Cytosolic and Acrosomal pH Regulation in Mammalian Sperm
Abstract
:1. Introduction
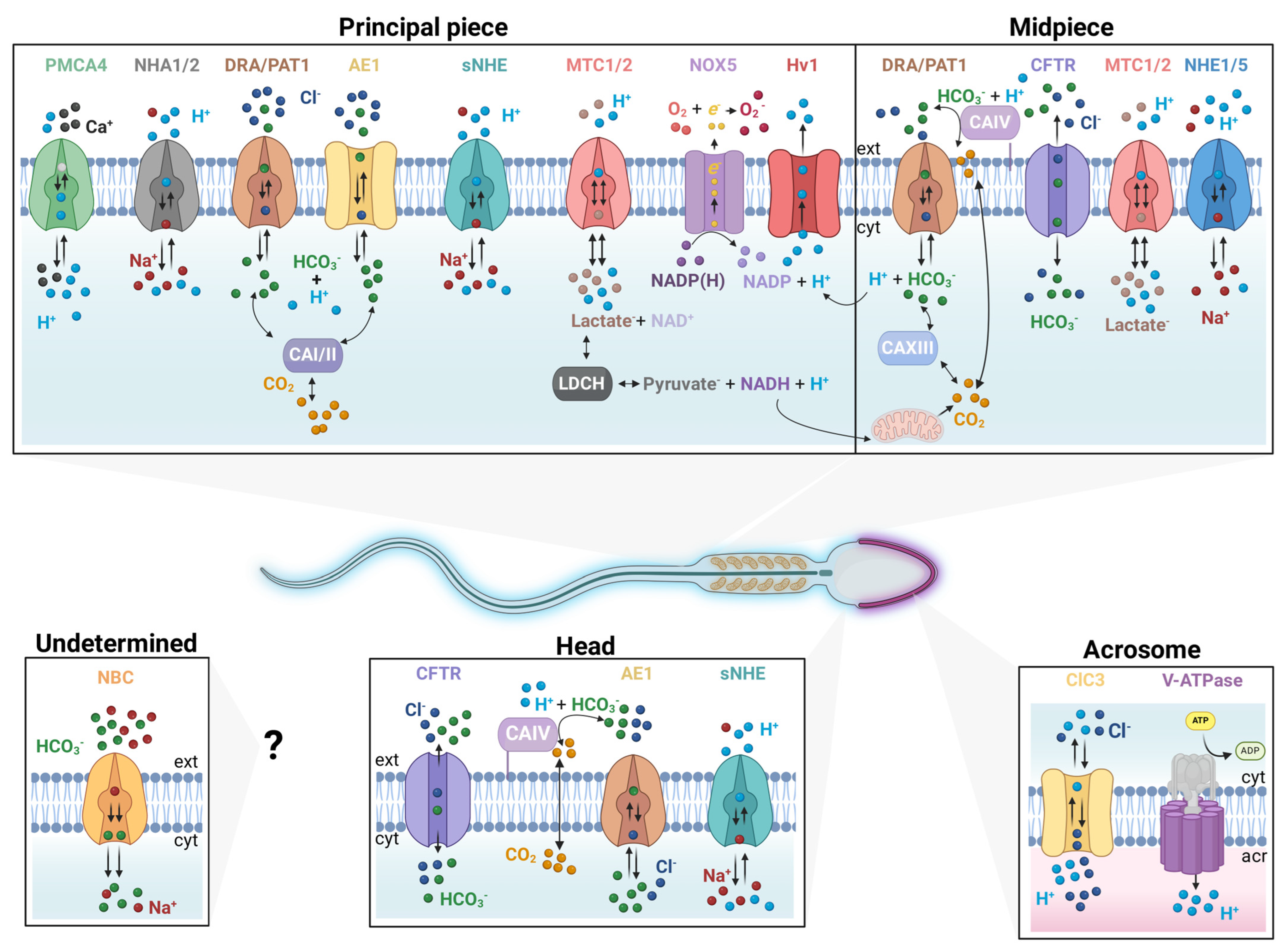
2. Ion Transporters That Regulate Cytosolic and Acrosomal pH
2.1. The CO2/HCO3− Pair and Carbonic Anhydrases (CAs)
2.2. HCO3− Transporters
2.2.1. Solute Carrier Family 4 (SLC4)
2.2.2. Solute Carrier Family 26 (SLC26)
2.2.3. The Cystic Fibrosis Transmembrane Conductance Regulator (CFTR)
2.3. Na+/H+ Exchangers (NHEs)
2.4. The Voltage-Gated H+ Channel (Hv1)
2.5. The Plasma Membrane Ca2+ ATPase (PMCA) Pump
2.6. Monocarboxylate Transporters (MCTs/SLC16A)
2.7. Acrosome pH (pHa) Regulation
2.8. The Vacuolar ATPase (V-ATPase)
2.9. Role of Cl− Channels in pHa Maintenance
2.10. Role of [Ca2+]i in pHa Regulation
2.11. Role of HCO3− in pHa Regulation
2.12. Role of Na+/H+ Exchanger (NHE) in pHa Regulation
3. Role of pHa during Sperm Mammalian Capacitation
4. Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and Regulators of Intracellular PH. Nat. Rev. Mol. Cell Biol. 2009, 11, 50–61. [Google Scholar] [CrossRef]
- Ruffin, V.A.; Salameh, A.I.; Boron, W.F.; Parker, M.D. Intracellular PH Regulation by Acid-Base Transporters in Mammalian Neurons. Front. Physiol. 2014, 5, 74282. [Google Scholar] [CrossRef]
- Swietach, P.; Vaughan-Jones, R.D.; Harris, A.L.; Hulikova, A. The Chemistry, Physiology and Pathology of PH in Cancer. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130099. [Google Scholar] [CrossRef]
- Freeman, S.A.; Grinstein, S.; Orlowski, J. Determinants, Maintenance, and Function of Organellar PH. Physiol. Rev. 2023, 103, 515–606. [Google Scholar] [CrossRef]
- Hamamah, S.; Magnoux, E.; Royere, D.; Barthelemy, C.; Dacheux, J.L.; Gatti, J.L. Internal PH of Human Spermatozoa: Effect of Ions, Human Follicular Fluid and Progesterone. Mol. Hum. Reprod. 1996, 2, 219–224. [Google Scholar] [CrossRef]
- Darszon, A.; Nishigaki, T.; Beltran, C.; Treviño, C.L. Calcium Channels in the Development, Maturation, and Function of Spermatozoa. Physiol. Rev. 2011, 91, 1305–1355. [Google Scholar] [CrossRef]
- Gervasi, M.G.; Visconti, P.E. Chang’s Meaning of Capacitation: A Molecular Perspective. Mol. Reprod. Dev. 2016, 83, 860–874. [Google Scholar] [CrossRef]
- Chang, M. Fertilizing Capacity of Spermatozoa Deposited into the Fallopian Tubes. Nature 1951, 168, 697–698. [Google Scholar] [CrossRef]
- Austin, C.R. Observations on the Penetration of the Sperm in the Mammalian Egg. Aust. J. Sci. Res. B. 1951, 4, 581–596. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.K.; Chen, L.M. The Physiology of Bicarbonate Transporters in Mammalian Reproduction. Biol. Reprod. 2012, 86, 99–100. [Google Scholar] [CrossRef]
- Ng, K.Y.B.; Mingels, R.; Morgan, H.; Macklon, N.; Cheong, Y. In Vivo Oxygen, Temperature and PH Dynamics in the Female Reproductive Tract and Their Importance in Human Conception: A Systematic Review. Hum. Reprod. Update 2018, 24, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Navarro, B.; Perez, G.; Jackson, A.C.; Hsu, S.; Shi, Q.; Tilly, J.L.; Clapham, D.E. A Sperm Ion Channel Required for Sperm Motility and Male Fertility. Nature 2001, 413, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, M.; Wei, A.; Yuan, A.; Gaut, J.; Saito, M.; Salkoff, L. Slo3, a Novel PH-Sensitive K+ Channel from Mammalian Spermatocytes. J. Biol. Chem. 1998, 273, 3509–3516. [Google Scholar] [CrossRef]
- Santi, C.M.; Martínez-López, P.; de la Vega-Beltrán, J.L.; Butler, A.; Alisio, A.; Darszon, A.; Salkoff, L. The SLO3 Sperm-Specific Potassium Channel Plays a Vital Role in Male Fertility. FEBS Lett. 2010, 584, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, M.D.; Yuan, P.; Hsiung, Y.; MacKinnon, R. Functional and Structural Analysis of the Human SLO3 PH- and Voltage-Gated K+ Channel. Proc. Natl. Acad. Sci. USA 2012, 109, 19274–19279. