Synergistic Effect of PBz/Epoxy/PCLA Composite Films with Improved Thermal Properties
Abstract
:1. Introduction
2. Materials
3. Methods
3.1. Synthesis of Benzoxazine Monomer (6-Allyl-8-methoxy-3-ethylamine-3,4-dihydro-1,3-benzoxazine) (Eu-Bzo)
3.2. Preparation of PBz/Epoxy/PCLA Films
3.3. Instrumentation Methods
4. Results and Discussion
4.1. Structure Analysis of Eu-Bzo
4.2. Polymerization Behavior of Bzo/Epoxy/CPLA Blends
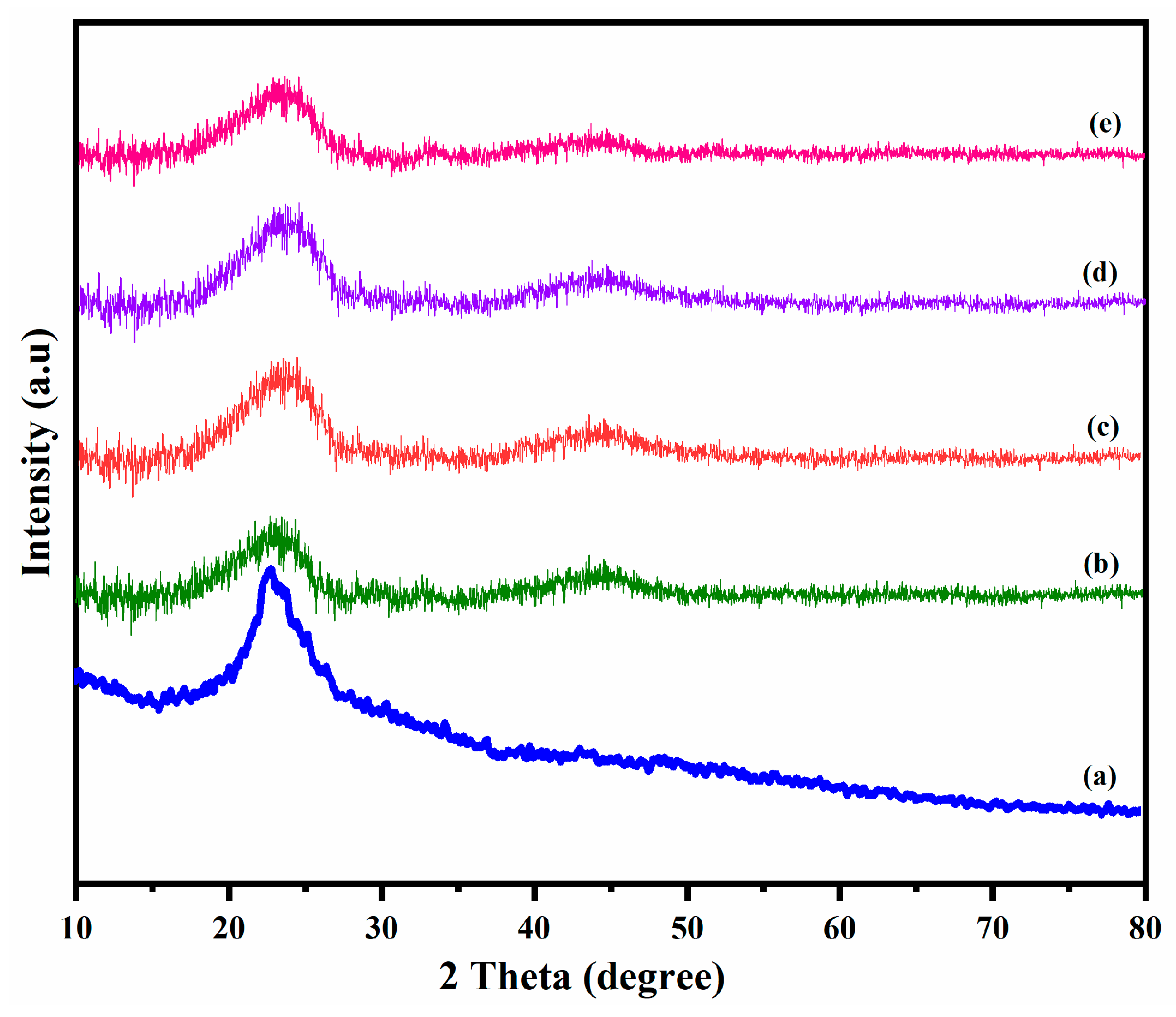
4.3. Structural Analysis of PBz/Epoxy/PCLA Films
4.4. Microscopic Exploration of PBz/Epoxy/PCLA Films
4.5. Optical Image of PBz/Epoxy/PCLA Films
4.6. Hydrophobicity of PBz/Epoxy/PCLA Films
4.7. Thermal Stability of PBz/Epoxy/PCLA Composite Films
4.8. Flame Retardancy of PBz/Epoxy/PCLA Composite Films
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alper, K.; Tekin, K.; Ragauskas, A.J. Sustainable Energy and Fuels from Biomass: A Review Focusing on Hydrothermal Biomass. Sustain. Energy Fuels 2020, 4, 4390–4414. [Google Scholar] [CrossRef]
- Matsumura, S.; Hlil, A.R.; Lepiller, C.; Gaudet, J.; Guay, D.; Shi, Z.; Holdcroft, S.; Hay, A.S. Stability and Utility of Pyridyl Disulfide Functionality in RAFT and Conventional Radical Polymerizations. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 7207–7224. [Google Scholar]
- Hong, M.; Chen, E.Y.X. Future Directions for Sustainable Polymers. Trends Chem. 2019, 1, 148–151. [Google Scholar] [CrossRef]
- Kiskan, B. Adapting benzoxazine Chemistry for Unconventional Applications. React. Funct. Polym. 2018, 129, 76–88. [Google Scholar] [CrossRef]
- Periyasamy, T.; Asrafali, S.P.; Muthusamy, S.; Kim, S.C. Replacing Bisphenol-A with Bisguaiacol-F to Synthesize Polybenzoxazines for a Pollution-Free Environment. New J. Chem. 2016, 40, 9313–9319. [Google Scholar] [CrossRef]
- Sawaryn, C.; Landfester, K.; Taden, A. Cationic Polybenzoxazines. A Novel Polyelectrolyte Class with Adjustable Solubility and Unique Hydrogen-Bonding Capabilities. Macromolecules 2011, 44, 7668–7674. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Parveen, A.S.; Sarojadevi, M. Synthesis and Copolymerization of Fully biobased benzoxazines from Renewable Resources. ACS Sustain. Chem. Eng. 2014, 2, 2790–2801. [Google Scholar] [CrossRef]
- Rybyan, A.A.; Bilichenko, J.V.; Kireev, V.V.; Kolenchenko, A.A.; Chistyakov, E.M. Curing of DER-331 Epoxy Resin with Arylaminocyclotriphosphazenes Based on o-, m-, and p-methylanilines. Polymers 2022, 14, 5334. [Google Scholar] [CrossRef] [PubMed]
