Facile Fabrication of Porous Adsorbent with Multiple Amine Groups for Efficient and Selective Removal of Amaranth and Tartrazine Dyes from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Characterization
2.3. Preparation of Amine-Rich Porous Adsorbent (ARPA)
2.4. Adsorption Experiments
2.5. Selective Adsorption Experiments
2.6. Regeneration Study
3. Results and Discussion
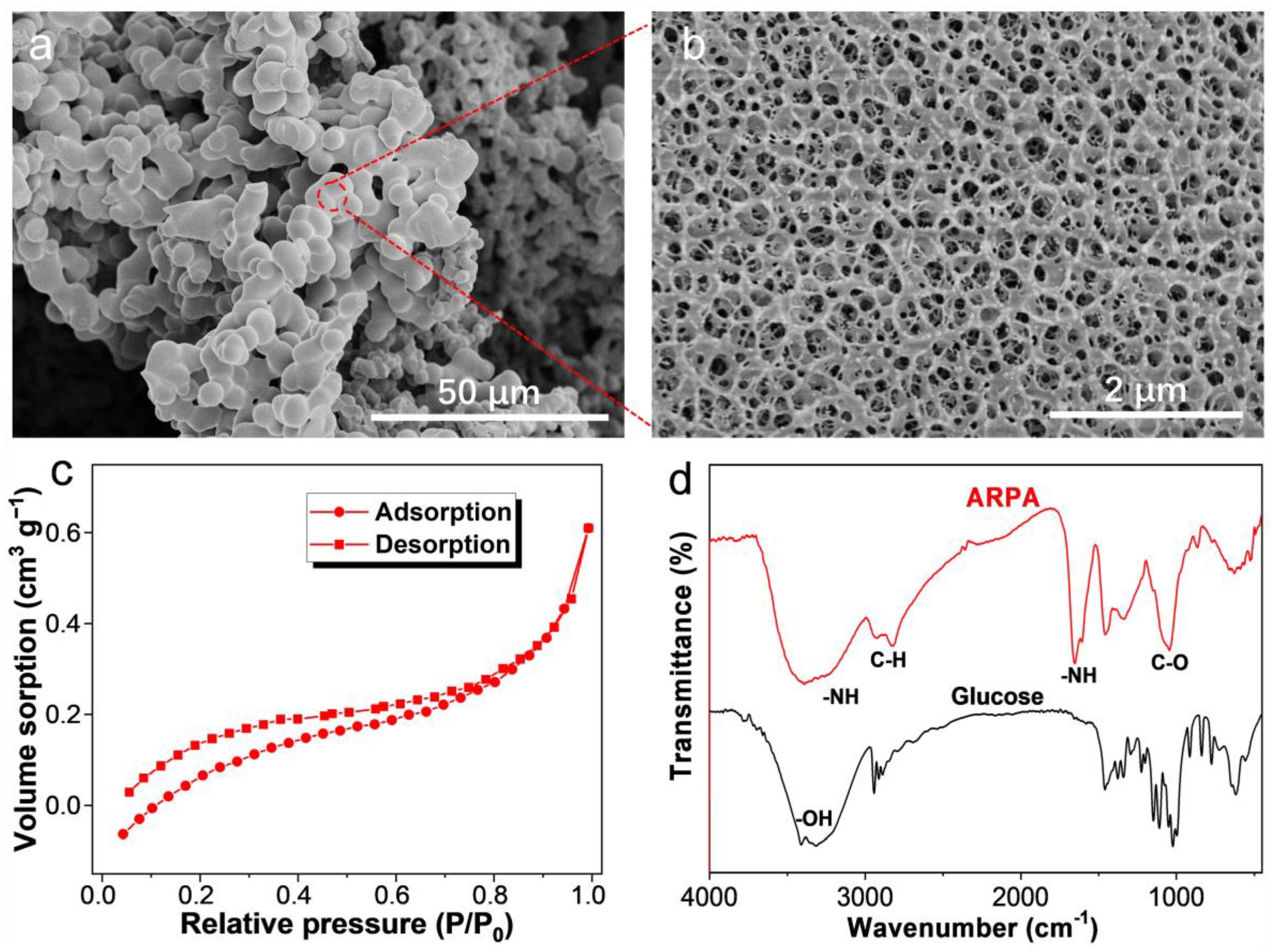
3.1. Preparation and Characterization of ARPA
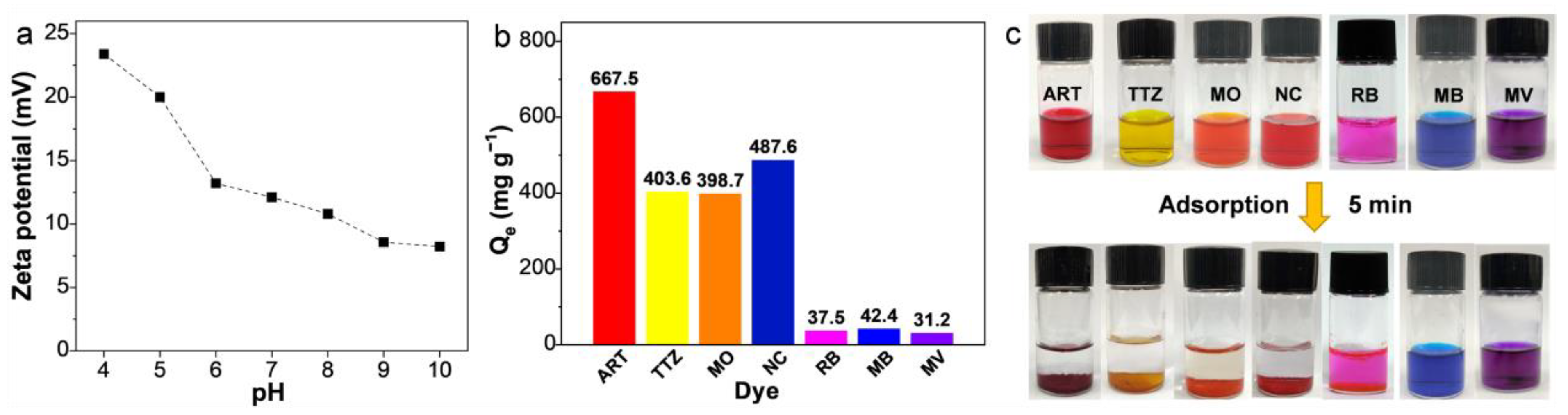
3.2. Adsorption Performance of ARPA for Different Dyes
3.3. Effects of Initial Dye Concentration, Solution pH, and Ionic Strength
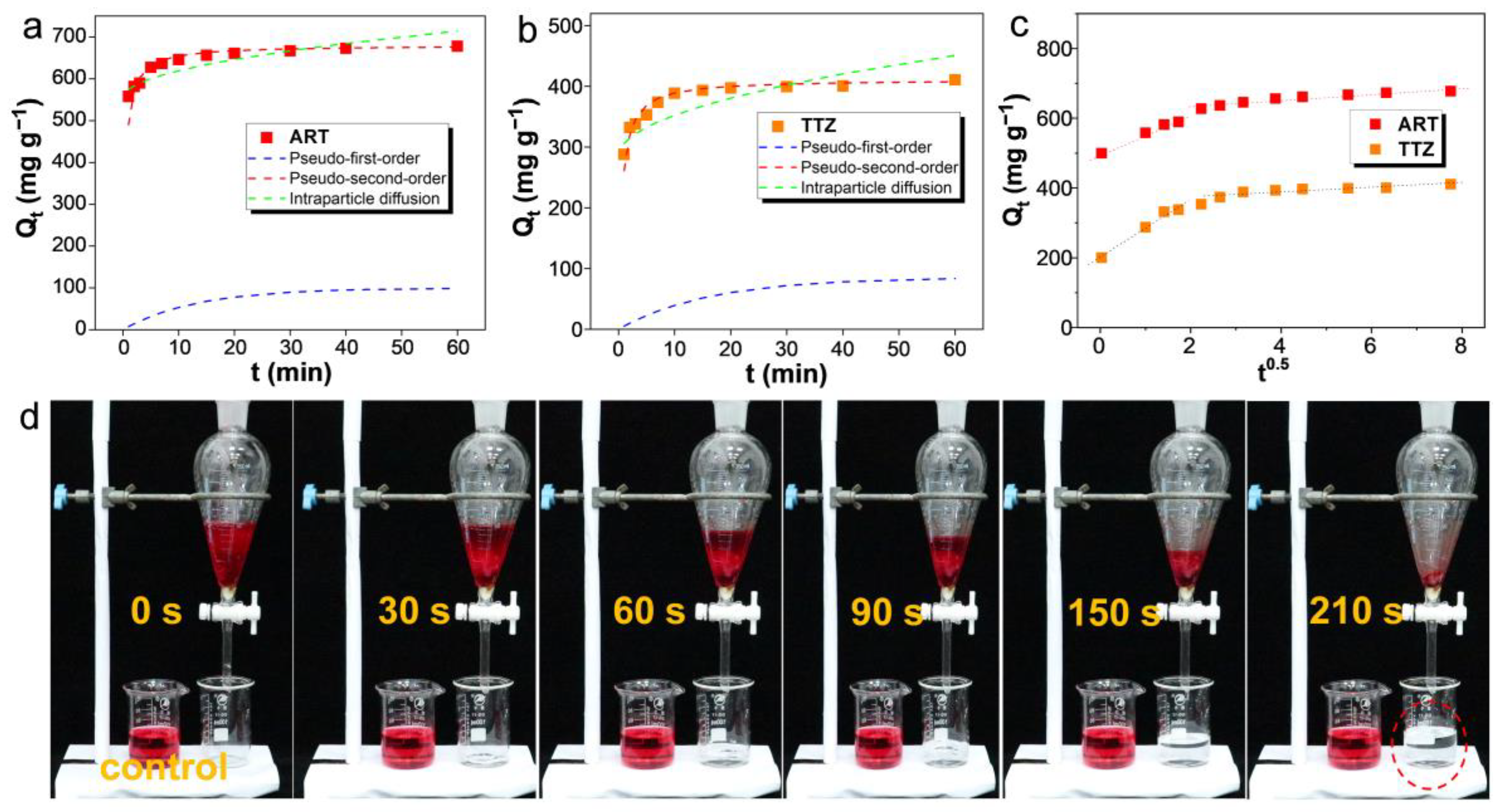
3.4. Adsorption Kinetics
3.5. Adsorption Isotherms
3.6. Adsorption Thermodynamic
3.7. Regeneration Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, N.; Huang, L.-Y.; Xiong, Y.-S.; Tian, R.; Yin, J.-Y.; Cao, D.-Y.; Hu, D.-B.; Lu, H.-Q.; Li, W.; Li, K. Micro-mechanism insights into the adsorption of anionic dyes using quaternary ammonium-functionalised chitosan aerogels. Carbohyd. Polym. 2023, 313, 120855. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid. Interfac. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Li, X.; Li, P.; Chen, W.; Ren, J.; Wu, W. Preparation and adsorption properties of lignin/cellulose hydrogel. Materials 2023, 16, 4260. [Google Scholar] [CrossRef]
- Duan, Y.; Song, Y.; Zhou, L. Facile synthesis of polyamidoamine dendrimer gel with multiple amine groups as a super adsorbent for highly efficient and selective removal of anionic dyes. J. Colloid. Interf. Sci. 2019, 546, 351–360. [Google Scholar] [CrossRef]
- Sen, T.K.; Afroze, S.; Ang, H.M. Equilibrium, kinetics and mechanism of removal of methylene blue from aqueous solution by adsorption onto pine cone biomass of pinus radiata. Water. Air. Soil. Poll. 2011, 218, 499–515. [Google Scholar] [CrossRef]
- Li, Y.; Gao, H.; Wang, C.; Zhang, X.; Zhou, H. One-step fabrication of chitosan-Fe(OH)3 beads for efficient adsorption of anionic dyes. Int. J. Biol. Macromol. 2018, 117, 30–41. [Google Scholar]
- Shimizu, T.; De Silva, K.K.H.; Hara, M.; Yoshimura, M. Facile synthesis of carbon nanotubes and cellulose nanofiber incorporated graphene aerogels for selective organic dye adsorption. Appl. Surf. Sci. 2022, 600, 154098. [Google Scholar] [CrossRef]
