Fiber-Reinforced Coal Gangue-Based Alumina Aerogel Composites with Highly Thermal Stability by Ambient Pressure Drying
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials and Methods
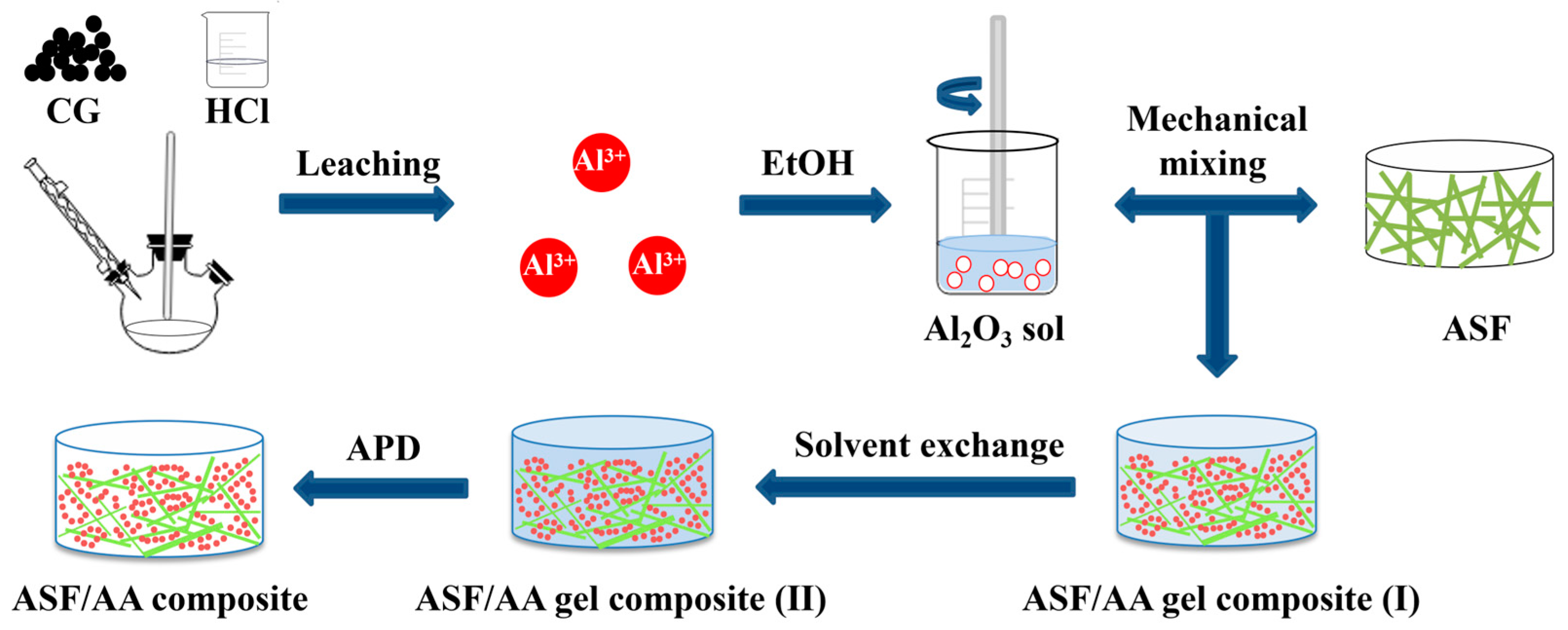
2.2. Preparation of AlCl3 Precursor
2.3. Preparation of Aluminum Silicate Fiber/Al2O3 Aerogel
2.4. Properties and Characterizations
3. Results and Discussion
3.1. Microstructure and Properties of Coal Gangue
3.2. Effects of Various Parameters on Alumina-Dissolution Rate of Coal Gangue
3.2.1. Effect of Temperature on Alumina-Dissolution Rate of Coal Gangue
3.2.2. Effect of Holding Time on Alumina-Dissolution Rate of Coal Gangue
3.3. Microstructure and Properties of Aluminum Silicate Fiber/Al2O3 Aerogel
3.4. Thermal Stability Property
4. Conclusions
- (1)
- The calcination temperature is 800 °C, the holding time is 0.5 h, and the mass ratio of acid waste is 0.96:1. Under this condition, most of the Al2O3 in CG can be extracted, and the extraction rate reaches 81.02%.
- (2)
- The final prepared ASF/AA has similar low density (0.26 g/cm3) and low thermal conductivity (0.047 W/(m·K)) at room temperature compared to the supercritical dried fiber/aerogel material.
