Preparation and Hydration Properties of Sodium Silicate-Activated Municipal Solid Waste Incineration Bottom Ash Composite Ground-Granulated Blast Furnace Slag Cementitious Materials
Abstract
:1. Introduction
2. Methods
2.1. Experimental Materials
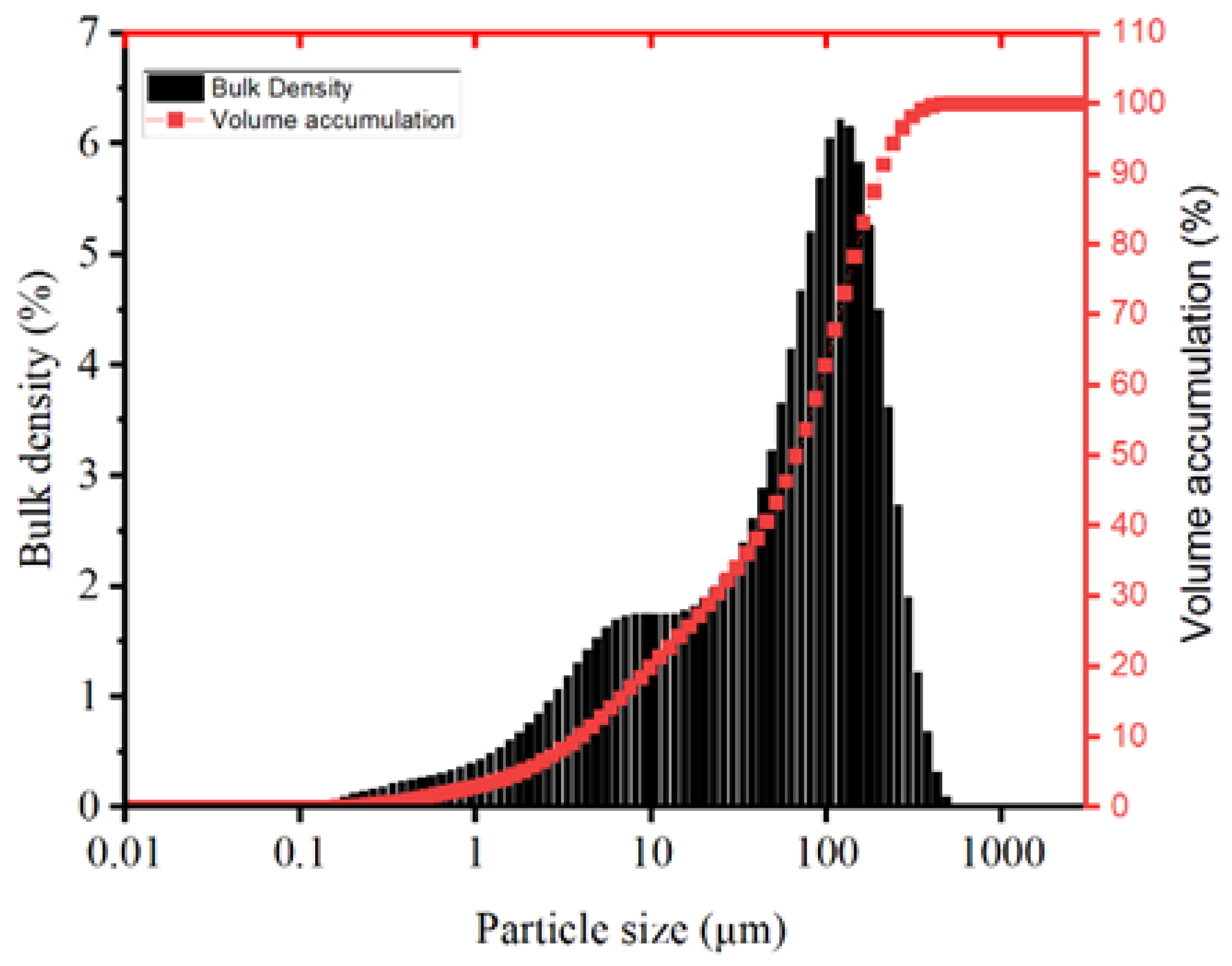
2.1.1. MSWIBA and GGBS
2.1.2. Sodium Silicate
2.1.3. Other Materials
2.2. Methods
2.2.1. Material Mixing
2.2.2. Experimental Methods
- Compressive strength
- 2.
- Isothermal calorimetry experiment
- 3.