- Zghal, S.; Jedidi, I.; Cretin, M.; Cerneaux, S.; Abdelmouleh, M. Adsorptive removal of Rhodamine B dye using carbon graphite/CNT composites as adsorbents: Kinetics, isotherms and thermodynamic Study. Materials 2023, 16, 1015. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.; Wen, B.; Li, Y.; Zhou, B.; Wang, Z.; Yang, S.; Zhao, R. Nitrogen-rich covalent triazine frameworks for high-efficient removal of anion dyes and the synergistic adsorption of cationic dyes. Chemosphere 2021, 272, 129622. [Google Scholar] [CrossRef]
- Singh, N.B.; Nagpal, G.; Agrawal, S. Water purification by using adsorbents: A Review. Environ. Technol. Inno. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Inyinbor, A.A.; Adekola, F.A.; Olatunji, G.A. Kinetics, isotherms and thermodynamic modeling of liquid phase adsorption of Rhodamine B dye onto Raphia hookerie fruit epicarp. Water. Resour. Ind. 2016, 15, 14–27. [Google Scholar] [CrossRef]
- Chinoune, K.; Bentaleb, K.; Bouberka, Z.; Nadim, A.; Maschke, U. Adsorption of reactive dyes from aqueous solution by dirty bentonite. Appl. Clay. Sci. 2016, 123, 64–75. [Google Scholar] [CrossRef]
- Song, Y.; Tan, J.; Wang, G.; Zhou, L. Superior amine-rich gel adsorbent from peach gum polysaccharide for highly efficient removal of anionic dyes. Carbohyd. Polym. 2018, 199, 178–185. [Google Scholar] [CrossRef]
- Liu, C.; Omer, A.M.; Ouyang, X.K. Adsorptive removal of cationic methylene blue dye using carboxymethyl cellulose/k-carrageenan/activated montmorillonite composite beads: Isotherm and kinetic studies. Int. J. Biol. Macromol. 2018, 106, 823–833. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, F.; Ouyang, X.K.; Yang, L.Y.; Wang, Y. Adsorption of Pb(II) from aqueous solution by mussel shell-based adsorbent: Preparation, characterization, and adsorption performance. Materials 2021, 14, 741. [Google Scholar] [CrossRef]
