Daidzein and Equol: Ex Vivo and In Silico Approaches Targeting COX-2, iNOS, and the Canonical Inflammasome Signaling Pathway
Abstract
:1. Introduction
2. Results
2.1. Cell Viability
2.2. DZ and EQ Down-Regulated COX-2 Expression and PGE2 Levels
2.3. Effects of DZ and EQ on Nitrite Production and iNOS Overexpression
2.4. DZ and EQ Down-Regulated Inflammatory Mediators Induced by LPS
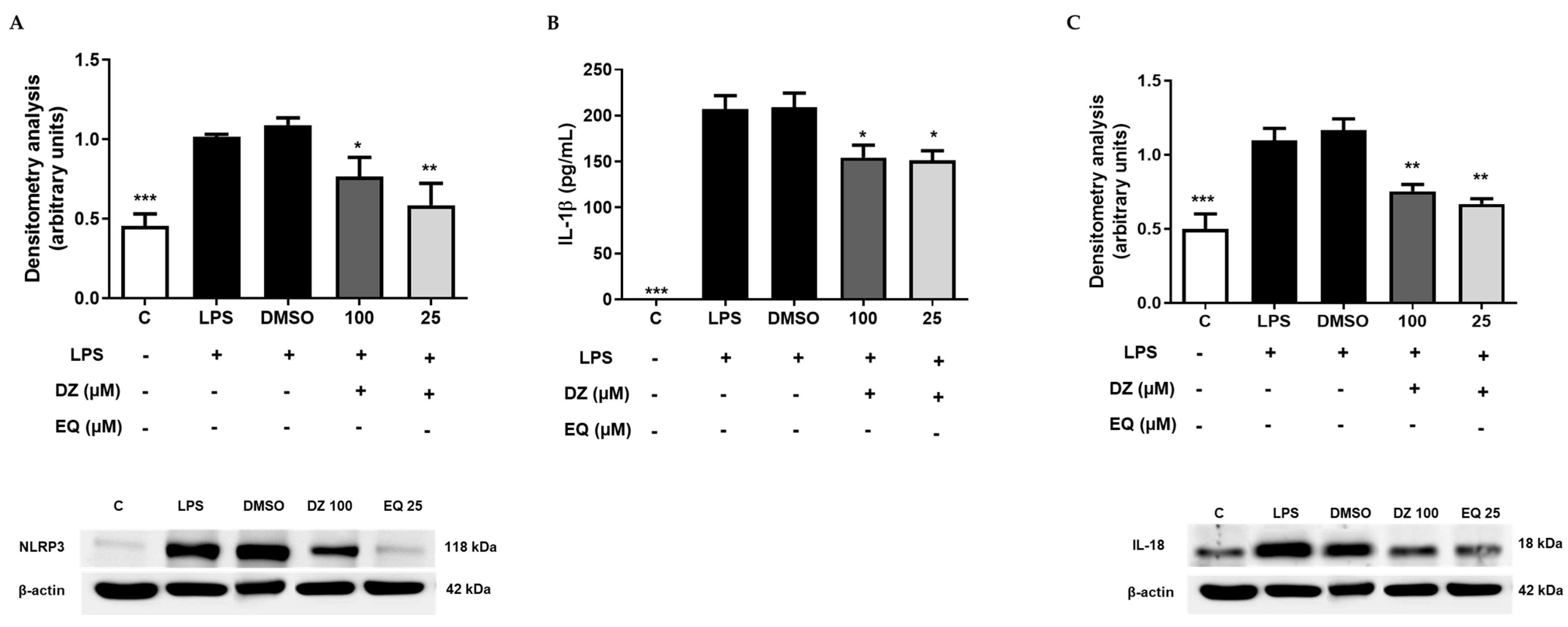
2.5. DZ and EQ Inhibit the Canonical Inflammasome Signaling Pathway
2.6. Docking Studies of DZ and S-EQ on COX-2
2.7. Docking Studies of DZ and EQ on iNOS
2.8. Docking Results for NLRP3
2.9. Docking Results for ASC and Caspase 1
2.10. Docking Results for NF-κB
2.11. Log p Value Estimation and Electrostatic Potential Map of DZ and EQ
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Ex Vivo Evaluation
4.2.1. Animals
4.2.2. Obtention of Murine Peritoneal Macrophages Cultures
4.2.3. Cell Viability
4.2.4. Measurement of Nitrite Production
4.2.5. Inflammation Mediators’ Quantification
4.2.6. Isolation of Cytoplasmic Proteins and Immunoblotting Detection
4.2.7. Statistical Evaluation
4.3. In Silico Evaluation
4.3.1. Protein Models
4.3.2. Docking Analysis
4.3.3. Log p Value Estimation and Electrostatic Potential Map Characterization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome Activation and Regulation: Toward a Better Understanding of Complex Mechanisms. Cell Discov. 2020, 6, 36. [Google Scholar] [CrossRef]
- Hao, L.Y.; Liu, X.; Franchi, L. Inflammasomes in Inflammatory Bowel Disease Pathogenesis. Curr. Opin. Gastroenterol. 2013, 29, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Tschopp, J. The Inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Li, Z.; Guo, J.; Bi, L. Role of the NLRP3 inflammasome in autoimmune diseases. Biomed. Pharmacother. 2020, 130, 110542. [Google Scholar] [CrossRef] [PubMed]
- Ciraci, C.; Janczy, J.R.; Sutterwala, F.S.; Cassel, S.L. Control of Innate and Adaptive Immunity by the Inflammasome. Microbes Infect. 2012, 14, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Gross, O.; Thomas, C.J.; Guarda, G.; Tschopp, J. The Inflammasome: An Integrated View. Immunol Rev. 2011, 243, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.K.; Wen, H.; Ting, J.P.Y. The Inflammasome NLRs in Immunity, Inflammation, and Associated Diseases. Annu. Rev. Immunol. 2011, 29, 707–735. [Google Scholar] [CrossRef]
- Yu, H.B.; Finlay, B.B. The Caspase-1 Inflammasome: A Pilot of Innate Immune Responses. Cell Host Microbe 2008, 4, 198–208. [Google Scholar] [CrossRef]
- Huang, Z.; Hoffmann, F.K.W.; Fay, J.D.; Hashimoto, A.C.; Chapagain, M.L.; Kaufusi, P.H.; Hoffmann, P.R. Stimulation of Unprimed Macrophages with Immune Complexes Triggers a Low Output of Nitric Oxide by Calcium-Dependent Neuronal Nitric-Oxide Synthase. J. Biol. Chem. 2012, 287, 4492–4502. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, C.A.A.; Kovacs-Nolan, J.; Mine, Y. Bioactive Dietary Peptides and Amino Acids in Inflammatory Bowel Disease. Amino Acids 2015, 47, 2127–2141. [Google Scholar] [CrossRef]