- Orlov, A.; Konstantinova, A.; Korotkov, R.; Yudaev, P.; Mezhuev, Y.; Terekhov, I.; Gurevich, L.; Chistyakov, E. Epoxy Compositions with Reduced Flammability Based on DER-354 Resin and a Curing Agent Containing Aminophosphazenes Synthesized in Bulk Isophoronediamine. Polymers 2022, 14, 3592. [Google Scholar] [CrossRef]
- Konstantinova, A.; Yudaev, P.; Orlov, A.; Loban, O.; Lukashov, N.; Chistyakov, E. Aryloxyphosphazene-Modified and Graphite-Filled Epoxy Compositions with Reduced Flammability and Electrically Conductive Properties. J. Compos. Sci. 2023, 7, 417. [Google Scholar] [CrossRef]
- Koz, B.; Kiskan, B.; Yagci, Y. A novel benzoxazine monomer with methacrylate functionality and its thermally curable (co)polymers. Polym. Bull. 2011, 66, 165–174. [Google Scholar] [CrossRef]
- Yagci, Y.; Kiskan, B.; Ghosh, N.N. Recent advancement on polybenzoxazine—A newly developed high performance thermoset. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 5565–5576. [Google Scholar] [CrossRef]
- Li, X.; Xia, Y.; Xu, W.; Ran, Q.; Gu, Y. The curing procedure for a benzoxazine-cyanate-epoxy system and the properties of the terpolymer. Polym. Chem. 2012, 3, 1629–1633. [Google Scholar] [CrossRef]
- Barry, J.; Fritz, M.; Brender, J.R.; Smith, P.E.S.; Lee, D.-K.; Ramamoorthy, A. Determining the Effects of Lipophilic Drugs on Membrane Structure by Solid-State NMR Spectroscopy: The Case of the Antioxidant Curcumin. J. Am. Chem. Soc. 2009, 131, 4490–4498. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.N.; Ko, W.-K.; Moon, H.-J.; Kim, H.-J.; Lee, S.J.; Lee, J.B. Inhibition of osteoclast differentiation by gold nanoparticles functionalized with cyclodextrin curcumin complexes. ACS Nano 2014, 8, 12049–12062. [Google Scholar] [CrossRef]
- Mohamed Mydeen, K.; Arumugam, H.; Krishnasamy, B.; Sathy Srikandan, S.; Muthukaruppan, A. Sustainable L-Tyrosine Based Bio-benzoxazines for Efficient Protection of Mild Steel Surfaces from Marine Environment. Int. J. Adhes. Adhes. 2023, 125, 103405. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Shakila Parveen, A.; Atchudan, R.; Kim, S.C. Sustainability and Antimicrobial Assessments of Bio Based Polybenzoxazine Film. Eur. Polym. J. 2018, 109, 248–256. [Google Scholar] [CrossRef]
- Gupta, N.; Kumar, B.; Ansari, M.; Dilsad, M.; Arihant, I. Biomass Conversion of Agricultural Waste Residues for Different Applications: A Comprehensive Review. Environ. Sci. Pollut. Res. 2022, 73622–73647. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil—New Insights into Selected Therapeutic Applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef]
- Lu, G.; Dai, J.; Liu, J.; Tian, S.; Xu, Y.; Teng, N.; Liu, X. A New Sight into Bio-Based Polybenzoxazine: From Tunable Thermal and Mechanical Properties to Excellent Marine Antifouling Performance. ACS Omega 2020, 5, 3763–3773. [Google Scholar] [CrossRef]