- Zeng, S.; Tan, J.; Xu, X.; Huang, X.; Zhou, L. Facile synthesis of amphiphilic peach gum polysaccharide as a robust host for efficient encapsulation of methylene blue and methyl orange dyes from water. Int. J. Biol. Macromol. 2020, 154, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Chowdhury, M.; Rahman, M.M. Biobased amphoteric aerogel derived from amine-modified clay-enriched chitosan/alginate for adsorption of organic dyes and chromium (VI) ions from aqueous solution. Mater. Today Sustain. 2021, 13, 100077. [Google Scholar] [CrossRef]
- Roşu, M.C.; Socaci, C.; Floare-Avram, V.; Borodi, G.; Pogăcean, F.; Coroş, M.; Măgeruşan, L.; Pruneanu, S. Photocatalytic performance of graphene/TiO2-Ag composites on amaranth dye degradation. Mater. Chem. Phys. 2016, 179, 232–241. [Google Scholar] [CrossRef]
- Huang, B.; Yang, C.; Zeng, H.; Zhou, L. Multivalent iron-based magnetic porous biochar from peach gum polysaccharide as a heterogeneous Fenton catalyst for degradation of dye pollutants. Int. J. Biol. Macromol. 2023, 253, 126753. [Google Scholar] [CrossRef]
- Hassan, M.M.; Carr, C.M. Biomass-derived porous carbonaceous materials and their composites as adsorbents for cationic and anionic dyes: A review. Chemosphere 2021, 265, 129087. [Google Scholar] [CrossRef]
- Ouassif, H.; Moujahid, E.M.; Lahkale, R.; Sadik, R.; Bouragba, F.Z.; Sabbar, E.M.; Diouri, M. Zinc-Aluminum layered double hydroxide: High efficient removal by adsorption of tartrazine dye from aqueous solution. Surf. Interfaces 2020, 18, 100401. [Google Scholar] [CrossRef]
- Ranjbari, S.; Ayati, A.; Tanhaei, B.; Al-Othman, A.; Karimi, F. The surfactant-ionic liquid bi-functionalization of chitosan beads for their adsorption performance improvement toward Tartrazine. Environ. Res. 2022, 204, 111961. [Google Scholar] [CrossRef]
- de Lima Barizão, A.C.; Silva, M.F.; Andrade, M.; Brito, F.C.; Gomes, R.G.; Bergamasco, R. Green synthesis of iron oxide nanoparticles for tartrazine and bordeaux red dye removal. J. Environ. Chem. Eng. 2020, 8, 103618. [Google Scholar] [CrossRef]
- Alcantara-Cobos, A.; Gutiérrez-Segura, E.; Solache-Ríos, M.; Amaya-Chávez, A.; Solís-Casados, D.A. Tartrazine removal by ZnO nanoparticles and a zeolite-ZnO nanoparticles composite and the phytotoxicity of ZnO nanoparticles. Micropor. Mesopor. Mat. 2020, 302, 110212. [Google Scholar] [CrossRef]
- Mosoarca, G.; Vancea, C.; Popa, S.; Dan, M.; Boran, S. A novel high-efficiency natural biosorbent material obtained from sour cherry (Prunus cerasus) leaf biomass for cationic dyes adsorption. Materials 2023, 16, 4252. [Google Scholar] [CrossRef]