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; King, S.M.; Quill, T.A.; Doolittle, L.K.; Garbers, D.L. A New Sperm-Specific Na+/H+ Exchanger Required for Sperm Motility and Fertility. Nat. Cell Biol. 2003, 5, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Garduño, S.; Chávez, J.C.; Matamoros-Volante, A.; Sánchez-Guevara, Y.; Torres, P.; Treviño, C.L.; Nishigaki, T. Hyperpolarization Induces Cytosolic Alkalization of Mouse Sperm Flagellum Probably through Sperm Na+/H+ Exchanger. Reproduction 2022, 164, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Cavarocchi, E.; Whitfield, M.; Chargui, A.; Stouvenel, L.; Lorès, P.; Coutton, C.; Arnoult, C.; Santulli, P.; Patrat, C.; Thierry-Mieg, N.; et al. The Sodium/Proton Exchanger SLC9C1 (SNHE) Is Essential for Human Sperm Motility and Fertility. Clin. Genet. 2021, 99, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Takagi, M.; Okamura, Y. A Voltage Sensor-Domain Protein Is a Voltage-Gated Proton Channel. Science 2006, 312, 589–592. [Google Scholar] [CrossRef]
- Lishko, P.V.; Botchkina, I.L.; Fedorenko, A.; Kirichok, Y. Acid Extrusion from Human Spermatozoa Is Mediated by Flagellar Voltage-Gated Proton Channel. Cell 2010, 140, 327–337. [Google Scholar] [CrossRef]
- Carlson, A.E.; Westenbroek, R.E.; Quill, T.; Ren, D.; Clapham, D.E.; Hille, B.; Garbers, D.L.; Babcock, D.F. CatSper1 Required for Evoked Ca2+ Entry and Control of Flagellar Function in Sperm. Proc. Natl. Acad. Sci. USA 2003, 100, 14864–14868. [Google Scholar] [CrossRef]
- Wang, D.; Hu, J.; Bobulescu, I.A.; Quill, T.A.; McLeroy, P.; Moe, O.W.; Garbers, D.L. A Sperm-Specific Na+/H+ Exchanger (SNHE) Is Critical for Expression and in Vivo Bicarbonate Regulation of the Soluble Adenylyl Cyclase (SAC). Proc. Natl. Acad. Sci. USA 2007, 104, 9325–9330. [Google Scholar] [CrossRef]
- Hess, K.C.; Jones, B.H.; Marquez, B.; Chen, Y.; Ord, T.S.; Kamenetsky, M.; Miyamoto, C.; Zippin, J.H.; Kopf, G.S.; Suarez, S.S.; et al. The “Soluble” Adenylyl Cyclase in Sperm Mediates Multiple Signaling Events Required for Fertilization. Dev. Cell 2005, 9, 249–259. [Google Scholar] [CrossRef]
- Esposito, G.; Jaiswal, B.S.; Xie, F.; Krajnc-Franken, M.A.M.; Robben, T.J.A.A.; Strik, A.M.; Kuil, C.; Philipsen, R.L.A.; Van Duin, M.; Conti, M.; et al. Mice Deficient for Soluble Adenylyl Cyclase Are Infertile Because of a Severe Sperm-Motility Defect. Proc. Natl. Acad. Sci. USA 2004, 101, 2993–2998. [Google Scholar] [CrossRef]
- Peralta-Arias, R.D.; Vívenes, C.Y.; Camejo, M.I.; Piñero, S.; Proverbio, T.; Martínez, E.; Marín, R.; Proverbio, F. ATPases, Ion Exchangers and Human Sperm Motility. Reproduction 2015, 149, 475–484. [Google Scholar] [CrossRef]
- Darszon, A.; Nishigaki, T.; López-González, I.; Visconti, P.E.; Treviño, C.L. Differences and Similarities: The Richness of Comparative Sperm Physiology. Physiology 2020, 35, 196–208. [Google Scholar] [CrossRef]
- Delgado-Bermúdez, A.; Yeste, M.; Bonet, S.; Pinart, E. A Review on the Role of Bicarbonate and Proton Transporters during Sperm Capacitation in Mammals. Int. J. Mol. Sci. 2022, 23, 6333. [Google Scholar] [CrossRef]
- Casey, J.R. Why Bicarbonate? This Paper Is One of a Selection of Papers Published in This Special Issue, Entitled CSBMCB—Membrane Proteins in Health and Disease. 2006, 84, 930–939. [Google Scholar] [CrossRef]
- Theparambil, S.M.; Ruminot, I.; Schneider, H.P.; Shull, G.E.; Deitmer, J.W. The Electrogenic Sodium Bicarbonate Cotransporter NBCe1 Is a High-Affinity Bicarbonate Carrier in Cortical Astrocytes. J. Neurosci. 2014, 34, 1148–1157. [Google Scholar] [CrossRef]
- Supuran, C. Carbonic Anhydrases An Overview. Curr. Pharm. Des. 2008, 14, 603–614. [Google Scholar] [CrossRef]
- Nocentini, A.; Supuran, C.T.; Capasso, C. An Overview on the Recently Discovered Iota-Carbonic Anhydrases. J. Enzyme Inhib. Med. Chem. 2021, 36, 1988–1995. [Google Scholar] [CrossRef]
- Alvarez, B.V.; Vilas, G.L.; Casey, J.R. Metabolon Disruption: A Mechanism That Regulates Bicarbonate Transport. EMBO J. 2005, 24, 2499–2511. [Google Scholar] [CrossRef]
- Sterling, D.; Reithmeier, R.A.F.; Casey, J.R. A Transport Metabolon: Functional Interaction of Carbonic Anhydrase Ii and Chloride/Bicarbonate Exchangers. J. Biol. Chem. 2001, 276, 47886–47894. [Google Scholar] [CrossRef]
- Ali Akbar, S.; Nicolaides, K.; Brown, P. Carbonic Anhydrase Isoenzymes CAI and CAII in Semen, Decidua, Chorionic Villi and Various Fetal Tissues. Early Hum. Dev. 1998, 51, 205–211. [Google Scholar] [CrossRef]