- Chang, W.T.; Huang, W.C.; Liou, C.J. Evaluation of the Anti-Inflammatory Effects of Phloretin and Phlorizin in Lipopolysaccharide-Stimulated Mouse Macrophages. Food Chem. 2012, 134, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kang, N.; Park, S.Y.; Cheong, S.H.; Chang, K.J.; Kim, S.H.; Um, J.H.; Han, E.J.; Kim, E.A.; Jeon, Y.-J.; et al. Xylose-Taurine Reduced Suppresses the Inflammatory Responses in Lipopolysaccharide-Stimulated Raw264.7 Macrophages. In Taurine 10. Advances in Experimental Medicine and Biology; Lee, D.H., Schaffer, S.W., Park, E., Kim, H.W., Eds.; Springer: Dordrecht, The Netherlands, 2017; Volume 975, ISBN 978-94-024-1079-2. [Google Scholar]
- Morris, G.P.; Beck, P.L.; Herridge, M.S.; Depew, W.T.; Szewczuk, M.R.; Wallace, J.L. Hapten -Induced Model of Chronic Inflammation and Ulceration in the Rat Colon. Gastroenterology 1989, 96, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Wang, P.; Qi, J.; Zhang, L.; Gao, C. TLR-Induced NF-ΚB Activation Regulates NLRP3 Expression in Murine Macrophages. FEBS Lett. 2012, 586, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.; Ghosh, S. Regulation of the NF-ΚB-Mediated Transcription of Inflammatory Genes. Front. Immunol. 2014, 5, 71. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Kleinert, H.; Schwarz, P.M.; Förstermann, U. Regulation of the Expression of Inducible Nitric Oxide Synthase. Biol. Chem. 2003, 384, 1343–1364. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Shin, S.; Kim, H.; Han, S.; Kim, K.; Kwon, J.; Kwak, J.H.; Lee, C.K.; Ha, N.J.; Yim, D.; et al. Anti-Inflammatory Function of Arctiin by Inhibiting COX-2 Expression via NF-ΚB Pathways. J. Inflamm. 2011, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-B.; Han, A.-R.; Park, E.-Y.; Kim, J.-Y.; Cho, W.; Lee, J.; Seo, E.-K.; Lee, K.-T. Inhibition of LPS-Induced INOS, COX-2 and Cytokines Expression by Poncirin through the NF-κB Inactivation in RAW 264.7 Macrophage Cells. Biol. Pharm. Bull. 2007, 30, 2345–2351. [Google Scholar] [CrossRef] [PubMed]
- Suhail, M.; Rehan, M.; Tarique, M.; Tabrez, S.; Husain, A.; Zughaibi, T.A. Targeting a Transcription Factor NF-ΚB by Green Tea Catechins Using in Silico and in Vitro Studies in Pancreatic Cancer. Front. Nutr. 2023, 9, 1078642. [Google Scholar] [CrossRef]
- Babu, P.V.A.; Liu, D.; Gilbert, E.R. Recent Advances in Understanding the Anti-Diabetic Actions of Dietary Flavonoids. J. Nutr. Biochem. 2013, 24, 1777–1789. [Google Scholar] [CrossRef]