- Özdemir, Y.; Doğan, M.; Alkan, M. Adsorption of cationic dyes from aqueous solutions by sepiolite. Micropor. Mesopor. Mat. 2006, 96, 419–427. [Google Scholar] [CrossRef]
- Dotto, G.L.; Rodrigues, F.K.; Tanabe, E.H.; Fröhlich, R.; Bertuol, D.A.; Martins, T.R.; Foletto, E.L. Development of chitosan/bentonite hybrid composite to remove hazardous anionic and cationic dyes from colored effluents. J. Environ. Chem. Eng. 2016, 4, 3230–3239. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Meng, D.; Zhou, L. Facile synthesis of low-cost magnetic biosorbent from peach gum polysaccharide for selective and efficient removal of cationic dyes. Int. J. Biol. Macromol. 2018, 107, 1871–1878. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, J.; He, B.; Zhang, F.; Li, H. Peach gum for efficient removal of methylene blue and methyl violet dyes from aqueous solution. Carbohyd. Polym. 2014, 101, 574–581. [Google Scholar] [CrossRef]
- Likhitha, M.; Sailaja, R.R.N.; Priyambika, V.S.; Ravibabu, M.V. Microwave assisted synthesis of guar gum grafted sodium acrylate/cloisite superabsorbent nanocomposites: Reaction parameters and swelling characteristics. Int. J. Biol. Macromol. 2014, 65, 500–508. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, S.; Duan, H.; He, J.; Luo, Y. Removal of anionic and cationic dyes using porous chitosan/carboxymethyl cellulose-PEG hydrogels: Optimization, adsorption kinetics, isotherm and thermodynamics studies. Int. J. Biol. Macromol. 2023, 231, 123213. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, A.C.; Shojaosadati, S.A. Magnetic pectin-Chlorella vulgaris biosorbent for the adsorption of dyes. J. Environ. Chem. Eng. 2019, 7, 103062. [Google Scholar] [CrossRef]
- Al-Ahmed, Z.A.; Hassan, A.A.; El-Khouly, S.M.; El-Shafey, S.E. TEMPO-oxidized cellulose nanofibers/TiO2 nanocomposite as new adsorbent for Brilliant Blue dye removal. Polym. Bull. 2020, 77, 6213–6226. [Google Scholar] [CrossRef]
- Zhang, Y.; Hong, X.; Cao, X.; Huang, X.; Hu, B.; Ding, S.; Lin, H. Functional porous organic polymers with conjugated triaryl triazine as the core for superfast adsorption removal of organic dyes. ACS Appl. Mater. Interfaces 2021, 13, 6359–6366. [Google Scholar] [CrossRef] [PubMed]