- Hydration product analysis
3. Results and Discussion
3.1. Mechanical Properties
3.1.1. Mechanical Properties of Different MSWIBA Doping Levels
3.1.2. Mechanical Properties of Different Sodium Silicate Dosages
3.2. Hydration Process
3.2.1. Cumulative Heat
3.2.2. Heat Flow
3.2.3. Hydration Exothermic Equivalent Age Modeling
3.3. Hydration Products and Properties
3.3.1. XRD Analysis
3.3.2. Fourier-Transform Infrared (FTIR) Analysis
3.3.3. Scanning Electron Microscopy
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, R.V.; de Brito, J.; Lynn, C.J.; Dhir, R.K. Environmental impacts of the use of bottom ashes from municipal solid waste incineration: A review. Resour. Conserv. Recycl. 2019, 140, 23–35. [Google Scholar] [CrossRef]
- Barros, R.M. 4-Municipal solid waste ash. In Sustainable Concrete Made with Ashes and Dust from Different Sources; Siddique, R., Belarbi, R., Eds.; Woodhead Publishing: Cambridge, UK, 2022; pp. 93–177. [Google Scholar]
- Stegemann, J.A.; Schneider, J.; Baetz, B.W.; Murphy, K.L. Lysimeter washing of MSW incinerator bottom ash. Waste Manag. Res. 1995, 13, 149–165. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Li, Z.; Xie, G.; Zhang, W.; Jin, H.; Xing, F. Investigation of the cyclic separation of dioxins from municipal solid waste incineration fly ash by using fat. J. Clean. Prod. 2024, 450, 141840. [Google Scholar] [CrossRef]
- Joseph, A.M.; Snellings, R.; Van den Heede, P.; Matthys, S.; De Belie, N. The Use of Municipal Solid Waste Incineration Ash in Various Building Materials: A Belgian Point of View. Materials 2018, 11, 141. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Mills-Beale, J.; Yang, X.; Dai, Q. Alternative Materials for Sustainable Transportation; Michigan Department of Transportation: St. Joseph, MI, USA, 2012.
- Provis, J.L. Geopolymers and other alkali activated materials: Why, how, and what? Mater. Struct. 2014, 47, 11–25. [Google Scholar] [CrossRef]
- Ali, M.B.; Saidur, R.; Hossain, M.S. A review on emission analysis in cement industries. Renew. Sustain. Energy Rev. 2011, 15, 2252–2261. [Google Scholar] [CrossRef]
- Wang, J.; Zhai, Z.; Jing, Y.; Zhang, C. Influence analysis of building types and climate zones on energetic, economic and environmental performances of BCHP systems. Appl. Energy 2011, 88, 3097–3112. [Google Scholar] [CrossRef]
- González-Torres, M.; Pérez-Lombard, L.; Coronel, J.F.; Maestre, I.R.; Yan, D. A review on buildings energy information: Trends, end-uses, fuels and drivers. Energy Rep. 2022, 8, 626–637. [Google Scholar] [CrossRef]
- Alves, B.I.; Marvila, M.T.; Linhares Júnior, J.A.; Vieira, C.M.; Alexandre, J.; de Azevedo, A.R. Alkaline Activation of Binders: A Comparative Study. Materials 2024, 17, 667. [Google Scholar] [CrossRef]
- Gao, X.; Yuan, B.; Yu, Q.L.; Brouwers, H.J.H. Characterization and application of municipal solid waste incineration (MSWI) bottom ash and waste granite powder in alkali activated slag. J. Clean. Prod. 2017, 164, 410–419. [Google Scholar] [CrossRef]
- Tang, P.; Chen, W.; Xuan, D.; Zuo, Y.; Poon, C.S. Investigation of cementitious properties of different constituents in municipal solid waste incineration bottom ash as supplementary cementitious materials. J. Clean. Prod. 2020, 258, 120675. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Nagaishi, M.; Kisu, K.; Nakamura, Y.; Ikeda, K. Preparation of monolithic geopolymer materials from urban waste incineration slags. J.-Ceram. Soc. Jpn. 2013, 121, 847–854. [Google Scholar] [CrossRef]
- Łach, M.; Mierzwiński, D.; Korniejenko, K.; Mikuła, J.; Hebda, M. Geopolymers as a material suitable for immobilization of fly ash from municipal waste incineration plants. J. Air Waste Manag. Assoc. 2018, 68, 1190–1197. [Google Scholar] [CrossRef]
- Peceño, B.; Luna-Galiano, Y.; Varela, F.; Alonso-Fariñas, B.; Leiva, C. Study of a Fire-Resistant Plate Containing Fly Ashes Generated from Municipal Waste Incinerator: Fire and Mechanical Characteristics and Environmental Life Cycle Assessment. Materials 2024, 17, 1813. [Google Scholar] [CrossRef]
- Vilarinho, I.S.; Guimarães, G.; Labrincha, J.A.; Seabra, M.P. Development of Eco-Mortars with the Incorporation of Municipal Solid Wastes Incineration Ash. Materials 2023, 16, 6933. [Google Scholar] [CrossRef]