- Sánchez-Calvo, J.M.; Rodríguez-Iglesias, M.A.; Molinillo, J.M.G.; Macías, F.A. Soy Isoflavones and Their Relationship with Microflora: Beneficial Effects on Human Health in Equol Producers. Phytochem. Rev. 2013, 12, 979–1000. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Brown, N.M.; Lydeking-Olsen, E. Critical Review the Clinical Importance of the Metabolite Equol-A Clue to the Effectiveness of Soy and Its Isoflavones. J. Nutr. 2002, 132, 3577–3584. [Google Scholar] [CrossRef]
- Magee, P.J. Is Equol Production Beneficial to Health? Proc. Nutr. Soc. 2011, 70, 10–18. [Google Scholar] [CrossRef]
- Kuek, A.; Hazleman, B.L.; Östör, A.J.K. Immune-Mediated Inflammatory Diseases (IMIDs) and Biologic Therapy: A Medical Revolution. Postgrad. Med. J. 2007, 83, 251–260. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Gravallese, E.M. Immune-Mediated Inflammatory Disease Therapeutics: Past, Present and Future. Nat. Rev. Immunol. 2021, 21, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Cheong, S.H.; Furuhashi, K.; Ito, K.; Nagaoka, M.; Yonezawa, T.; Miura, Y.; Yagasaki, K. Daidzein Promotes Glucose Uptake through Glucose Transporter 4 Translocation to Plasma Membrane in L6 Myocytes and Improves Glucose Homeostasis in Type 2 Diabetic Model Mice. J. Nutr. Biochem. 2014, 25, 136–143. [Google Scholar] [CrossRef]
- Singh, H.; Singh, S.; Srivastava, A.; Tandon, P.; Bharti, P.; Kumar, S.; Maurya, R. Conformational Analysis and Vibrational Study of Daidzein by Using FT-IR and FT-Raman Spectroscopies and DFT Calculations. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 120, 405–415. [Google Scholar] [CrossRef]
- Arora, A.; Nair, M.G.; Strasburg, G.M. Antioxidant Activities of Isoflavones and Their Biological Metabolites in a Liposomal System. Arcives Biochem. Biophys. 1998, 356, 133–141. [Google Scholar] [CrossRef]
- Lephart, E.D. Skin Aging and Oxidative Stress: Equol’s Anti-Aging Effects via Biochemical and Molecular Mechanisms. Ageing Res. Rev. 2016, 31, 36–54. [Google Scholar] [CrossRef]
- Liu, T.H.; Tsai, T.Y. Effects of Equol on Deoxycorticosterone Acetate Salt-Induced Hypertension and Associated Vascular Dementia in Rats. Food Funct. 2016, 7, 3444–3457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, G.; Gurley, E.C.; Zhou, H. Flavonoid Apigenin Inhibits Lipopolysaccharide-Induced Inflammatory Response through Multiple Mechanisms in Macrophages. PLoS ONE 2014, 9, e107072. [Google Scholar] [CrossRef] [PubMed]
- Oroz, J.; Barrera-Vilarmau, S.; Alfonso, C.; Rivas, G.; De Alba, E. ASC Pyrin Domain Self-Associates and Binds NLRP3 Protein Using Equivalent Binding Interfaces. J. Biol. Chem. 2016, 291, 19487–19501. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Ruth, H.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Software News and Updates AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Vajjhala, P.R.; Kaiser, S.; Smith, S.J.; Ong, Q.R.; Soh, S.L.; Stacey, K.J.; Hill, J.M. Identification of Multifaceted Binding Modes for Pyrin and ASC Pyrin Domains Gives Insights into Pyrin Inflammasome Assembly. J. Biol. Chem. 2014, 289, 23504–23519. [Google Scholar] [CrossRef] [PubMed]
