Differential Studies on the Structure of Lignin–Carbohydrate Complexes (LCC) in Alkali-Extracted Plant Hemicelluloses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Extraction of Hemicellulose via the Alkali Method
2.3. Enrichment of LCC
2.4. Determination of Sugar Fraction
2.5. Lignin Content Determination
2.6. Molecular Weight Detection
2.7. Nuclear Magnetic Resonance Spectroscopy Detection
3. Results and Discussion
3.1. Analysis of LCC Components in Different Raw Materials
3.2. Analysis of the Molecular Weight of LCC in Gramineae
3.3. Two-Dimensional NMR Spectroscopy of LCC Enrichment in Gramineae
3.3.1. NMR Analysis of Carbohydrate Fractions
3.3.2. NMR Analysis of the Lignin Fraction
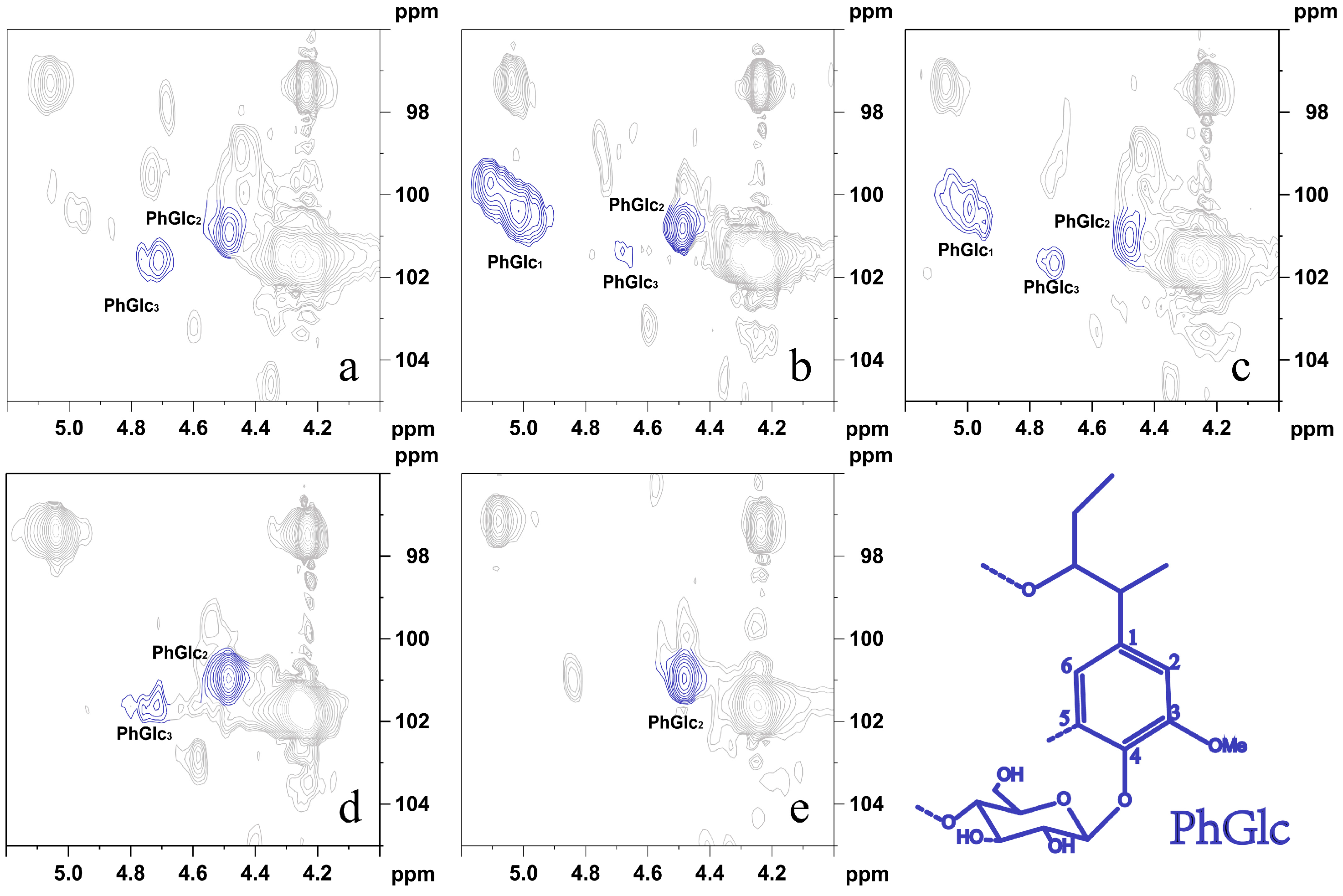
3.3.3. NMR Analysis of LC Bonding Components
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Madyaratri, E.W.; Ridho, M.R.; Aristri, M.A.; Lubis, M.A.R.; Iswanto, A.H.; Nawawi, D.S.; Antov, P.; Kristak, L.; Majlingova, A.; Fatriasari, W. Recent Advances in the Development of Fire-Resistant Biocomposites—A Review. Polymers 2022, 14, 362. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, L.; Deng, B.; Huang, C.; Zhu, J.; Liang, L.; He, X.; Wei, Y.; Qin, C.; Liang, C.; et al. Application and prospect of organic acid pretreatment in lignocellulosic biomass separation: A review. Int. J. Biol. Macromol. 2022, 222, 1400–1413. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Guo, X.; Ma, Z.; Gong, J.; Wang, H.; Lv, Y. Efficient Extraction and Structural Characterization of Hemicellulose from Sugarcane Bagasse Pith. Polymers 2020, 12, 608. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, J.; Pang, S.; Yao, S.; Zhu, C.; Zhao, J.; Liu, Y.; Liang, C.; Qin, C. High-Efficiency and High-Quality Extraction of Hemicellulose of Bamboo by Freeze-Thaw Assisted Two-Step Alkali Treatment. Int. J. Mol. Sci. 2022, 23, 8612. [Google Scholar] [CrossRef] [PubMed]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Satlewal, A.; Agrawal, R.; Bhagia, S.; Sangoro, J.; Ragauskas, A.J. Natural deep eutectic solvents for lignocellulosic biomass pretreatment: Recent developments, challenges and novel opportunities. Biotechnol. Adv. 2018, 36, 2032–2050. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Wang, X.; Liang, C.; Liu, X.; Wang, S. Purification of hemicellulose from sugarcane bagasse alkaline hydrolysate using an aromatic-selective adsorption resin. Carbohydr. Polym. 2019, 225, 115216. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, D.; Leitch, M.; Fatehi, P. Lignin-carbohydrate complexes: Properties, applications, analyses, and methods of extraction: A review. Biotechnol. Biofuels 2018, 11, 269. [Google Scholar] [CrossRef]