- (3)
- ASF/AA has good high-temperature resistance. After two hours of heat treatment at 1200 °C, the thermal conductivity stayed low (0.071 W/(m·K)). The composite’s high-temperature thermal stability is enhanced by the mullite phase that forms after heat treatment above 1000 °C. The infrared imaging pictures show that the composite material has a strong ability to insulate against heat.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Wang, J. Comprehensive utilization and environmental risks of coal gangue: A review. J. Clean. Prod. 2019, 239, 117946. [Google Scholar] [CrossRef]
- Liang, Y.C.; Liang, H.D.; Zhu, S.Q. Mercury emission from spontaneously ignited coal gangue hill in Wuda coalfield, Inner Mongolia, China. Fuel 2016, 182, 525–530. [Google Scholar] [CrossRef]
- Li, Q.; Lv, L.; Zhao, X.; Wang, Y.; Wang, Y. Cost-effective microwave-assisted hydrothermal rapid synthesis of analcime-activated carbon composite from coal gangue used for Pb2+ adsorption. Environ. Sci. Pollut. Res. 2022, 29, 77788–77799. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H.; Zhou, Q.; Qi, T.; Liu, G.; Peng, Z. Efficient separation of silica and alumina in simulated CFB slag by reduction roasting-alkaline leaching process. Waste Manag. 2019, 87, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Zhang, Y.; Zhao, X.; Yang, N.; Wang, Z.; Guo, Z.; Tong, J.; Zhang, Y.; Liu, Z. Temperature Distribution Regularity and Dynamic Evolution of Spontaneous Combustion Coal Gangue Dump: Case Study of Yinying Coal Mine in Shanxi, China. Sustainability 2023, 15, 6362. [Google Scholar] [CrossRef]
- Li, L.; Long, G.; Bai, C.; Ma, K.; Wang, M.; Zhang, S. Utilization of Coal Gangue Aggregate for Railway Roadbed Construction in Practice. Sustainability 2020, 12, 4583. [Google Scholar] [CrossRef]
- Li, Y.; Yao, Y.; Liu, X.; Sun, H.; Ni, W. Improvement on pozzolanic reactivity of coal gangue by integrated thermal and chemical activation. Fuel 2013, 109, 527–533. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, Z.; Gao, Y.; Liu, J.; Wang, D.; Liu, H.; Zhang, Y.; Li, L. Synthesis and CO2 adsorption performance of high Si/Al ratio DDR zeolites prepared from silica fume. J. Environ. Chem. Eng. 2023, 11, 110837. [Google Scholar] [CrossRef]
- Xiong, J.; Zang, L.; Zha, J.; Mahmood, Q.; He, Z. Phosphate Removal from Secondary Effluents Using Coal Gangue Loaded with Zirconium Oxide. Sustainability 2019, 11, 2453. [Google Scholar] [CrossRef]
- Cai, H.; Jiang, Y.; Chen, Q.; Zhang, S.; Li, L.; Feng, J.; Feng, J. Sintering behavior of SiO2 aerogel composites reinforced by mullite fibers via in-situ rapid heating TEM observations. J. Eur. Ceram. Soc. 2020, 40, 127–135. [Google Scholar] [CrossRef]
- Koebel, M.; Rigacci, A.; Achard, P. Aerogel-based thermal superinsulation: An overview. J. Sol-Gel Sci. Technol. 2012, 63, 315–339. [Google Scholar] [CrossRef]
- Lamy-Mendes, A.; Pontinha, A.D.R.; Alves, P.; Santos, P.; Durães, L. Progress in silica aerogel-containing materials for buildings’ thermal insulation. Constr. Build. Mater. 2021, 286, 122815. [Google Scholar] [CrossRef]
- Xie, F.; Dai, X.; Zhuo, L.; Dai, Q.; He, C.; Lu, Z. Robust BNNS/ANF aerogel skeleton-based PEG composite phase change materials with high latent heat for efficient thermal management. Compos. Struct. 2023, 323, 117479. [Google Scholar] [CrossRef]
- Kotov, E.V.; Nemova, D.; Sergeev, V.; Dontsova, A.; Koriakovtseva, T.; Andreeva, D. Thermal Performance Assessment of Aerogel Application in Additive Construction of Energy-Efficient Buildings. Sustainability 2024, 16, 2398. [Google Scholar] [CrossRef]