- Stanzione, F.; Giangreco, I.; Cole, J.C. Use of molecular docking computational tools in drug discovery. Progress. Med. Chem. 2021, 60, 273–343. [Google Scholar]
- Ahamada, M.M.; Jia, Y.; Wu, X. Macrophage Polarization and Plasticity in Systemic Lupus Erythematosus. Front. Immunol. 2021, 12, 734008. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Campitiello, R.; Gotelli, E.; Soldano, S. The Role of M1/M2 Macrophage Polarization in Rheumatoid Arthritis Synovitis. Front. Immunol. 2022, 13, 867260. [Google Scholar] [CrossRef] [PubMed]
- Castejón, M.L.; Montoya, T.; Ortega-Vidal, J.; Altarejos, J.; Alarcón-de-la-Lastra, C. Ligstroside aglycon, an extra virgin olive oil secoiridoid, prevents inflammation by regulation of MAPKs, JAK/STAT, NF-κB, Nrf2/HO-1, and NLRP3 inflammasome signaling pathways in LPS-stimulated murine peritoneal macrophages. Food Funct. 2022, 13, 10200–10209. [Google Scholar] [CrossRef]
- Cárdeno, A.; Sánchez-Hidalgo, M.; Aparicio-Soto, M.; Sánchez-Fidalgo, S.; Alarcón-De-La-Lastra, C. Extra Virgin Olive Oil Polyphenolic Extracts Downregulate Inflammatory Responses in LPS-Activated Murine Peritoneal Macrophages Suppressing NFκB and MAPK Signalling Pathways. Food Funct. 2014, 5, 1270–1277. [Google Scholar] [CrossRef]
- Blay, M.; Espinel, A.E.; Delgado, M.A.; Baiges, I.; Bladé, C.; Arola, L.; Salvadó, J. Isoflavone Effect on Gene Expression Profile and Biomarkers of Inflammation. J. Pharm. Biomed. Anal. 2010, 51, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Soto, M.; Sánchez-Fidalgo, S.; González-Benjumea, A.; Maya, I.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C. Naturally Occurring Hydroxytyrosol Derivatives: Hydroxytyrosyl Acetate and 3,4-Dihydroxyphenylglycol Modulate Inflammatory Response in Murine Peritoneal Macrophages. Potential Utility as New Dietary Supplements. J. Agric. Food Chem. 2015, 63, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xue, M.; Geng, Z.; Chen, P. Cellular Physiology Cellular Physiology Cellular Physiology Cellular Physiology the Suppressive Effects of Bursopentine (BP5) on Oxidative Stress and NF-κB Activation in Lipopolysaccharide-Activated Murine Peritoneal Macrophages. Cell. Physiol. Biochem. 2012, 19, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Rafii, F.; Jackson, L.D.; Ross, I.; Heinze, T.M.; Lewis, S.M.; Aidoo, A.; Lyn-Cook, L.; Manjanatha, M. Comparative Medicine Metabolism of Daidzein by Fecal Bacteria in Rats. Comp. Med. 2007, 57, 282–286. [Google Scholar] [PubMed]
- Márquez-Flores, Y.K.; Campos-Aldrete, M.E.; Sagado-Zamora, H.; Correa-Basurto, J.; Meléndez-Camargo, M.E. Acute and Chronic Anti-Inflammatory Evaluation of Imidazo[1,2-α]Pyridine Carboxylic Acid Derivatives and Docking Analysis. Med. Chem. Res. 2012, 21, 3491–3498. [Google Scholar] [CrossRef]