- José, O.; Torres-Rodríguez, P.; Forero-Quintero, L.S.; Chávez, J.C.; De la Vega-Beltrán, J.L.; Carta, F.; Supuran, C.T.; Deitmer, J.W.; Treviño, C.L. Carbonic Anhydrases and Their Functional Differences in Human and Mouse Sperm Physiology. Biochem. Biophys. Res. Commun. 2015, 468, 713–718. [Google Scholar] [CrossRef]
- Parkkila, S.; Kaunisto, K.; Kellokumpu, S.; Rajaniemi, H. A High Activity Carbonic Anhydrase Isoenzyme (CA II) Is Present in Mammalian Spermatozoa. Histochemistry 1991, 95, 477–482. [Google Scholar] [CrossRef]
- Wandernoth, P.M.; Raubuch, M.; Mannowetz, N.; Becker, H.M.; Deitmer, J.W.; Sly, W.S.; Wennemuth, G. Role of Carbonic Anhydrase IV in the Bicarbonate-Mediated Activation of Murine and Human Sperm. PLoS ONE 2010, 5, e15061. [Google Scholar] [CrossRef]
- Wandernoth, P.M.; Mannowetz, N.; Szczyrba, J.; Grannemann, L.; Wolf, A.; Becker, H.M.; Sly, W.S.; Wennemuth, G. Normal Fertility Requires the Expression of Carbonic Anhydrases II and IV in Sperm. J. Biol. Chem. 2015, 290, 29202–29216. [Google Scholar] [CrossRef]
- Grahn, E.; Kaufmann, S.V.; Askarova, M.; Ninov, M.; Welp, L.M.; Berger, T.K.; Urlaub, H.; Kaupp, U.B. Control of Intracellular PH and Bicarbonate by CO2 Diffusion into Human Sperm. Nat. Commun. 2023, 14, 1–17. [Google Scholar] [CrossRef]
- Ekstedt, E.; Holm, L.; Ridderstråle, Y. Carbonic Anhydrase in Mouse Testis and Epididymis; Transfer of Isozyme IV to Spermatozoa during Passage. J. Mol. Histol. 2004, 35, 167–173. [Google Scholar] [CrossRef]
- Mezquita, P. Novel Transcripts of Carbonic Anhydrase II in Mouse and Human Testis. Mol. Hum. Reprod. 1999, 5, 199–205. [Google Scholar] [CrossRef]
- Lehtonen, J.; Shen, B.; Vihinen, M.; Casini, A.; Scozzafava, A.; Supuran, C.T.; Parkkila, A.-K.; Saarnio, J.; Kivelä, A.J.; Waheed, A.; et al. Characterization of CA XIII, a Novel Member of the Carbonic Anhydrase Isozyme Family. J. Biol. Chem. 2004, 279, 2719–2727. [Google Scholar] [CrossRef]
- Donà, G.; Tibaldi, E.; Andrisani, A.; Ambrosini, G.; Sabbadin, C.; Pagano, M.A.; Brunati, A.M.; Armanini, D.; Ragazzi, E.; Bordin, L. Human Sperm Capacitation Involves the Regulation of the Tyr-Phosphorylation Level of the Anion Exchanger 1 (AE1). Int. J. Mol. Sci. 2020, 21, 4063. [Google Scholar] [CrossRef]
- Medina, J.F.; Recalde, S.; Prieto, J.; Lecanda, J.; Saez, E.; Funk, C.D.; Vecino, P.; van Roon, M.A.; Ottenhoff, R.; Bosma, P.J.; et al. Anion Exchanger 2 Is Essential for Spermiogenesis in Mice. Proc. Natl. Acad. Sci. USA 2003, 100, 15847–15852. [Google Scholar] [CrossRef]
- Demarco, I.A.; Espinosa, F.; Edwards, J.; Sosnik, J.; de la Vega-Beltrán, J.L.; Hockensmith, J.W.; Kopf, G.S.; Darszon, A.; Visconti, P.E. Involvement of a Na+/HCO Cotransporter in Mouse Sperm Capacitation. J. Biol. Chem. 2003, 278, 7001–7009. [Google Scholar] [CrossRef]
- Chávez, J.C.; Hernández-González, E.O.; Wertheimer, E.; Visconti, P.E.; Darszon, A.; Treviño, C.L. Participation of the Cl−/HCO3 Exchangers SLC26A3 and SLC26A6, the Cl− Channel CFTR, and the Regulatory Factor SLC9A3R1 in Mouse Sperm Capacitation. Biol. Reprod. 2012, 86, 14–15. [Google Scholar] [CrossRef]
- Chen, W.Y.; Xu, W.M.; Chen, Z.H.; Ni, Y.; Yuan, Y.Y.; Zhou, S.C.; Zhou, W.W.; Tsang, L.L.; Chung, Y.W.; Höglund, P.; et al. Cl− Is Required for HCO3− Entry Necessary for Sperm Capacitation in Guinea Pig: Involvement of a Cl−/HCO3− Exchanger (SLC26A3) and CFTR1. Biol. Reprod. 2009, 80, 115–123. [Google Scholar] [CrossRef]
- Hihnala, S.; Kujala, M.; Toppari, J.; Kere, J.; Holmberg, C.; Höglund, P. Expression of SLC26A3, CFTR and NHE3 in the Human Male Reproductive Tract: Role in Male Subfertility Caused by Congenital Chloride Diarrhoea. Mol. Hum. Reprod. 2006, 12, 107–111. [Google Scholar] [CrossRef]
- Li, C.Y.; Jiang, L.Y.; Chen, W.Y.; Li, K.; Sheng, H.Q.; Ni, Y.; Lu, J.X.; Xu, W.X.; Zhang, S.Y.; Shi, Q.X. CFTR Is Essential for Sperm Fertilizing Capacity and Is Correlated with Sperm Quality in Humans. Hum. Reprod. 2010, 25, 317–327. [Google Scholar] [CrossRef]
- Matamoros-Volante, A.; Treviño, C.L. Capacitation-Associated Alkalization in Human Sperm Is Differentially Controlled at the Subcellular Level. J. Cell Sci. 2020, 133, jcs238816. [Google Scholar] [CrossRef]