- Chen, J.; Jian, R.; Yang, K.; Bai, W.; Huang, C.; Lin, Y.; Zheng, B.; Wei, F.; Lin, Q.; Xu, Y. Urushiol-Based benzoxazine Copper Polymer with Low Surface Energy, Strong Substrate Adhesion and Antibacterial for Marine Antifouling Application. J. Clean. Prod. 2021, 318, 128527. [Google Scholar] [CrossRef]
- Ma, W.; Ding, Y.; Zhang, M.; Gao, S.; Li, Y.; Huang, C.; Fu, G. Nature-Inspired Chemistry toward Hierarchical superhydrophobic, Antibacterial and Biocompatible Nanofibrous Membranes for Effective UV-Shielding, Self-Cleaning and Oil-Water Separation. J. Hazard. Mater. 2020, 384, 121476. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.; Shi, Z.; Mei, J.; Wang, X.; Yan, D.; Cui, Z. Novel High-k Polymers as Dielectric Layers for Organic Thin-Film Transistors. Polym. Chem. 2015, 6, 6651–6658. [Google Scholar] [CrossRef]
- Lyu, Y.; Ishida, H. Natural-Sourced benzoxazine Resins, Homopolymers, Blends and Composites: A Review of Their Synthesis Manufacturing and Applications. Prog. Polym. Sci. 2019, 99, 101168. [Google Scholar] [CrossRef]
- Selvaraj, K.; Arumugam, H.; Muthukaruppan, A.; Kannaiyan, S.K.; Krishnan, S.; Peethambaram, P.; Magaraphan, R.; Kannaiyan, D. Supercapacitor and High k Properties of CNT–PbS Reinforced Quinoxaline Amine Based Polybenzoxazine Composites. Soft Matter 2022, 18, 8779–8791. [Google Scholar] [CrossRef] [PubMed]
- Madesh, P.; Ramachandran, S.; Appasamy, S.; Krishnasamy, B.; Muthu, K.; Muthukaruppan, A. Valorization of Agricultural Waste to Polybenzoxazine-Carbon Composites: Studies on Microstructure, Thermal and Dielectric Properties. Eur. Polym. J. 2023, 197, 112355. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, H.; Lan, T.; Liu, X.; Ran, Q. High Electromagnetic Interference Shielding Effectiveness Achieved by Multiple Internal Reflection and Absorption in Polybenzoxazine/Graphene Foams. J. Appl. Polym. Sci. 2021, 138, 51318. [Google Scholar] [CrossRef]
- Li, B.; Salcedo-galan, F.; Xidas, P.I.; Manias, E. Improving Electrical Breakdown Strength of Polymer Nanocomposites by Tailoring Hybrid-Filler Structure for High- Voltage Dielectric Applications. ACS Appl. Nano Mater. 2018, 1, 4401. [Google Scholar] [CrossRef]
- Wang, C.; Sun, J.; Liu, X.; Sudo, A.; Endo, T. Synthesis and copolymerization of fully bio-based benzoxazines from guaiacol, furfurylamine and stearylamine. Green Chem. 2012, 14, 2799–2806. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Shakila Parveen, A.; Sarojadevi, M. New benzoxazines containing polyhedral oligomeric silsesquioxane from eugenol, guaiacol and vanillin. New J. Chem. 2015, 39, 1691–1702. [Google Scholar]