- Ricciotti, E.; Fitzgerald, G.A. Prostaglandins and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Saranya, A.; Manikandan, S.; Ramanathan, T.; Kesavanarayanan, K.S.; The, L.K.; Salleh, M.Z. Phytochemical Screening and In Silico Investigation of 2-Benzoxazolinone from Acanthus Ilicifolius Linn. as Dual Inhibitors of Cyclooxygenase-2 and 5-Lipooxygenase Enzymes. Int. J. Phytomedicine 2014, 6, 455–463. [Google Scholar]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-Inflammatory Effects of Flavonoids: Genistein, Kaempferol, Quercetin, and Daidzein Inhibit STAT-1 and NF-ΚB Activations, Whereas Flavone, Isorhamnetin, Naringenin, and Pelargonidin Inhibit Only NF-ΚB Activation along with Their Inhibitory Effect on iNOS Expression and NO Production in Activated Macrophages. Mediat. Inflamm. 2007, 2007, 045673. [Google Scholar] [CrossRef]
- Subedi, L.; Ji, E.; Shin, D.; Jin, J.; Yeo, J.H.; Kim, S.Y. Equol, a Dietary Daidzein Gut Metabolite Attenuates Microglial Activation and Potentiates Neuroprotection in Vitro. Nutrients 2017, 9, 207. [Google Scholar] [CrossRef]
- Huang, S.S.; Su, S.Y.; Chang, J.S.; Lin, H.J.; Wu, W.T.; Deng, J.S.; Huang, G.J. Antioxidants, Anti-Inflammatory, and Antidiabetic Effects of the Aqueous Extracts from Glycine Species and Its Bioactive Compounds. Bot. Stud. 2016, 57, 38. [Google Scholar] [CrossRef]
- Khan, M.N.A.; Cho, J.Y.; Lee, M.C.; Kang, J.Y.; Nam, G.P.; Fujii, H.; Hong, Y.K. Isolation of Two Anti-Inflammatory and One pro-Inflammatory Polyunsaturated Fatty Acids from the Brown Seaweed Undaria Pinnatifida. J. Agric. Food Chem. 2007, 55, 6984–6988. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Kamiie, K.; Kidachi, Y.; Noshita, T.; Umetsu, H.; Fuke, Y.; Ryoyama, K. Structural Basis for the Interaction of 6-(Methylsulfinyl) Hexyl Isothiocyanate with Inducible Nitric Oxide Synthase. Int. J. Comput. Bioinform. Silico Model. 2014, 3, 426–432. [Google Scholar]
- Kang, J.S.; Yoon, Y.D.; Han, M.H.; Han, S.B.; Lee, K.; Kang, M.R.; Moon, E.Y.; Jeon, Y.J.; Park, S.K.; Kim, H.M. Estrogen Receptor-Independent Inhibition of Tumor Necrosis Factor-α Gene Expression by Phytoestrogen Equol Is Mediated by Blocking Nuclear Factor-ΚB Activation in Mouse Macrophages. Biochem. Pharmacol. 2005, 71, 136–143. [Google Scholar] [CrossRef]
- Opal, S.M.; Depalo, V.A. Anti-Inflammatory Cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef]
- Montoya, T.; Castejón, M.L.; Sánchez-Hidalgo, M.; González-Benjumea, A.; Fernández-Bolaños, J.G.; Alarcón De-La-Lastra, C. Oleocanthal Modulates LPS-Induced Murine Peritoneal Macrophages Activation via Regulation of Inflammasome, Nrf-2/HO-1, and MAPKs Signaling Pathways. J. Agric. Food Chem. 2019, 67, 5552–5559. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.J.; McDermott, M.F.; Kanneganti, T.D. Inflammasomes and Autoimmunity. Trends Mol. Med. 2011, 17, 57–64. [Google Scholar] [CrossRef]