- Malaiškienė, J.; Spudulis, E.; Stonys, R. The Effect of Milled Municipal Solid Waste Incineration Bottom Ash on Cement Hydration and Mortar Properties. Materials 2023, 16, 2528. [Google Scholar] [CrossRef]
- Thomas, M.; Ślosarczyk, A. Effect of Municipal Solid Waste Slag on the Durability of Cementitious Composites in Terms of Resistance to Freeze–Thaw Cycling. Materials 2023, 16, 626. [Google Scholar] [CrossRef]
- Poranek, N.; Łaźniewska-Piekarczyk, B.; Lombardi, L.; Czajkowski, A.; Bogacka, M.; Pikoń, K. Green Deal and Circular Economy of Bottom Ash Waste Management in Building Industry—Alkali (NaOH) Pre-Treatment. Materials 2022, 15, 3487. [Google Scholar] [CrossRef]
- Czop, M.; Łaźniewska-Piekarczyk, B. Use of Slag from the Combustion of Solid Municipal Waste as a Partial Replacement of Cement in Mortar and Concrete. Materials 2020, 13, 1593. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.L.; Lin, D.F. Hydration characteristics of municipal solid waste incinerator bottom ash slag as a pozzolanic material for use in cement. Cem. Concr. Compos. 2006, 28, 817–823. [Google Scholar] [CrossRef]
- Lynn, C.J.; Dhir, R.K.; Ghataora, G.S. Municipal incinerated bottom ash characteristics and potential for use as aggregate in concrete. Constr. Build. Mater. 2016, 127, 504–517. [Google Scholar] [CrossRef]
- Pouhet, R.; Cyr, M. Carbonation in the pore solution of metakaolin-based geopolymer. Cem. Concr. Res. 2016, 88, 227–235. [Google Scholar] [CrossRef]
- Ryu, G.S.; Lee, Y.B.; Koh, K.T.; Chung, Y.S. The mechanical properties of fly ash-based geopolymer concrete with alkaline activators. Constr. Build. Mater. 2013, 47, 409–418. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Li, J.; Hou, Z.; Jin, C. Use of slaked lime and Portland cement to improve the resistance of MSWI bottom ash-GBFS geopolymer concrete against carbonation. Constr. Build. Mater. 2018, 166, 290–300. [Google Scholar] [CrossRef]
- Xiaolong, Z.; Shiyu, Z.; Hui, L.; Yingliang, Z. Disposal of mine tailings via geopolymerization. J. Clean. Prod. 2021, 284, 124756. [Google Scholar] [CrossRef]
- Krivenko, P.; Pushkareva, E.; Chirkova, V. Processes of the calcium silicate hydration in the presence of alkali-metal compounds. Izv. Vyss. Uchebnykh Zaved. Khimiya I Khimicheskaya Tekhnologiya 1985, 28, 70–74. [Google Scholar]
- Provis, J.L.; Duxson, P.; Van Deventer, J.S.J. Geopolymer Technology and the Search for a Low-CO2 Alternative to Concrete; American Institute of Chemical Engineers: Salt Lake City, UT, USA, 2007. [Google Scholar]
- Davidovits, J. Geopolymers and geopolymeric materials. J. Therm. Anal. 1989, 35, 429–441. [Google Scholar] [CrossRef]
- Moussadik, A.; Ouzoun, F.; Ez-zaki, H.; Saadi, M.; Diouri, A. Mineralogical study of a binder based on alkali-activated coal gangue. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Setlak, K.; Mikuła, J.; Łach, M. Application of Industrial Waste Materials by Alkaline Activation for Use as Geopolymer Binders. Materials 2023, 16, 7651. [Google Scholar] [CrossRef]
- Kwek, S.Y.; Awang, H.; Cheah, C.B.J.M. Influence of liquid-to-solid and alkaline activator (Sodium silicate to sodium hydroxide) ratios on fresh and hardened properties of alkali-activated palm oil fuel ash geopolymer. Materials 2021, 14, 4253. [Google Scholar] [CrossRef]
- Sun, K.; Peng, X.; Wang, S.; Zeng, L.; Ran, P.; Ji, G.J.C.; Materials, B. Effect of nano-SiO2 on the efflorescence of an alkali-activated metakaolin mortar. Constr. Build. Mater. 2020, 253, 118952. [Google Scholar] [CrossRef]
- Adeleke, B.O.; Kinuthia, J.M.; Oti, J.; Pirrie, D.; Power, M. Mechanical and Microstructural Investigation of Geopolymer Concrete Incorporating Recycled Waste Plastic Aggregate. Materials 2024, 17, 1340. [Google Scholar] [CrossRef]
- Sirotti, M.; Delsaute, B.; Staquet, S. New Experimental Evidence for Drying Shrinkage of Alkali-Activated Slag with Sodium Hydroxide. Materials 2023, 16, 5659. [Google Scholar] [CrossRef]
- Naqi, A.; Delsaute, B.; Königsberger, M.; Staquet, S. Effect of Solution-to-Binder Ratio and Alkalinity on Setting and Early-Age Properties of Alkali-Activated Slag-Fly Ash Binders. Materials 2023, 16, 373. [Google Scholar] [CrossRef]
- GB/T 203-2008; Granulated Blastfurnace Slag Used for Cement Production. National Cement Standardization Committee: Beijing, China, 2008.