- Baumann, T.F.; Kucheyev, S.O.; Gash, A.E.; Satcher, J.H. Facile synthesis of a cryHstalline, high-surface-area SnO2 aerogel. Adv. Mater. 2005, 17, 1546–1548. [Google Scholar] [CrossRef]
- Tokudome, Y.; Nakanishi, K.; Kanamori, K.; Fujita, K.; Akamatsu, H.; Hanada, T. Structural characterization of hierarchically porous alumina aerogel and xerogel monoliths. J. Colloid. Interf. Sci. 2009, 338, 506–513. [Google Scholar] [CrossRef]
- Bao, B.Y.; Wang, I.; Haines, T.; Ren, L.; Tian, H.; Hu, J. Methane decomposition for the production of COx-free hydrogen and carbon nanotubes over transition metal aerogel catalysts. Abstr. Pap. Am. Chem. Soc. 2018, 256. [Google Scholar]
- Gao, B.; Yuan, G.; Ren, L. Polydiacetylene-functionalized alumina aerogels as visually observable sensing materials for detecting VOCs concentration. J. Mater. Sci. 2018, 53, 6698–6706. [Google Scholar] [CrossRef]
- Hurwitz, F.I.; Gallagher, M.; Olin, T.C.; Shave, M.K.; Ittes, M.A.; Olafson, K.N.; Fields, M.G.; Rogers, R.B.; Guo, H.Q. Optimization of Alumina and Aluminosilicate Aerogel Structure for High-Temperature Performance. Int. J. Appl. Glass Sci. 2014, 5, 276–286. [Google Scholar] [CrossRef]
- Hayase, G.; Nonomura, K.; Hasegawa, G.; Kanamori, K.; Nakanishi, K. Ultralow-Density, Transparent, Superamphiphobic Boehmite Nanofiber Aerogels and Their Alumina Derivatives. Chem. Mater. 2015, 27, 3–5. [Google Scholar] [CrossRef]
- Zhang, Z.; Scherer, G.W. Supercritical drying of cementitious materials. Cem. Concr. Res. 2017, 99, 137–154. [Google Scholar] [CrossRef]
- Liu, F.; Jiang, Y.; Peng, F.; Feng, J.; Li, L.; Feng, J. Fiber-reinforced alumina-carbon core-shell aerogel composite with heat-induced gradient structure for thermal protection up to 1800 °C. Chem. Eng. J. 2023, 461, 141721. [Google Scholar] [CrossRef]
- Wang, H.; Quan, X.; Yin, L.; Jin, X.; Pan, Y.; Wu, C.; Huang, H.; Hong, C.; Zhang, X. Lightweight quartz fiber fabric reinforced phenolic aerogel with surface densified and graded structure for high temperature thermal protection. Compos. Part. A Appl. Sci. Manuf. 2022, 159, 107022. [Google Scholar] [CrossRef]
- Yang, X.; Wei, J.; Shi, D.; Sun, Y.; Lv, S.; Feng, J.; Jiang, Y. Comparative investigation of creep behavior of ceramic fiber-reinforced alumina and silica aerogel. Mater. Sci. Eng. A 2014, 609, 125–130. [Google Scholar] [CrossRef]
- Kim, C.-Y.; Lee, J.-K.; Kim, B.-I. Synthesis and pore analysis of aerogel-glass fiber composites by ambient drying method. Colloid. Surf. A 2008, 313, 179–182. [Google Scholar] [CrossRef]
- Zhang, R.; Hou, X.; Ye, C.; Wang, B. Enhanced mechanical and thermal properties of anisotropic fibrous porous mullite-zirconia composites produced using sol-gel impregnation. J. Alloys Compd. 2017, 699, 511–516. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, F.; Yao, H.; Dong, J.; Long, D. High-temperature Insulation Property of Opacifier-doped Al2O3-SiO2 Aerogel/Mullite Fiber Composites. J. Inorg. Mater. 2018, 33, 969–975. [Google Scholar]
- Du, D.; Sun, L.; Sun, C.; Liu, Z.; Guo, X.; Zhang, Y. Synthesis of alumina aerogel enriched carboxymethyl cellulose with polymodal pore size distribution by ambient pressure drying. Cellulose 2023, 30, 11369–11385. [Google Scholar] [CrossRef]
- Han, Y.; Wu, Y.; Huang, S.; Zhang, H.; Liang, Z.; Guan, X.; Wu, S. Convenient and rapid preparation of aerogels dried at ambient pressure. J. Non-Cryst. Solids 2023, 622, 122665. [Google Scholar] [CrossRef]