- Sari Yilmaz, M. Graphene oxide/hollow mesoporous silica composite for selective adsorption of methylene blue. Micropor. Mesopor. Mat. 2022, 330, 111570. [Google Scholar] [CrossRef]
- Gao, P.; Chen, D.; Chen, W.; Sun, J.; Wang, G.; Zhou, L. Facile synthesis of amine-crosslinked starch as an efficient biosorbent for adsorptive removal of anionic organic pollutants from water. Int. J. Biol. Macromol. 2021, 191, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Zhou, L. Two birds with one stone: One-pot hydrothermal treatment of dragon fruit peel to simultaneously prepare efficient biochar adsorbent and fluorescent carbon quantum dots. Ind. Crop. Prod. 2024, 210, 118086. [Google Scholar] [CrossRef]
- Liu, M.; Zheng, J.; Wang, L.; Hu, Z.; Lan, S.; Rao, W.; Liu, Y.; Xie, Y.; Yu, C. Ultrafast and selective adsorption of anionic dyes with amine-functionalized glucose-based adsorbents. J. Mol. Struct. 2022, 1263, 133150. [Google Scholar] [CrossRef]
- El-Sewify, I.M.; Ma, S. Recent development of metal–organic frameworks for water purification. Langmuir 2024, 40, 5060–5076. [Google Scholar] [CrossRef]
- Gomaa, H.; Shenashen, M.A.; Elbaz, A.; Kawada, S.; El-Nasr, T.A.S.; Cheira, M.F.; Eid, A.I.; El-Safty, S.A. Inorganic-organic mesoporous hybrid segregators for selective and sensitive extraction of precious elements from urban mining. J. Colloid. Interf. Sci. 2021, 604, 61–79. [Google Scholar] [CrossRef]
- Shi, Y.; Chang, Q.; Zhang, T.; Song, G.; Sun, Y.; Ding, G. A review on selective dye adsorption by different mechanisms. J. Environ. Chem. Eng. 2022, 10, 108639. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Abdelfattah, A.M.; Tharwat, R.M.; Nabil, G.M. Adsorption of negatively charged food tartrazine and sunset yellow dyes onto positively charged triethylenetetramine biochar: Optimization, kinetics and thermodynamic study. J. Mol. Liq. 2020, 318, 114297. [Google Scholar] [CrossRef]
- Amaku, J.F.; Taziwa, R. Thermodynamics, kinetics and isothermal studies of tartrazine adsorption onto microcline/MWCNTs nanocomposite and the regeneration potentials. Sci. Rep. 2023, 13, 9872. [Google Scholar] [CrossRef]