- Puga Molina, L.C.; Pinto, N.A.; Torres Rodríguez, P.; Romarowski, A.; Vicens Sanchez, A.; Visconti, P.E.; Darszon, A.; Treviño, C.L.; Buffone, M.G. Essential Role of CFTR in PKA-Dependent Phosphorylation, Alkalinization, and Hyperpolarization During Human Sperm Capacitation. J. Cell. Physiol. 2017, 232, 1404–1414. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhou, C.X.; Shi, Q.X.; Yuan, Y.Y.; Yu, M.K.; Ajonuma, L.C.; Ho, L.S.; Lo, P.S.; Tsang, L.L.; Liu, Y.; et al. Involvement of CFTR in Uterine Bicarbonate Secretion and the Fertilizing Capacity of Sperm. Nat. Cell Biol. 2003, 5, 902–906. [Google Scholar] [CrossRef]
- Xu, W.M.; Shi, Q.X.; Chen, W.Y.; Zhou, C.X.; Ni, Y.; Rowlands, D.K.; Yi Liu, G.; Zhu, H.; Ma, Z.G.; Wang, X.F.; et al. Cystic Fibrosis Transmembrane Conductance Regulator Is Vital to Sperm Fertilizing Capacity and Male Fertility. Proc. Natl. Acad. Sci. USA 2007, 104, 9816–9821. [Google Scholar] [CrossRef]
- Novero, A.G.; Rodríguez, P.T.; Beltrán, J.L.D.L.V.; Schiavi-Ehrenhaus, L.J.; Luque, G.M.; Carruba, M.; Stival, C.; Gentile, I.; Ritagliati, C.; Santi, C.M.; et al. The Sodium-Proton Exchangers SNHE and NHE1 Control Plasma Membrane Hyperpolarization in Mouse Sperm. bioRxiv 2024, 38, 547–554. [Google Scholar] [CrossRef]
- Woo, A.L.; James, P.F.; Lingrel, J.B. Roles of the Na,K-ATPase A4 Isoform and the Na+/H+ Exchanger in Sperm Motility. Mol. Reprod. Dev. 2002, 62, 348–356. [Google Scholar] [CrossRef]
- Balbach, M.; Hamzeh, H.; Jikeli, J.F.; Brenker, C.; Schiffer, C.; Hansen, J.N.; Neugebauer, P.; Trötschel, C.; Jovine, L.; Han, L.; et al. Molecular Mechanism Underlying the Action of Zona-Pellucida Glycoproteins on Mouse Sperm. Front. Cell Dev. Biol. 2020, 8, 572735. [Google Scholar] [CrossRef]
- Chen, S.-R.; Chen, M.; Deng, S.-L.; Hao, X.-X.; Wang, X.-X.; Liu, Y.-X. Sodium–Hydrogen Exchanger NHA1 and NHA2 Control Sperm Motility and Male Fertility. Cell Death Dis. 2016, 7, e2152. [Google Scholar] [CrossRef]
- Gardner, C.C.; James, P.F. The SLC9C2 Gene Product (Na+/H+ Exchanger Isoform 11; NHE11) Is a Testis-Specific Protein Localized to the Head of Mature Mammalian Sperm. Int. J. Mol. Sci. 2023, 24, 5329. [Google Scholar] [CrossRef]
- Miller, M.R.; Kenny, S.J.; Mannowetz, N.; Mansell, S.A.; Wojcik, M.; Mendoza, S.; Zucker, R.S.; Xu, K.; Lishko, P.V. Asymmetrically Positioned Flagellar Control Units Regulate Human Sperm Rotation. Cell Rep. 2018, 24, 2606–2613. [Google Scholar] [CrossRef]
- Zhao, R.; Kennedy, K.; De Blas, G.A.; Orta, G.; Pavarotti, M.A.; Arias, R.J.; de la Vega-Beltrán, J.L.; Li, Q.; Dai, H.; Perozo, E.; et al. Role of Human Hv1 Channels in Sperm Capacitation and White Blood Cell Respiratory Burst Established by a Designed Peptide Inhibitor. Proc. Natl. Acad. Sci. USA 2018, 115, E11847–E11856. [Google Scholar] [CrossRef]
- Zhao, R.; Dai, H.; Arias, R.J.; De Blas, G.A.; Orta, G.; Pavarotti, M.A.; Shen, R.; Perozo, E.; Mayorga, L.S.; Darszon, A.; et al. Direct Activation of the Proton Channel by Albumin Leads to Human Sperm Capacitation and Sustained Release of Inflammatory Mediators by Neutrophils. Nat. Commun. 2021, 12. [Google Scholar] [CrossRef]
- Brini, M.; Carafoli, E. Calcium Pumps in Health and Disease. Physiol. Rev. 2009, 89, 1341–1378. [Google Scholar] [CrossRef]
- Okunade, G.W.; Miller, M.L.; Pyne, G.J.; Sutliff, R.L.; O’Connor, K.T.; Neumann, J.C.; Andringa, A.; Miller, D.A.; Prasad, V.; Doetschman, T.; et al. Targeted Ablation of Plasma Membrane Ca2+-ATPase (PMCA) 1 and 4 Indicates a Major Housekeeping Function for PMCA1 and a Critical Role in Hyperactivated Sperm Motility and Male Fertility for PMCA4. J. Biol. Chem. 2004, 279, 33742–33750. [Google Scholar] [CrossRef]
- Mannowetz, N.; Wandernoth, P.M.; Wennemuth, G. Glucose Is a PH-Dependent Motor for Sperm Beat Frequency during Early Activation. PLoS ONE 2012, 7, e41030. [Google Scholar] [CrossRef]
- Chen, C.; Maekawa, M.; Yamatoya, K.; Nozaki, M.; Ito, C.; Iwanaga, T.; Toshimori, K. Interaction between Basigin and Monocarboxylate Transporter 2 in the Mouse Testes and Spermatozoa. Asian J. Androl. 2016, 18, 600–606. [Google Scholar] [CrossRef]