- Masters, S.L. Specific Inflammasomes in Complex Diseases. Clin. Immunol. 2013, 147, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, X.; Cheng, K.; Zhang, X.; Lu, J.; Hu, R. X-11-5-27, a Daidzein Derivative, Inhibits NLRP3 Inflammasome Activity via Promoting Autophagy. Exp. Cell Res. 2017, 360, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Flores, Y.K.; Villegas, I.; Cárdeno, A.; Rosillo, M.T.; Alarcón-de-la-Lastra, C. Apigenin Supplementation Protects the Development of Dextran Sulfate Sodium-Induced Murine Experimental Colitis by Inhibiting Canonical and Non-Canonical Inflammasome Signaling Pathways. J. Nutr. Biochem. 2016, 30, 143–252. [Google Scholar] [CrossRef]
- Franchi, L.; Eigenbrod, T.; Muñoz-Planillo, R.; Nuñez, G. The Inflammasome: A Caspase-1-Activation Platform That Regulates Immune Responses and Disease Pathogenesis. Nat. Immunol. 2009, 10, 241–247. [Google Scholar] [CrossRef]
- Kersse, K.; Lamkanfi, M.; Bertrand, M.J.M.; Berghe, T.V.; Vandenabeele, P. Interaction Patches of Procaspase-1 Caspase Recruitment Domains (CARDs) Are Differently Involved in Procaspase-1 Activation and Receptor-Interacting Protein 2 (RIP2)-Dependent Nuclear Factor ΚB Signaling. J. Biol. Chem. 2011, 286, 35874–35882. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.E.; Yang, G.; Kim, N.D.; Jeong, S.; Jung, Y.; Choi, J.Y.; Park, H.H.; Lee, J.Y. Targeting ASC in NLRP3 Inflammasome by Caffeic Acid Phenethyl Ester: A Novel Strategy to Treat Acute Gout. Sci. Rep. 2016, 6, 38622. [Google Scholar] [CrossRef] [PubMed]
- Pantsar, T.; Poso, A. Binding Affinity via Docking: Fact and Fiction. Molecules 2018, 23, 1899. [Google Scholar] [CrossRef] [PubMed]
- Bakht, M.A.; Alajmi, M.F.; Alam, P.; Alam, A.; Alam, P.; Aljarba, T.M. Theoretical and Experimental Study on Lipophilicity and Wound Healing Activity of Ginger Compounds. Asian Pac. J. Trop. Biomed. 2014, 4, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.A.; Lai, J.F.; Halm, B.M. Absorption, Distribution, Metabolism, and Excretion of Isoflavonoids after Soy Intake. Arch. Biochem. Biophys. 2014, 559, 24–28. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Kanatsu, J.; Toh, M.; Naka, A.; Kondo, K.; Iida, K. The Dietary Isoflavone Daidzein Reduces Expression of Pro-Inflammatory Genes through PPARα/γ and JNK Pathways in Adipocyte and Macrophage Co-Cultures. PLoS ONE 2016, 11, 0149676. [Google Scholar] [CrossRef]