- Shewale, P.M.; Rao, A.V.; Rao, A.P. Effect of different trimethyl silylating agents on the hydrophobic and physical properties of silica aerogels. Appl. Surf. Sci. 2008, 254, 6902–6907. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, W.-Z.; Wang, X.; Li, Y.-M.; Tao, W.-Q. Thermal conductivity of fiber and opacifier loaded silica aerogel composite. Int. J. Heat. Mass. Tran. 2017, 115, 21–31. [Google Scholar] [CrossRef]
- Karlina, A.I.; Karlina, Y.I.; Gladkikh, V.A. Analysis of Experience in the Use of Micro- and Nanoadditives from Silicon Production Waste in Concrete Technologies. Minerals 2023, 13, 1525. [Google Scholar] [CrossRef]
- Liu, F.; Xie, M.; Yu, G.; Ke, C.; Zhao, H. Study on Calcination Catalysis and the Desilication Mechanism for Coal Gangue. ACS Sustain. Chem. Eng. 2021, 9, 10318–10325. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Liu, C.; Shen, L. Comprehensive Extraction of Silica and Alumina from High-Alumina Coal Gangue (HACG): Hematite Involved Roasting—Alkaline Leaching—Bayer Digestion Process. J. Sustain. Metall. 2021, 7, 1686–1698. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, H.; Zhang, H.; Zhang, P.; Bei, R. Aluminum extraction from activated coal gangue with carbide slag. J. Anal. Appl. Pyrolysis 2022, 163, 105504. [Google Scholar] [CrossRef]
- Xiao, J.; Li, F.; Zhong, Q.; Bao, H.; Wang, B.; Huang, J.; Zhang, Y. Separation of aluminum and silica from coal gangue by elevated temperature acid leaching for the preparation of alumina and SiC. Hydrometallurgy 2015, 155, 118–124. [Google Scholar] [CrossRef]
- Peng, F.; Jiang, Y.; Feng, J.; Cai, H.; Feng, J.; Li, L. Thermally insulating, fiber-reinforced alumina-silica aerogel composites with ultra-low shrinkage up to 1500 °C. Chem. Eng. J. 2021, 411, 128402. [Google Scholar] [CrossRef]
- Mizushima, Y.; Hori, M. Preparation of heat-resistant alumina aerogels. J. Mater. Res. 1993, 8, 2993–2999. [Google Scholar] [CrossRef]
- Zou, W.; Wang, X.; Wu, Y.; Zu, G.; Zou, L.; Zhang, R.; Yao, X.; Shen, J. Highly thermally stable alumina-based aerogels modified by partially hydrolyzed aluminum tri-sec-butoxide. J. Sol-Gel Sci. Technol. 2017, 84, 507–514. [Google Scholar] [CrossRef]
- Wu, X.; Shao, G.; Cui, S.; Wang, L.; Shen, X. Synthesis of a novel Al2O3-SiO2 composite aerogel with high specific surface area at elevated temperatures using inexpensive inorganic salt of aluminum. Ceram. Int. 2016, 42, 874–882. [Google Scholar] [CrossRef]
- Peng, F.; Jiang, Y.; Feng, J.; Feng, J.; Li, L. Foreign element doping and thermal stability of alumina aerogels. J. Am. Ceram. Soc. 2022, 105, 2288–2299. [Google Scholar] [CrossRef]
- Wu, Z.-G.; Zhao, Y.-X.; Liu, D.-S. The synthesis and characterization of mesoporous silica–zirconia aerogels. Microporous Mesoporous Mater. 2004, 68, 127–132. [Google Scholar] [CrossRef]
- Li, D.-Y.; Lin, Y.-S.; Li, Y.-C.; Shieh, D.-L.; Lin, J.-L. Synthesis of mesoporous pseudoboehmite and alumina templated with 1-hexadecyl-2,3-dimethyl-imidazolium chloride. Microporous Mesoporous Mater. 2008, 108, 276–282. [Google Scholar] [CrossRef]
- Ren, T.-Z.; Yuan, Z.-Y.; Su, B.-L. Microwave-Assisted Preparation of Hierarchical Mesoporous−Macroporous Boehmite AlOOH and γ-Al2O3. Langmuir 2004, 20, 1531–1534. [Google Scholar] [CrossRef] [PubMed]
- Aravind, P.R.; Mukundan, P.; Krishna Pillai, P.; Warrier, K.G.K. Mesoporous silica–alumina aerogels with high thermal pore stability through hybrid sol–gel route followed by subcritical drying. Microporous Mesoporous Mater. 2006, 96, 14–20. [Google Scholar] [CrossRef]