- Odet, F.; Duan, C.; Willis, W.; Goulding, E.; Kung, A.; Eddy, M.; Goldberg, E. Lactate Dehydrogenase-C4 (LDH-C4) Is Essential for Sperm Function. Biol. Reprod. 2008, 78, 187. [Google Scholar] [CrossRef]
- Carrasquel Martínez, G.; Aldana, A.; Carneiro, J.; Treviño, C.L.; Darszon, A. Acrosomal Alkalinization Occurs during Human Sperm Capacitation. Mol. Hum. Reprod. 2022, 28, gaac005. [Google Scholar] [CrossRef]
- Sun-Wada, G.-H.; Imai-Senga, Y.; Yamamoto, A.; Murata, Y.; Hirata, T.; Wada, Y.; Futai, M. A Proton Pump ATPase with Testis-Specific E1-Subunit Isoform Required for Acrosome Acidification. J. Biol. Chem. 2002, 277, 18098–18105. [Google Scholar] [CrossRef]
- Yeung, C.H.; Barfield, J.P.; Cooper, T.G. Chloride Channels in Physiological Volume Regulation of Human Spermatozoa. Biol. Reprod. 2005, 73, 1057–1063. [Google Scholar] [CrossRef]
- Zhong, J.; Dong, J.; Ruan, W.; Duan, X. Potential Theranostic Roles of SLC4 Molecules in Human Diseases. Int. J. Mol. Sci. 2023, 24, 15166. [Google Scholar] [CrossRef]
- Bernardino, R.L.; Carrageta, D.F.; Sousa, M.; Alves, M.G.; Oliveira, P.F. PH and Male Fertility: Making Sense on PH Homeodynamics throughout the Male Reproductive Tract. Cell. Mol. Life Sci. 2019, 76, 3783–3800. [Google Scholar] [CrossRef]
- Visconti, P.E.; Bailey, J.L.; Moore, G.D.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation of Mouse Spermatozoa: II. Protein Tyrosine Phosphorylation and Capacitation Are Regulated by a CAMP-Dependent Pathway. Development 1995, 121, 1139–1150. [Google Scholar] [CrossRef]
- Visconti, P.E.; Bailey, J.L.; Moore, G.D.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation of Mouse Spermatozoa: I. Correlation between the Capacitation State and Protein Tyrosine Phosphorylation. Development 1995, 121, 1129–1137. [Google Scholar] [CrossRef]
- Lis, C.P.M.; Pinto, N.A.; Torres, N.I.; Ana, L.G.C.; Luque, G.M.; Balestrini, P.A.; Romarowski, A.; Krapf, D.; Santi, C.M.; Treviño, C.L.; et al. CFTR/ENaC-Dependent Regulation of Membrane Potential during Human Sperm Capacitation Is Initiated by Bicarbonate Uptake through NBC. J. Biol. Chem. 2018, 293, 9924–9936. [Google Scholar] [CrossRef]
- Touré, A. Importance of Slc26 Transmembrane Anion Exchangers in Sperm Post-Testicular Maturation and Fertilization Potential. Front. Cell Dev. Biol. 2019, 7, 1–22. [Google Scholar] [CrossRef]
- Shcheynikov, N.; Wang, Y.; Park, M.; Ko, S.B.H.; Dorwart, M.; Naruse, S.; Thomas, P.J.; Muallem, S. Coupling Modes and Stoichiometry of Cl−/HCO3− Exchange by Slc26a3 and Slc26a6. J. Gen. Physiol. 2006, 127, 511–524. [Google Scholar] [CrossRef]
- Ko, S.B.H.; Zeng, W.; Dorwart, M.R.; Luo, X.; Kim, K.H.; Millen, L.; Goto, H.; Naruse, S.; Soyombo, A.; Thomas, P.J.; et al. Gating of CFTR by the STAS Domain of SLC26 Transporters. Nat. Cell Biol. 2004, 6, 343–350. [Google Scholar] [CrossRef]
- Touré, A.; Morin, L.; Pineau, C.; Becq, F.; Dorseuil, O.; Gacon, G. Tat1, a Novel Sulfate Transporter Specifically Expressed in Human Male Germ Cells and Potentially Linked to RhoGTPase Signaling. J. Biol. Chem. 2001, 276, 20309–20315. [Google Scholar] [CrossRef]
- Dirami, T.; Rode, B.; Jollivet, M.; Da Silva, N.; Escalier, D.; Gaitch, N.; Norez, C.; Tuffery, P.; Wolf, J.P.; Becq, F.; et al. Missense Mutations in SLC26A8, Encoding a Sperm-Specific Activator of CFTR, Are Associated with Human Asthenozoospermia. Am. J. Hum. Genet. 2013, 92, 760–766. [Google Scholar] [CrossRef]
- Schweinfest, C.W.; Spyropoulos, D.D.; Henderson, K.W.; Kim, J.H.; Chapman, J.M.; Barone, S.; Worrell, R.T.; Wang, Z.; Soleimani, M. Slc26a3 (Dra)-Deficient Mice Display Chloride-Losing Diarrhea, Enhanced Colonic Proliferation, and Distinct Up-Regulation of Ion Transporters in the Colon. J. Biol. Chem. 2006, 281, 37962–37971. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, S.; Eklund, R.; Lähdetie, J.; Mikkola, M.; Hovatta, O.; Kere, J. Mutational Analysis of the Human SLC26A8 Gene: Exclusion as a Candidate for Male Infertility Due to Primary Spermatogenic Failure. Mol. Hum. Reprod. 