- Scuro, L.S.; Simioni, P.U.; Grabriel, D.L.; Saviani, E.E.; Modolo, L.V.; Tamashiro, W.M.; Salgado, I. Suppression of Nitric Oxide Production in Mouse Macrophages by Soybean Flavonoids Accumulated in Response to Nitroprusside and Fungal Elicitation. BMC Biochem. 2004, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Yoon, Y.D.; Han, M.H.; Han, S.B.; Lee, K.; Park, S.K.; Kim, H.M. Equol Inhibits Nitric Oxide Production and Inducible Nitric Oxide Synthase Gene Expression through Down-Regulating the Activation of Akt. Int. Immunopharmacol. 2007, 7, 491–499. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. JNCI J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Moorcroft, M.J.; Davis, J.; Compton, R.G. Detection and Determination of Nitrate and Nitrite: A Review. Talanta 2001, 54, 785–803. [Google Scholar] [CrossRef]
- Sánchez-Hidalgo, M.; Martín, A.R.; Villegas, I.; Alarcón De La Lastra, C. Rosiglitazone, an Agonist of Peroxisome Proliferator-Activated Receptor Gamma, Reduces Chronic Colonic Inflammation in Rats. Biochem. Pharmacol. 2005, 69, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- National Library of Medicine. Standard Protein BLAST. Maryland, USA. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=Proteins (accessed on 18 January 2023).
- Protein Data Bank [WWW Document]. Available online: https://www.rcsb.org/ (accessed on 5 January 2023).
- Šali, A.; Blundell, T.L. Comparative Protein Modelling by Satisfaction of Spatial Restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef] [PubMed]
- Expanding the Limits of Computational Chemistry. News Products Support. 2018. Available online: https://gaussian.com/products/ (accessed on 5 January 2023).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009; Available online: https://gaussian.com/glossary/g09/ (accessed on 5 January 2023).
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Konwar, B.K. Molecular Docking Studies of Quercetin and Its Analogues against Human Inducible Nitric Oxide Synthase. Springerplus 2012, 1, 69. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- VCCLAB Virtual Computational Chemistry Laboratory. 2005. Available online: https://vcclab.org/ (accessed on 23 March 2023).
- Tetko, I.V.; Gasteiger, J.; Todeschini, R.; Mauri, A.; Livingstone, D.; Ertl, P.; Palyulin, V.A.; Radchenko, E.V.; Zefirov, N.S.; Makarenko, A.S.; et al. Virtual computational chemistry laboratory—design and description. J. Comput. Aid. Mol. Des. 2005, 19, 453–463. [Google Scholar] [CrossRef]








| Daidzein | S-Equol | |||||
|---|---|---|---|---|---|---|
 |  | |||||
| Protein | ΔG * | Ki ** | Residue Interactions | ΔG * | Ki ** | Residue Interactions |
| COX-2 | −8.61 | 0.49 | Ile124, Asp125, Thr129, Thr149, Arg150, Ala151, Asn375, Arg376, Ala378, Arg469, Phe529 | −8.57 | 0.52 | Ile124, Ser126, Pro128, Asn375, Arg376, Ile377, Ala378, Phe381, Lys532, Gly533, Phe529 |
| iNOS | −7.56 | 2.85 | Gln263, Tyr347, Pro350, Ala351, Val352, Phe369, Asn370, Tyr373, Glu377, Asp382, Arg388, Hem510 | −8.60 | 0.50 | Pro350, Ala351, Val352, Gly371, Trp372, Tyr373, Glu377, Asp382, Hem510 |
| NLRP3 | −7.80 | 1.91 | Ser374, Glu375, Arg675, Gly719, Leu720, Ser752, Leu753, Gly754, Asp795, Pro796, Gly915, Leu916, Ser917, Leu810, Gly811, Phe1113 | −7.50 | 3.17 | Leu187, Leu188, Ala189, Ile190, Glu197, Pro199, Val200, Ser201, Ile203, Pro211, Asp212, Leu822 |