- Cui, S.; Wei, X.; Chen, X.; Xie, Y. Investigation of chemical linkages between lignin and carbohydrates in cultured poplar cambium tissues via double isotope labeling. Int. J. Biol. Macromol. 2023, 231, 123250. [Google Scholar] [CrossRef]
- Wang, X.; Han, J.; Pang, S.; Li, J.; Zhao, J.; Qin, C.; Yao, S.; Liu, Y.; Liang, C. Structural enrichment and identification of lignin-carbohydrate complex in alkaline stabilized system. Carbohydr. Polym. 2022, 296, 119873. [Google Scholar] [CrossRef]
- Feng, N.; Ren, L.; Wu, H.; Wu, Q.; Xie, Y. New insights on structure of lignin-carbohydrate complex from hot water pretreatment liquor. Carbohydr. Polym. 2019, 224, 115130. [Google Scholar] [CrossRef] [PubMed]
- Geng, W.; Narron, R.; Jiang, X.; Pawlak, J.J.; Chang, H.-m.; Park, S.; Jameel, H.; Venditti, R.A. The influence of lignin content and structure on hemicellulose alkaline extraction for non-wood and hardwood lignocellulosic biomass. Cellulose 2019, 26, 3219–3230. [Google Scholar] [CrossRef]
- Zhao, B.-C.; Chen, B.-Y.; Yang, S.; Yuan, T.-Q.; Charlton, A.; Sun, R.-C. Structural Variation of Lignin and Lignin–Carbohydrate Complex in Eucalyptus grandis × E. urophylla during Its Growth Process. ACS Sustain. Chem. Eng. 2016, 5, 1113–1122. [Google Scholar] [CrossRef]
- Balakshin, M.; Capanema, E.; Gracz, H.; Chang, H.M.; Jameel, H. Quantification of lignin-carbohydrate linkages with high-resolution NMR spectroscopy. Planta 2011, 233, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Puitel, A.C.; Suditu, G.D.; Danu, M.; Ailiesei, G.L.; Nechita, M.T. An Experimental Study on the Hot Alkali Extraction of Xylan-Based Hemicelluloses from Wheat Straw and Corn Stalks and Optimization Methods. Polymers 2022, 14, 1662. [Google Scholar] [CrossRef]
- Huang, C.; He, J.; Li, X.; Min, D.; Yong, Q. Facilitating the enzymatic saccharification of pulped bamboo residues by degrading the remained xylan and lignin-carbohydrates complexes. Bioresour. Technol. 2015, 192, 471–477. [Google Scholar] [CrossRef]
- Yang, H.; Xie, Y.; Zheng, X.; Pu, Y.; Huang, F.; Meng, X.; Wu, W.; Ragauskas, A.; Yao, L. Comparative study of lignin characteristics from wheat straw obtained by soda-AQ and kraft pretreatment and effect on the following enzymatic hydrolysis process. Bioresour. Technol. 2016, 207, 361–369. [Google Scholar] [CrossRef]
