s24103146