- Xing, B.-L.; Guo, H.; Chen, L.-J.; Chen, Z.-F.; Zhang, C.-X.; Huang, G.-X.; Xie, W.; Yu, J.-L. Lignite-derived high surface area mesoporous activated carbons for electrochemical capacitors. Fuel Process. Technol. 2015, 138, 734–742. [Google Scholar] [CrossRef]
- Wu, X.; Shao, G.; Shen, X.; Cui, S.; Wang, L. Novel Al2O3–SiO2 composite aerogels with high specific surface area at elevated temperatures with different alumina/silica molar ratios prepared by a non-alkoxide sol-gel method. RSC Adv. 2016, 6, 5611–5620. [Google Scholar] [CrossRef]
- Aripin, H.; Mitsudo, S.; Prima, E.S.; Sudiana, I.N.; Kikuchi, H.; Sano, S.; Sabchevski, S. Crystalline mullite formation from mixtures of alumina and a novel material—Silica xerogel converted from sago waste ash. Ceram. Int. 2015, 41, 6488–6497. [Google Scholar] [CrossRef]
- Li, J.-Y.; Tian, B.-H.; Li, X.-X.; Wang, Z.; Cui, L.-P.; Liang, D.-D.; Wang, S.-L.; Liu, Y.-H.; Ou, H.-A.; Liang, H.-X. Energy effective utilization of circulating fluidized bed fly ash to prepare silicon-aluminum composite aerogel and gypsum. Waste Manag. 2023, 172, 162–170. [Google Scholar] [CrossRef]
- Yi, Z.; Zhang, X.; Yan, L.; Huyan, X.; Zhang, T.; Liu, S.; Guo, A.; Liu, J.; Hou, F. Super-insulated, flexible, and high resilient mullite fiber reinforced silica aerogel composites by interfacial modification with nanoscale mullite whisker. Compos. Part. B Eng. 2022, 230, 109549. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Ding, Y.-D.; Wang, F.; Deng, Z.-P. Thermal insulation material based on SiO2 aerogel. Constr. Build. Mater. 2016, 122, 548–555. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Gao, M.; Liu, B.; Zhao, P.; Yi, X.; Shen, X.; Xu, Y. Mechanical strengths and thermal properties of titania-doped alumina aerogels and the application as high-temperature thermal insulator. J. Sol-Gel Sci. Technol. 2019, 91, 514–522. [Google Scholar] [CrossRef]
- Hernandez, C.; Pierre, A.C. Evolution of the Texture and Structure of SiO2–Al2O3 Xerogels and Aerogels as a Function of the Si to Al Molar Ratio. J. Sol-Gel Sci. Technol. 2001, 20, 227–243. [Google Scholar] [CrossRef]
- Zhang, E.; Zhang, W.; Lv, T.; Li, J.; Dai, J.; Zhang, F.; Zhao, Y.; Yang, J.; Li, W.; Zhang, H. Insulating and Robust Ceramic Nanorod Aerogels with High-Temperature Resistance over 1400 °C. ACS Appl. Mater. Interfaces 2021, 13, 20548–20558. [Google Scholar] [CrossRef]







| Composition | SiO2 | Al2O3 | Fe2O3 | CaO | TiO2 | K2O | Na2O | F * |
|---|---|---|---|---|---|---|---|---|
| Precent (%) | 51.72 | 35.66 | 4.45 | 2.59 | 2.11 | 1.25 | 0.13 | 12.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bo, K.; Liu, H.; Zhang, Y.; Wang, Y. Fiber-Reinforced Coal Gangue-Based Alumina Aerogel Composites with Highly Thermal Stability by Ambient Pressure Drying. Sustainability 2024, 16, 4032. https://doi.org/10.3390/su16104032
Bo K, Liu H, Zhang Y, Wang Y. Fiber-Reinforced Coal Gangue-Based Alumina Aerogel Composites with Highly Thermal Stability by Ambient Pressure Drying. Sustainability. 2024; 16(10):4032. https://doi.org/10.3390/su16104032
Chicago/Turabian StyleBo, Kai, Hongwei Liu, Yanlan Zhang, and Yongzhen Wang. 2024. "Fiber-Reinforced Coal Gangue-Based Alumina Aerogel Composites with Highly Thermal Stability by Ambient Pressure Drying" Sustainability 16, no. 10: 4032. https://doi.org/10.3390/su16104032