- Thirukumaran, P.; Shakila Parveen, A.; Sarojadevi, M. Synthesis and characterization of novel bio-based benzoxazines from eugenol. RSC Adv. 2014, 4, 7959–7966. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Ranjith Kumar, M.; Shakila Parveen, A.; Atchudan, R.; Kim, S.-C. Sustainability and antimicrobial assessments of apigenin based polybenzoxazine film. Polymer 2019, 172, 100–109. [Google Scholar] [CrossRef]
- Agag, T.; Arza, C.R.; Maurer, F.H.H.; Ishida, H. Primary amine-functional benzoxazine monomers and their use for amide-containing monomeric benzoxazines. Macromolecules 2010, 43, 2748–2758. [Google Scholar] [CrossRef]
- Takeichi, T.; Yong, G.; Rimdusit, S. Performance improvement of polybenzoxazine by alloying with polyimide: Effect of preparation method on the properties. Polymer 2005, 46, 4909–4916. [Google Scholar] [CrossRef]
- Aydogan, B.; Sureka, D.; Kiskan, B.; Yagci, Y. Polysiloxane-containing benzoxazine moieties in the main chain. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 5156–5162. [Google Scholar] [CrossRef]
- Demir, K.D.; Kiskan, B.; Yagci, Y. Thermally Curable Acetylene-Containing Main-Chain Benzoxazine Polymers via Sonogashira Coupling Reaction. Macromolecules 2011, 44, 1801–1807. [Google Scholar] [CrossRef]
- Takeichi, T.; Agag, T.; Zeidam, R. Preparation of properties of polybenzoxazine/poly(imidesiloxane) alloys: In situ ring-opening polymerization of benzoxazine in the presence of soluble poly(imide-siloxane)s. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 2633–2641. [Google Scholar] [CrossRef]
- Chong, A.M.; Salazar, S.A.; Stanzione, J.F. Multifunctional biobased benzoxazines Blended with an Epoxy Resin for Tunable High-Performance Properties. ACS Sustain. Chem. Eng. 2021, 9, 5768–5775. [Google Scholar]
- Mohamed Mydeen, K.; Arumugam, H.; Krishnasamy, B.; Subramani, D.; Muthukaruppan, A. Nonylphenol-Based Polybenzoxazine Composites: Hydrophobic Coating, Ultra-Low-k and Anticorrosion Applications. J. Mater. Sci. 2023, 58, 10340–10358. [Google Scholar] [CrossRef]
- Dogan, Y.E.; Satilmis, B.; Uyar, T. Synthesis and Characterization of Bio-Based benzoxazines Derived from Thymol. J. Appl. Polym. Sci. 2019, 136, 47371. [Google Scholar] [CrossRef]
- Zhang, S.; Ran, Q.; Gu, Y. Polymerization Mechanism of 1,3-benzoxazine Catalyzed by PCl5 and Rearrangement of Chemical Structures. Eur. Polym. J. 2021, 142, 110133. [Google Scholar] [CrossRef]
- Yao, H.; Lu, X.; Xin, Z.; Li, X.; Chen, C.; Cao, Y. Colloids and Surfaces A: Physicochemical and Engineering Aspects Two Novel Eugenol-Based Difunctional benzoxazines: Synthesis and Properties. Colloids Surf. A 2021, 616, 126209. [Google Scholar] [CrossRef]