- Deng, S.; Long, J.; Dai, X.; Wang, G.; Zhou, L. Simultaneous detection and adsorptive removal of Cr(VI) ions by fluorescent sulfur quantum dots embedded in chitosan hydrogels. ACS. Appl. Nano. Mater. 2023, 6, 1817–1827. [Google Scholar] [CrossRef]
- Radwan, A.; Mohamed, S.O.; Khalil, M.M.; El-Sewify, I.M. Effective adsorption of fluorescent congo red azo dye from aqueous solution by green synthesized nanosphere ZnO/CuO composite using propolis as bee byproduct extract. Sci. Rep. 2024, 14, 9061. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, S.; Epifano, F.; Palumbo, L.; Collevecchio, C.; Bastianini, M.; Cardellini, F.; Spogli, R.; Genovese, S. Efficient removal of tartrazine from aqueous solutions by solid sorbents. Sep. Purif. Technol. 2022, 290, 120910. [Google Scholar] [CrossRef]
- Liao, H.; Wang, Z. Adsorption removal of amaranth by nanoparticles-composed Cu2O microspheres. J. Alloy. Compd. 2018, 769, 1088–1095. [Google Scholar] [CrossRef]
- Monier, M.; Abdel-Latif, D.A. Preparation of cross-linked magnetic chitosan-phenylthiourea resin for adsorption of Hg(II), Cd(II) and Zn(II) ions from aqueous solutions. J. Hazard. Mater. 2012, 209, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, L.; Wang, X.; Zheng, W.; Hao, C.; Jiang, C.; Wu, J. Synthesis of aminated calcium lignosulfonate and its adsorption properties for azo dyes. Ind. Eng. Chem. 2018, 61, 321–330. [Google Scholar] [CrossRef]
- Shu, Q.; Qiu, W.; Luo, M.; Xiao, L. Morphology-controlled hydrothermal synthesis of copper selenides with orange juice for highly efficient cationic dyes adsorption. Mater. Today Sustain. 2022, 17, 100094. [Google Scholar] [CrossRef]
- Lentz, L.; Mayer, D.A.; Dogenski, M.; Ferreira, S.R.S. Hybrid aerogels of sodium alginate/graphene oxide as efficient adsorbents for wastewater treatment. Mater. Chem. Phys. 2022, 283, 125981. [Google Scholar] [CrossRef]
- Long, Y.; Xiao, L.; Cao, Q. Co-polymerization of catechol and polyethylenimine on magnetic nanoparticles for efficient selective removal of anionic dyes from water. Powder. Technol. 2017, 310, 24–34. [Google Scholar] [CrossRef]