2005, 11, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.P.; Gregory, R.J.; Thompson, S.; Souza, D.W.; Paul, S.; Mulligan, R.C.; Smith, A.E.; Welsh, M.J. Demonstration That CFTR Is a Chloride Channel by Alteration of Its Anion Selectivity. Science 1991, 253, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Berger, H.A.; Travis, S.M.; Welsh, M.J. Regulation of the Cystic Fibrosis Transmembrane Conductance Regulator Cl− Channel by Specific Protein Kinases and Protein Phosphatases. J. Biol. Chem. 1993, 268, 2037–2047. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S.; Jakubiczka, S.; Kropf, S.; Nickel, I.; Muschke, P.; Kleinstein, J. Increased Frequency of Cystic Fibrosis Transmembrane Conductance Regulator Gene Mutations in Infertile Males. Fertil. Steril. 2006, 85, 135–138. [Google Scholar] [CrossRef]
- Hernández-González, E.O.; Treviño, C.L.; Castellano, L.E.; de la Vega-Beltrán, J.L.; Ocampo, A.Y.; Wertheimer, E.; Visconti, P.E.; Darszon, A. Involvement of Cystic Fibrosis Transmembrane Conductance Regulator in Mouse Sperm Capacitation. J. Biol. Chem. 2007, 282, 24397–24406. [Google Scholar] [CrossRef] [PubMed]
- Figueiras-Fierro, D.; Acevedo, J.J.; Martínez-López, P.; Escoffier, J.; Sepúlveda, F.V.; Balderas, E.; Orta, G.; Visconti, P.E.; Darszon, A. Electrophysiological Evidence for the Presence of Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) in Mouse Sperm. J. Cell. Physiol. 2013, 228, 590–601. [Google Scholar] [CrossRef]
- Fuster, D.G.; Alexander, R.T. Traditional and Emerging Roles for the SLC9 Na+/H+ Exchangers. Pflugers Arch. Eur. J. Physiol. 2014, 466, 61–76. [Google Scholar] [CrossRef]
- Orlowski, J.; Grinstein, S. Diversity of the Mammalian Sodium/Proton Exchanger SLC9 Gene Family. Pflugers Arch. Eur. J. Physiol. 2004, 447, 549–565. [Google Scholar] [CrossRef]
- Attaphitaya, S.; Park, K.; Melvin, J.E. Molecular Cloning and Functional Expression of a Rat Na+/H+ Exchanger (NHE5) Highly Expressed in Brain. J. Biol. Chem. 1999, 274, 4383–4388. [Google Scholar] [CrossRef]
- Pedersen, S.F.; Counillon, L. The SLC9A-C Mammalian Na+/H+ Exchanger Family: Molecules, Mechanisms, and Physiology. Physiol. Rev. 2019, 99, 2015–2113. [Google Scholar] [CrossRef]
- Windler, F.; Bönigk, W.; Körschen, H.G.; Grahn, E.; Strünker, T.; Seifert, R.; Kaupp, U.B. The Solute Carrier SLC9C1 Is a Na+/H+-Exchanger Gated by an S4-Type Voltage-Sensor and Cyclic-Nucleotide Binding. Nat. Commun. 2018, 9, 2809. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Chen, C.; Han, D.; Xiong, X.; Kong, Y.; Wan, B.; Yu, L. Cloning of a Novel Human NHEDC1 (Na+/H+ Exchanger like Domain Containing 1) Gene Expressed Specifically in Testis. Mol. Biol. Rep. 2006, 33, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Fuster, D.G.; Zhang, J.; Shi, M.; Alexandru Bobulescu, I.; Andersson, S.; Moe, O.W. Characterization of the Sodium/Hydrogen Exchanger NHA2. J. Am. Soc. Nephrol. 2008, 19, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Bernardazzi, C.; Sheikh, I.A.; Xu, H.; Ghishan, F.K. The Physiological Function and Potential Role of the Ubiquitous Na+/H+ Exchanger Isoform 8 (NHE8): An Overview Data. Int. J. Mol. Sci. 2022, 23, 10857. [Google Scholar] [CrossRef] [PubMed]
- Oberheide, K.; Puchkov, D.; Jentsch, T.J. Loss of the Na+/H+ Exchanger NHE8 Causes Male Infertility in Mice by Disrupting Acrosome Formation. J. Biol. Chem. 2017, 292, 10845–10854. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Chiang, H.S.; Cheng, C.Y.; Wu, Y.N.; Lin, Y.C.; Liu, H.C.; Tsai, W.K.; Chen, Y.L.; Lin, Y.H. SLC9A3 Protein Is Critical for Acrosomal Formation in Postmeiotic Male Germ Cells. Int. J. Mol. Sci. 2017, 19, 103. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, H.; Li, J.; Zhao, Y.; Ghishan, F.K. Disruption of NHE8 Expression Impairs Leydig Cell Function in the Testes. Am. J. Physiol. Cell Physiol. 2015, 308, C330–C338. [Google Scholar] [CrossRef] [PubMed]
- Kalienkova, V.; Peter, M.F.; Rheinberger, J.; Paulino, C. Structures of a Sperm-Specific Solute Carrier Gated by Voltage and CAMP. Nature 2023, 623, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Yeo, H.; Mehta, V.; Gulati, A.; Drew, D. Structure and Electromechanical Coupling of a Voltage-Gated Na+/H+ Exchanger. Nature 2023, 623, 193–201. [Google Scholar] [CrossRef]