- Shah, M.; Srinivasan, H.; Arumugam, H.; Krishnasamy, B.; Muthukaruppan, A. Synthesis and Characterisation of Cycloaliphatic and Aromatic Amines Based cardanol benzoxazines: A Comparative Study. J. Mol. Struct. 2023, 1277, 134802. [Google Scholar] [CrossRef]
- Tudu, B.K.; Sinhamahapatra, A.; Kumar, A. Surface Modification of Cotton Fabric Using TiO2 Nanoparticles for Self-Cleaning, Oil-Water Separation, Antistain, Anti-Water Absorption, and Antibacterial Properties. ACS Omega 2020, 5, 7850–7860. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Chen, X.; Chen, C.; Hu, J.; Zhou, C.; Cai, X.; Wang, W.; Zheng, C.; Zhang, P.; Cheng, J.; et al. Durably Antibacterial and Bacterially Antiadhesive Cotton Fabrics Coated by Cationic Fluorinated Polymers. ACS Appl. Mater. Interfaces 2018, 10, 6124–6136. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, Q.; Yin, X.; Xu, J.; Cai, Y.; Han, L.; Huang, H.; Zhou, Y.; Tan, Y.; Wang, L.; et al. Fabrication of superhydrophobic and Superoleophilic Polybenzoxazine-Based Cotton Fabric for Oil–Water Separation. Cellulose 2018, 25, 6691–6704. [Google Scholar] [CrossRef]
- van Krevelen, D.W. Some Basic Aspects of Flame Resistance of Polymeric Materials. Polymer 1975, 16, 615–620. [Google Scholar] [CrossRef]
- Li, C.; Kang, N.J.; Labrandero, S.D.; Wan, J.; Lez, C.G.; Wang, D.Y. Synergistic Effect of Carbon Nanotube and Polyethersulfone on Flame Retardancy of Carbon Fiber Reinforced Epoxy Composites. Ind. Eng. Chem. Res. 2014, 53, 1040–1047. [Google Scholar] [CrossRef]










| S.No. | Samples | Ti (°C) | T5 (°C) | T10 (°C) | CY (%) | LOI |
|---|---|---|---|---|---|---|
| 1. | PBz/epoxy | 258 | 336 | 394 | 36.2 | 31.9 |
| 2. | PBz/epoxy/CPLA10 | 273 | 358 | 407 | 38.4 | 32.8 |
| 3. | PBz/epoxy/CPLA30 | 296 | 383 | 438 | 46.7 | 36.2 |
| 4. | PBz/epoxy/CPLA50 | 284 | 362 | 419 | 42.3 | 34.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Periyasamy, T.; Asrafali, S.P.; Kim, S.; Lee, J. Synergistic Effect of PBz/Epoxy/PCLA Composite Films with Improved Thermal Properties. Sustainability 2024, 16, 3991. https://doi.org/10.3390/su16103991
Periyasamy T, Asrafali SP, Kim S, Lee J. Synergistic Effect of PBz/Epoxy/PCLA Composite Films with Improved Thermal Properties. Sustainability. 2024; 16(10):3991. https://doi.org/10.3390/su16103991
Chicago/Turabian StylePeriyasamy, Thirukumaran, Shakila Parveen Asrafali, Seongcheol Kim, and Jaewoong Lee. 2024. "Synergistic Effect of PBz/Epoxy/PCLA Composite Films with Improved Thermal Properties" Sustainability 16, no. 10: 3991. https://doi.org/10.3390/su16103991