- Wang, X.; Xie, P.; He, L.; Liang, Y.; Zhang, L.; Miao, Y.; Liu, Z. Ultralight, mechanically enhanced, and thermally improved graphene-cellulose-polyethyleneimine aerogels for the adsorption of anionic and cationic dyes. Nanomaterials 2022, 12, 1727. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Woo, M.W.; Chen, H.; Dang, X.; Mansouri, S.; Shan, Z. Hydrogel beads based on oxidized corn starch cross-linked with gelatin for tartrazine adsorption from aqueous environments. Polym. J. 2017, 49, 549–555. [Google Scholar] [CrossRef]
- Singh, S.; Arputharaj, E.; Dahms, H.U.; Patel, A.K.; Huang, Y.L. Chitosan-based nanocomposites for removal of Cr(VI) and synthetic food colorants from wastewater. Bioresource. Technol. 2022, 351, 127018. [Google Scholar] [CrossRef]
- Lin, K.Y.A.; Wu, C.H. Efficient adsorptive removal of toxic amaranth dye from water using a zeolitic imidazolate framework. Water. Environ. Res. 2018, 90, 1947–1955. [Google Scholar] [CrossRef] [PubMed]
- Karanfil, D.Y.; Coşkun, R.; Delibaş, A. Aminated magnetic polymeric resin for removal of anthraquinone and azo dyes from aqueous solutions. J. Polym. Res. 2022, 29, 87. [Google Scholar] [CrossRef]
- Tan, J.; Song, Y.; Huang, X.; Zhou, L. Facile functionalization of natural peach gum polysaccharide with multiple amine groups for highly efficient removal of toxic hexavalent chromium (Cr(VI)) ions from water. ACS Omega 2018, 3, 17309–17318. [Google Scholar] [CrossRef]
- Prabakaran, E.; Pillay, K.; Brink, H. Hydrothermal synthesis of magnetic-biochar nanocomposite derived from avocado peel and its performance as an adsorbent for the removal of methylene blue from wastewater. Mater. Today Sustain. 2022, 18, 100123. [Google Scholar] [CrossRef]






| Dye | Chemical Structure | λmax (nm) | Molecular Weight (g mol−1) |
|---|---|---|---|
| ART |  | 521 | 604.47 |
| TTZ |  | 426 | 534.36 |
| MO |  | 463 | 327.33 |
| MB |  | 662 | 319.85 |
| MV |  | 583 | 407.99 |
| RB |  | 554 | 479.01 |
| Kinetic Model | Parameter | Dye | |
|---|---|---|---|
| ART | TTZ | ||
| Pseudo-first-order: ln(Qe − Qt) = lnQe − k1t | k1 (min−1) | 0.0762 | 0.0613 |
| Qe-cal (mg g−1) | 99.96 | 85.55 | |
| R2 | 0.9538 | 0.8570 | |
| Pseudo-second-order: t/Qt = 1/k2Qe2 + t/Qe | k2 (min−1) | 0.0038 | 0.0042 |
| Qe-cal (mg g−1) | 680.3 | 411.5 | |