- Dong, Y.; Li, H.; Ilie, A.; Gao, Y.; Boucher, A.; Zhang, X.C.; Orlowski, J.; Zhao, Y. Structural Basis of Autoinhibition of the Human NHE3-CHP1 Complex. Sci. Adv. 2022, 8, 3925. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, Y.; Ilie, A.; Kim, D.S.; Boucher, A.; Li, B.; Zhang, X.C.; Orlowski, J.; Zhao, Y. Structure and Mechanism of the Human NHE1-CHP1 Complex. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, I.; Matsuoka, R.; Meier, P.F.; Shutin, D.; Zhang, C.; Orellana, L.; Sexton, R.; Landreh, M.; Robinson, C.V.; Beckstein, O.; et al. Structure and Elevator Mechanism of the Mammalian Sodium/Proton Exchanger NHE9. EMBO J. 2020, 39, 4541–4559. [Google Scholar] [CrossRef] [PubMed]
- Wöhlert, D.; Kühlbrandt, W.; Yildiz, Ö. Structure and Substrate Ion Binding in the Sodium/Proton Antiporter PaNhaP. Elife 2014, 3, e03579. [Google Scholar] [CrossRef] [PubMed]
- Paulino, C.; Wöhlert, D.; Kapotova, E.; Yildiz, Ö.; Kühlbrandt, W. Structure and Transport Mechanism of the Sodium/Proton Antiporter MjNhaP1. Elife 2014, 3, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Kang, H.J.; von Ballmoos, C.; Newstead, S.; Uzdavinys, P.; Dotson, D.L.; Iwata, S.; Beckstein, O.; Cameron, A.D.; Drew, D. A Two-Domain Elevator Mechanism for Sodium/Proton Antiport. Nature 2013, 501, 573–577. [Google Scholar] [CrossRef]
- Hunte, C.; Screpanti, E.; Venturi, M.; Rimon, A.; Padan, E.; Michel, H. Structure of a Na+/H+ Antiporter and Insights into Mechanism of Action and Regulation by PH. Nature 2005, 435, 1197–1202. [Google Scholar] [CrossRef]
- Matsuoka, R.; Fudim, R.; Jung, S.; Zhang, C.; Bazzone, A.; Chatzikyriakidou, Y.; Robinson, C.V.; Nomura, N.; Iwata, S.; Landreh, M.; et al. Structure, Mechanism and Lipid-Mediated Remodeling of the Mammalian Na+/H+ Exchanger NHA2. Nat. Struct. Mol. Biol. 2022, 29, 108–120. [Google Scholar] [CrossRef]
- Florman, H.M.; Tombes, R.M.; First, N.L.; Babcock, D.F. An Adhesion-Associated Agonist from the Zona Pellucida Activates G Protein-Promoted Elevations of Internal Ca2+ and PH That Mediate Mammalian Sperm Acrosomal Exocytosis. Dev. Biol. 1989, 135, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Speer, K.F.; Allen-Waller, L.; Novikov, D.R.; Barott, K.L. Molecular Mechanisms of Sperm Motility Are Conserved in an Early-Branching Metazoan. Proc. Natl. Acad. Sci. USA 2021, 118, e2109993118. [Google Scholar] [CrossRef]
- Romero, F.; Nishigaki, T. Comparative Genomic Analysis Suggests That the Sperm-Specific Sodium/Proton Exchanger and Soluble Adenylyl Cyclase Are Key Regulators of CatSper among the Metazoa. Zool. Lett. 2019, 5, 25. [Google Scholar] [CrossRef]
- Arcos-Hernández, C.; Suárez-Delgado, E.; Islas, L.D.; Romero, F.; López-González, I.; Ai, H.; Nishigaki, T. How to Study a Highly Toxic Protein to Bacteria: A Case of Voltage Sensor Domain of Mouse Sperm-Specific Sodium/Proton Exchanger. Protein Expr. Purif. 2023, 201, 106172. [Google Scholar] [CrossRef] [PubMed]
- Chávez, J.C.; Darszon, A.; Treviño, C.L.; Nishigaki, T. Quantitative Intracellular PH Determinations in Single Live Mammalian Spermatozoa Using the Ratiometric Dye SNARF-5F. Front. Cell Dev. Biol. 2020, 7, 491365. [Google Scholar] [CrossRef]
- Thomas, R.C.; Meech, R.W. Hydrogen Ion Currents and Intracellular PH in Depolarized Voltage-Clamped Snail Neurones. Nature 1982, 299, 826–828. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, I.S.; Moran, M.M.; Chong, J.A.; Clapham, D.E. A Voltage-Gated Proton-Selective Channel Lacking the Pore Domain. Nature 2006, 440, 1213–1216. [Google Scholar] [CrossRef] [PubMed]
- Castillo, K.; Pupo, A.; Baez-Nieto, D.; Contreras, G.F.; Morera, F.J.; Neely, A.; Latorre, R.; Gonzalez, C. Voltage-Gated Proton (Hv1) Channels, a Singular Voltage Sensing Domain. FEBS Lett. 2015, 589, 3471–3478. [Google Scholar] [CrossRef] [PubMed]
- Musset, B.; Smith, S.M.E.; Rajan, S.; Morgan, D.; Cherny, V.V.; Decoursey, T.E. Aspartate 112 Is the Selectivity Filter of the Human Voltage-Gated Proton Channel. Nature 2011, 480, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Cherny, V.V.; Markin, V.S.; Decoursey, T.E. The Voltage-Activated Hydrogen Ion Conductance in Rat Alveolar Epithelial Cells Is Determined by the PH Gradient. J. Gen. Physiol. 1995, 105, 861–896. [Google Scholar] [CrossRef]