| ASC | −5.57 | 83.41 | Tyr36, Gly37, Ile39, Pro40, Phe59, Tyr60 | −5.95 | 43.81 | Lys109, Pro110, Leu112, His113, Phe114, Ile115, Asp116, Arg119, Arg160, Phe163, Ser164 |
| Pro-caspase 1 | −7.23 | 5.03 | Lys268, Asn269, Gly303, Thr309, Thr310, Glu312, Phe313, Glu314, Lys320 | −6.12 | 32.45 | Lys268, Thr309, Thr310, Glu311, Glu312, Glu314, Lys319 |
| Caspase 1 | −7.60 | 2.70 | Arg240, Cys285, Arg286, Ala284, Cys331, Glu241, Gly242, Gln257, Leu258, Ile282 | −6.42 | 19.80 | Arg179.Ser236, His237, Gln283, Cys285, Ser339, Trp340, Arg341 |
| NF-κB | −5.43 | 104.92 | P65: Cys197, Arg198 P50: Lys278, Thr304, Val306, His307, Arg308 | −5.44 | 102.86 | P65: Cys197, Arg198 P50: Lys278, Thr304, Val306, His307, Arg308 |
| Antibody | Type | Supplier | Dilution |
|---|---|---|---|
| Rabbit polyclonal anti-COX-2 | Primary | Cayman®, Ann Arbor, MI, USA | 1:2500 |
| Rabbit polyclonal anti-iNOS | Primary | Cayman®, Ann Arbor, MI, USA | 1:1000 |
| Rabbit polyclonal anti-IL-18 | Primary | Abcam plc | 1:200 |
| Rabbit anti-ASC | Primary | Santa Cruz Biotechnology®, Inc. | 1:100 |
| Rabbit anti-caspase 1 | Primary | Novus Biologicals, LLC | 1:400 |
| Mouse anti-NLRP3 | Primary | Novus Biologicals, LLC | 1:100 |
| Anti-rabbit | Secondary | Cayman®, Ann Arbor, MI, USA | 1:2500 |
| Anti-mouse | Secondary | Dako®, Atlanta, GA, USA | 1:2500 |
| Protein | UniProt | PDB (Resolution in Å/Quality Model) |
|---|---|---|
| NLRP3 | Q96P20 | NA a (98%) |
| ASC | Q9ULZ3 | 2KN6 |
| Pro-caspase 1 | P29466 | 3E4C (2.05/98%) |
| Caspase 1 | P29466 | 1RWK (2.30) |
| COX-2 | Q05769 | 5COX (3.00) |
| NF-κB | Q04206 and P19838 | 1NFI (2.70) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Márquez-Flores, Y.K.; Martínez-Galero, E.; Correa-Basurto, J.; Sixto-López, Y.; Villegas, I.; Rosillo, M.Á.; Cárdeno, A.; Alarcón-de-la-Lastra, C. Daidzein and Equol: Ex Vivo and In Silico Approaches Targeting COX-2, iNOS, and the Canonical Inflammasome Signaling Pathway. Pharmaceuticals 2024, 17, 647. https://doi.org/10.3390/ph17050647
Márquez-Flores YK, Martínez-Galero E, Correa-Basurto J, Sixto-López Y, Villegas I, Rosillo MÁ, Cárdeno A, Alarcón-de-la-Lastra C. Daidzein and Equol: Ex Vivo and In Silico Approaches Targeting COX-2, iNOS, and the Canonical Inflammasome Signaling Pathway. Pharmaceuticals. 2024; 17(5):647. https://doi.org/10.3390/ph17050647
Chicago/Turabian StyleMárquez-Flores, Yazmín K., Elizdath Martínez-Galero, José Correa-Basurto, Yudibeth Sixto-López, Isabel Villegas, María Á. Rosillo, Ana Cárdeno, and Catalina Alarcón-de-la-Lastra. 2024. "Daidzein and Equol: Ex Vivo and In Silico Approaches Targeting COX-2, iNOS, and the Canonical Inflammasome Signaling Pathway" Pharmaceuticals 17, no. 5: 647. https://doi.org/10.3390/ph17050647