- Xie, D.; Gan, T.; Su, C.; Han, Y.; Liu, Z.; Cao, Y. Structural characterization and antioxidant activity of water-soluble lignin-carbohydrate complexes (LCCs) isolated from wheat straw. Int. J. Biol. Macromol. 2020, 161, 315–324. [Google Scholar] [CrossRef]
- Ge, J.; Wu, Y.; Han, Y.; Qin, C.; Nie, S.; Liu, S.; Wang, S.; Yao, S. Effect of hydrothermal pretreatment on the demineralization and thermal degradation behavior of eucalyptus. Bioresour. Technol. 2020, 307, 123246. [Google Scholar] [CrossRef]
- Han, J.; You, X.; Wang, S.; Chen, C.; Yao, S.; Meng, C.; Liang, C.; Zhao, J. Chlorine dioxide oxidation of hemicellulose from alkaline hydrolysate bagasse to remove lignin unit in lignin-carbohydrate complex. Carbohydr. Polym. 2022, 277, 118817. [Google Scholar] [CrossRef]
- Miyamoto, T.; Mihashi, A.; Yamamura, M.; Tobimatsu, Y.; Suzuki, S.; Takada, R.; Kobayashi, Y.; Umezawa, T. Comparative analysis of lignin chemical structures of sugarcane bagasse pretreated by alkaline, hydrothermal, and dilute sulfuric acid methods. Ind. Crop. Prod. 2018, 121, 124–131. [Google Scholar] [CrossRef]
- Zhang, L.; Gellerstedt, G. Quantitative 2D HSQC NMR determination of polymer structures by selecting suitable internal standard references. Magn. Reson. Chem. 2007, 45, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.L.; Sun, S.L.; Xue, B.L.; Sun, R.C. Recent Advances in Characterization of Lignin Polymer by Solution-State Nuclear Magnetic Resonance (NMR) Methodology. Materials 2013, 6, 359–391. [Google Scholar] [CrossRef] [PubMed]
- Felisberto, M.H.F.; Beraldo, A.L.; Costa, M.S.; Boas, F.V.; Franco, C.M.L.; Clerici, M. Characterization of young bamboo culm starch from Dendrocalamus asper. Food Res. Int. 2019, 124, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Sun, S.C.; Wang, B.; Sun, S.F.; Shi, Q.; Zheng, L.; Wang, S.F.; Liu, S.J.; Li, M.F.; Cao, X.F.; et al. Effect of various pretreatments on improving cellulose enzymatic digestibility of tobacco stalk and the structural features of co-produced hemicelluloses. Bioresour. Technol. 2020, 297, 122471. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.C.; Xu, J.D.; Chen, B.Y.; Cao, X.F.; Yuan, T.Q.; Wang, S.F.; Charlton, A.; Sun, R.C. Selective precipitation and characterization of lignin-carbohydrate complexes (LCCs) from Eucalyptus. Planta 2018, 247, 1077–1087. [Google Scholar] [CrossRef]