- GB/T 18046-2017; Ground Granulated Blast Furnace Slag Used for Cement, Mortar and Concrete. National Cement Standardization Committee: Beijing, China, 2017.
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). National Standards—State Administration for Market Supervision and Administration: Beijing, China, 2021.
- Asadizadeh, M.; Hedayat, A.; Tunstall, L.; Gonzalez, J.A.V.; Alvarado, J.W.V.; Neira, M.T. The impact of slag on the process of geopolymerization and the mechanical performance of mine-tailings-based alkali-activated lightweight aggregates. Constr. Build. Mater. 2024, 411, 134347. [Google Scholar] [CrossRef]
- Zhao, J.; Long, B.; Yang, G.; Cheng, Z.; Liu, Q. Characteristics of alkali-activated slag powder mixing with seawater: Workability, hydration reaction kinetics and mechanism. Case Stud. Constr. Mater. 2022, 17, e01381. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Zhang, Y.; Gu, X. Research on hydration characteristics of OSR-GGBFS-FA alkali-activated materials. Constr. Build. Mater. 2024, 411, 134321. [Google Scholar] [CrossRef]
- Huang, G.; Yang, K.; Sun, Y.; Lu, Z.; Zhang, X.; Zuo, L.; Feng, Y.; Qian, R.; Qi, Y.; Ji, Y.; et al. Influence of NaOH content on the alkali conversion mechanism in MSWI bottom ash alkali-activated mortars. Constr. Build. Mater. 2020, 248, 118582. [Google Scholar] [CrossRef]
- Mitrović, A.; Zdujić, M. Preparation of pozzolanic addition by mechanical treatment of kaolin clay. Int. J. Miner. Process. 2014, 132, 59–66. [Google Scholar] [CrossRef]
- Li, B.; Liu, Z.; Sun, Q.; Yang, L. Preparation of alkali-activated nickel slag-based cemented tailings backfill: Workability, strength characteristics, localized deformation and hydration mechanism. Constr. Build. Mater. 2024, 411, 134639. [Google Scholar] [CrossRef]
- Ahmad, M.R.; Qian, L.-P.; Fang, Y.; Wang, A.; Dai, J.-G. A multiscale study on gel composition of hybrid alkali-activated materials partially utilizing air pollution control residue as an activator. Cem. Concr. Compos. 2023, 136, 104856. [Google Scholar] [CrossRef]









| Oxides | SiO2 | CaO | Al2O3 | Fe2O3 | P2O5 | Na2O | MgO | SO3 | K2O |
|---|---|---|---|---|---|---|---|---|---|
| MSWIBA | 37.79 | 28.60 | 9.34 | 5.94 | 5.22 | 3.25 | 2.74 | 2.33 | 1.52 |
| GGBS | 30.49 | 40.83 | 15.26 | 0.32 | 0.02 | 0.49 | 8.50 | 2.08 | 0.45 |
| Number | MSWIBA | Sodium Silicate | GGBS | W/C |
|---|---|---|---|---|
| B5S25 | 5 | 25 | 70 | 0.5 |
| B29S25 | 29 | 25 | 46 | 0.5 |
| B17S25 | 17 | 25 | 58 | 0.5 |
| B29S15 | 29 | 15 | 56 | 0.5 |
| B29S35 | 29 | 35 | 36 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, J.; Wu, G.; Xia, Y.; Liu, L. Preparation and Hydration Properties of Sodium Silicate-Activated Municipal Solid Waste Incineration Bottom Ash Composite Ground-Granulated Blast Furnace Slag Cementitious Materials. Materials 2024, 17, 2406. https://doi.org/10.3390/ma17102406
Deng J, Wu G, Xia Y, Liu L. Preparation and Hydration Properties of Sodium Silicate-Activated Municipal Solid Waste Incineration Bottom Ash Composite Ground-Granulated Blast Furnace Slag Cementitious Materials. Materials. 2024; 17(10):2406. https://doi.org/10.3390/ma17102406
Chicago/Turabian StyleDeng, Juan, Guoxiong Wu, Yuchao Xia, and Li Liu. 2024. "Preparation and Hydration Properties of Sodium Silicate-Activated Municipal Solid Waste Incineration Bottom Ash Composite Ground-Granulated Blast Furnace Slag Cementitious Materials" Materials 17, no. 10: 2406. https://doi.org/10.3390/ma17102406