| Qe-exp (mg g−1) | 667.5 | 404.8 | |
| R2 | 0.9991 | 0.9997 | |
| Intraparticle diffusion: Qt = kit0.5 + C | Ki (mg g−1 min−1/2) | 20.71 | 21.55 |
| C (mg g−1) | 2.235 | 4.225 | |
| R2 | 0.7685 | 0.7124 | |
| Isotherm Model | Parameters | ART | TTZ |
|---|---|---|---|
| Langmuir: Ce/Qe = Ce/Qm + 1/QmKL | Qm (mg g−1) | 675.68 | 534.76 |
| KL (L mg−1) | 0.0043 | 0.0027 | |
| R2 | 0.9984 | 0.9995 | |
| Freundlich: lnQe = lnKF + bFlnCe | KF (mg g−1) | 298.2 | 110.3 |
| bF | 0.0908 | 0.1799 | |
| R2 | 0.9121 | 0.7451 | |
| Temkin: Qe = RTlnat/bt + RTlnCe/bt | at (L g−1) | 32.26 | 0.1811 |
| bt (J mol−1) | 45.86 | 33.28 | |
| R2 | 0.8041 | 0.9635 |
| Adsorbents | Dye | Qmax (mg g−1) | Refs |
|---|---|---|---|
| Zeolitic Imidazolate Framework | ART | 121 | [56] |
| Fe3O4@catechol/PEI | ART | 146.2 | [52] |
| Graphene/Cellulose/Polyethyleneimine Aerogels | ART | 369.37 | [53] |
| Chitosan/bentonite composite | ART | 362.1 | [27] |
| ARPA | ART | 675.68 | This study |
| GO/CS hydrogel beads | TTZ | 293 | [54] |
| SA-GO aerogels | TTZ | 420.36 | [51] |
| Aminated magnetic polymeric resin | TTZ | 297 | [57] |
| PEI@AC@Fe3O4-CS/PVA composite | TTZ | 148 | [55] |
| ARPA | TTZ | 534.76 | This study |
| Dye | ΔH (kJ mol−1) | ΔS (J−1 mol−1 k−1) | ΔG (kJ mol−1) | ||
|---|---|---|---|---|---|
| 275 K | 298 K | 313 K | |||
| ART | 17.91 | 117.4 | −14.39 | −17.09 | −18.85 |
| TTZ | 8.612 | 98.20 | −18.39 | −20.65 | −22.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Liao, J.; Zeng, S.; Zhou, L. Facile Fabrication of Porous Adsorbent with Multiple Amine Groups for Efficient and Selective Removal of Amaranth and Tartrazine Dyes from Water. Materials 2024, 17, 2391. https://doi.org/10.3390/ma17102391
Chen Q, Liao J, Zeng S, Zhou L. Facile Fabrication of Porous Adsorbent with Multiple Amine Groups for Efficient and Selective Removal of Amaranth and Tartrazine Dyes from Water. Materials. 2024; 17(10):2391. https://doi.org/10.3390/ma17102391
Chicago/Turabian StyleChen, Qingli, Jie Liao, Sihua Zeng, and Li Zhou. 2024. "Facile Fabrication of Porous Adsorbent with Multiple Amine Groups for Efficient and Selective Removal of Amaranth and Tartrazine Dyes from Water" Materials 17, no. 10: 2391. https://doi.org/10.3390/ma17102391