- Su, C.; Gan, T.; Liu, Z.; Chen, Y.; Zhou, Q.; Xia, J.; Cao, Y. Enhancement of the antioxidant abilities of lignin and lignin-carbohydrate complex from wheat straw by moderate depolymerization via LiCl/DMSO solvent catalysis. Int. J. Biol. Macromol. 2021, 184, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Shi, Z.; Zhao, Y.; Deng, J.; Dong, M.; Liu, C.; Murugadoss, V.; Mai, X.; Guo, Z. Structural characterization of lignin and its carbohydrate complexes isolated from bamboo (Dendrocalamus sinicus). Int. J. Biol. Macromol. 2019, 126, 376–384. [Google Scholar] [CrossRef] [PubMed]
- You, T.-T.; Zhang, L.-M.; Zhou, S.-K.; Xu, F. Structural elucidation of lignin–carbohydrate complex (LCC) preparations and lignin from Arundo donax Linn. Ind. Crop. Prod. 2015, 71, 65–74. [Google Scholar] [CrossRef]
- Yao, H.Y.; Wang, J.Q.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. A review of NMR analysis in polysaccharide structure and conformation: Progress, challenge and perspective. Food Res. Int. 2021, 143, 110290. [Google Scholar] [CrossRef]
- Oliveira, L.; Evtuguin, D.V.; Cordeiro, N.; Silvestre, A.J.D.; Silva, A.M.S.; Torres, I.C. Structural characterization of lignin from leaf sheaths of “dwarf cavendish” banana plant. J. Agric. Food Chem. 2006, 54, 2598–2605. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.-Q.; Sun, S.-N.; Xu, F.; Sun, R.-C. Characterization of Lignin Structures and Lignin–Carbohydrate Complex (LCC) Linkages by Quantitative 13C and 2D HSQC NMR Spectroscopy. J. Agric. Food Chem. 2011, 59, 10604–10614. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.-P.; Hu, Y.-J.; Fu, G.-Q.; Sun, C.-X.; Li, M.-F.; Peng, F.; Sun, R.-C. Structural Differences between the Lignin-Carbohydrate Complexes (LCCs) from 2- and 24-Month-Old Bamboo (Neosinocalamus affinis). Int. J. Mol. Sci. 2017, 19, 1. [Google Scholar] [CrossRef]
- Balakshin, M.Y.; Capanema, E.A.; Chang, H.-M. MWL fraction with a high concentration of lignin-carbohydrate linkages: Isolation and 2D NMR spectroscopic analysis. Holzforschung 2007, 61, 1–7. [Google Scholar] [CrossRef]
- Giummarella, N.; Pu, Y.; Ragauskas, A.J.; Lawoko, M. A critical review on the analysis of lignin carbohydrate bonds. Green Chem. 2019, 21, 1573–1595. [Google Scholar] [CrossRef]
- Miyagawa, Y.; Mizukami, T.; Kamitakahara, H.; Takano, T. Synthesis and fundamental HSQC NMR data of monolignol β-glycosides, dihydromonolignol β-glycosides and p-hydroxybenzaldehyde derivative β-glycosides for the analysis of phenyl glycoside type lignin-carbohydrate complexes (LCCs). Holzforschung 2014, 68, 747–760. [Google Scholar] [CrossRef]
- Zhao, Y.; Shakeel, U.; Saif Ur Rehman, M.; Li, H.; Xu, X.; Xu, J. Lignin-carbohydrate complexes (LCCs) and its role in biorefinery. J. Clean. Prod. 2020, 253, 120076. [Google Scholar] [CrossRef]





| Sample | TS (%) | TL (%) | Ara (%) | Gal (%) | Glu (%) | Xyl (%) | Asl (%) | Kl (%) |
|---|---|---|---|---|---|---|---|---|
| H-Bag | 59.36 | 11.44 | 11.18 | 3.35 | 6.77 | 38.06 | 3.11 | 8.33 |
| H-Bam | 64.65 | 9.97 | 4.83 | 1.53 | 26.29 | 31.99 | 3.01 | 6.96 |
| H-Corn | 50.02 | 11.65 | 10.41 | 4.12 | 4.80 | 30.68 | 4.60 | 7.05 |
| H-Aca | 44.33 | 11.00 | 0.94 | 1.95 | 1.14 | 40.30 | 3.37 | 7.63 |
| H-Euc | 24.25 | 21.40 | 0.74 | 1.18 | 1.17 | 21.16 | 12.93 | 8.47 |
| LCC-Bag | 55.01 | 8.94 | 10.81 | 0.65 | 9.03 | 34.52 | 4.98 | 3.96 |
| LCC-Bam | 53.77 | 12.16 | 9.05 | 2.09 | 10.44 | 32.18 | 5.20 | 6.96 |
| LCC-Corn | 47.06 | 16.31 | 10.79 | 2.25 | 4.30 | 29.71 | 6.68 | 9.64 |
| LCC-Aca | 34.15 | 19.50 | 1.24 | 1.49 | 1.86 | 29.57 | 5.75 | 13.75 |
| LCC-Euc | 20.83 | 29.28 | 1.54 | 1.19 | 1.80 | 16.30 | 16.26 | 13.02 |
| Sample | Mw (g·mol−1) | Mn (g·mol−1) | Mw/Mn |
|---|---|---|---|
| LCC-Bag | 3587 | 2234 | 1.605 |
| LCC-Bam | 3229 | 2062 | 1.566 |
| LCC-Corn | 2826 | 1905 | 1.483 |
| LCC-Aca | 2198 | 1541 | 1.426 |
| LCC-Euc | 1567 | 970 | 1.615 |
| Features | LCC-Bag | LCC-Bam | LCC-Corn | LCC-Aca | LCC-Euc |
|---|---|---|---|---|---|
| G:S:H | 6.4:8.2:0.7 | 2.3:5.3:1.5 | 5.2:4.2:1.2 | 2.4:2.8:0.5 | 7.9:7.1:0.4 |
| β-O-4 | 40.63 | 45.57 | 22.13 | 55.19 | 48.07 |
| β-β | ND | ND | 5.44 | 5.28 | 6.50 |
| β-5 | ND | ND | 0.73 | ND | 2.96 |
| Signal | LCC-Bag | LCC-Bam | LCC-Corn | LCC-Aca | LCC-Euc |
|---|---|---|---|---|---|
| PhGlc | 14.24 | 25.06 | 10.75 | 16.89 | 12.56 |
| BE | 0.42 | ND | ND | ND | 0.14 |
| Signal | LCC-Bag | LCC-Bam | LCC-Corn | LCC-Aca | LCC-Euc |
|---|---|---|---|---|---|
| PhGlc1 | ND | 9.26 | 3.53 | ND | ND |
| PhGlc2 | 11.65 | 15.51 | 6.38 | 14.82 | 12.56 |
| PhGlc3 | 2.59 | 0.29 | 0.84 | 2.07 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, S.; Wang, X.; Pu, J.; Liang, C.; Yao, S.; Qin, C. Differential Studies on the Structure of Lignin–Carbohydrate Complexes (LCC) in Alkali-Extracted Plant Hemicelluloses. Polymers 2024, 16, 1403. https://doi.org/10.3390/polym16101403
Pang S, Wang X, Pu J, Liang C, Yao S, Qin C. Differential Studies on the Structure of Lignin–Carbohydrate Complexes (LCC) in Alkali-Extracted Plant Hemicelluloses. Polymers. 2024; 16(10):1403. https://doi.org/10.3390/polym16101403
Chicago/Turabian StylePang, Shuyu, Xin Wang, Jiali Pu, Chen Liang, Shuangquan Yao, and Chengrong Qin. 2024. "Differential Studies on the Structure of Lignin–Carbohydrate Complexes (LCC) in Alkali-Extracted Plant Hemicelluloses" Polymers 16, no. 10: 1403. https://doi.org/10.3390/polym16101403