- Decoursey, T.E. Voltage-Gated Proton Channels and Other Proton Transfer Pathways. Physiol. Rev. 2003, 83, 475–579. [Google Scholar] [CrossRef]
- Ramsey, I.S.; Ruchti, E.; Kaczmarek, J.S.; Clapham, D.E. Hv1 Proton Channels Are Required for High-Level NADPH Oxidase-Dependent Superoxide Production during the Phagocyte Respiratory Burst. Proc. Natl. Acad. Sci. USA 2009, 106, 7642–7647. [Google Scholar] [CrossRef]
- Mishra, A.K.; Kumar, A.; Yadav, S.; Anand, M.; Yadav, B.; Nigam, R.; Garg, S.K.; Swain, D.K. Functional Insights into Voltage Gated Proton Channel (Hv1) in Bull Spermatozoa. Theriogenology 2019, 136, 118–130. [Google Scholar] [CrossRef]
- Yeste, M.; Llavanera, M.; Mateo-Otero, Y.; Catalán, J.; Bonet, S.; Pinart, E. HVCN1 Channels Are Relevant for the Maintenance of Sperm Motility During In Vitro Capacitation of Pig Spermatozoa. Int. J. Mol. Sci. 2020, 21, 3255. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.K.; Fußhöller, D.M.; Goodwin, N.; Bönigk, W.; Müller, A.; Dokani Khesroshahi, N.; Brenker, C.; Wachten, D.; Krause, E.; Kaupp, U.B.; et al. Post-Translational Cleavage of Hv1 in Human Sperm Tunes PH- and Voltage-Dependent Gating. J. Physiol. 2017, 595, 1533–1546. [Google Scholar] [CrossRef] [PubMed]
- Lishko, P.V.; Botchkina, I.L.; Kirichok, Y. Progesterone Activates the Principal Ca2+ Channel of Human Sperm. Nature 2011, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Ritagliati, C.; Baro, C.; Stival, C.; Krapf, D. Mechanisms of Development Regulation Mechanisms and Implications of Sperm Membrane Hyperpolarization. Mech. Dev. 2018, 154, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Pathak, M.M.; Tran, T.; Hong, L.; Tombola, F.; Joós, B.; Morris, C.E. The Hv1 Proton Channel Responds to Mechanical Stimuli. J. Gen. Physiol. 2016, 148, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Peng, S.; Vance, J.; Tran, K.; Do, N.; Bui, N.; Gui, Z.; Wang, S. Structural Dynamics Determine Voltage and PH Gating in Human Voltage-Gated Proton Channel. Elife 2022, 11. [Google Scholar] [CrossRef]
- Ferreira, J.J.; Lybaert, P.; Puga-Molina, L.C.; Santi, C.M. Conserved Mechanism of Bicarbonate-Induced Sensitization of CatSper Channels in Human and Mouse Sperm. Front. Cell Dev. Biol. 2021, 9, 733653. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Úbeda, C.; Romero-Aguirregomezcorta, J.; Matás, C.; Visconti, P.E.; García-Vázquez, F.A. Manipulation of Bicarbonate Concentration in Sperm Capacitation Media Improves in Vitro Fertilisation Output in Porcine Species. J. Anim. Sci. Biotechnol. 2019, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Orta, G.; de la Vega-Beltran, J.L.; Martín-Hidalgo, D.; Santi, C.M.; Visconti, P.E.; Darszon, A. CatSper Channels Are Regulated by Protein Kinase A. J. Biol. Chem. 2018, 293, 16830–16841. [Google Scholar] [CrossRef]
- Carlson, A.E.; Hille, B.; Babcock, D.F. External Ca2+ Acts Upstream of Adenylyl Cyclase SACY in the Bicarbonate Signaled Activation of Sperm Motility. Dev. Biol. 2007, 312, 183. [Google Scholar] [CrossRef]
- Loiselle, F.B.; Casey, J.R. Measurement of Intracellular PH. Methods Mol. Biol. 2010, 637, 311–331. [Google Scholar] [CrossRef] [PubMed]
- Wennemuth, G.; Babcock, D.F.; Hille, B. Calcium Clearance Mechanisms of Mouse Sperm. J. Gen. Physiol. 2003, 122, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Schuh, K.; Cartwright, E.J.; Jankevics, E.; Bundschu, K.; Liebermann, J.; Williams, J.C.; Armesilla, A.L.; Emerson, M.; Oceandy, D.; Knobeloch, K.-P.; et al. Plasma Membrane Ca2+ ATPase 4 Is Required for Sperm Motility and Male Fertility. J. Biol. Chem. 2004, 279, 28220–28226. [Google Scholar] [CrossRef] [PubMed]
- Adijanto, J.; Philp, N.J. The SLC16A Family of Monocarboxylate Transporters (MCTs)—Physiology and Function in Cellular Metabolism, PH Homeostasis, and Fluid Transport. Curr. Top. Membr. 2012, 70, 275–312. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A.P.; Meredith, D. The SLC16 Gene Family—From Monocarboxylate Transporters (MCTs) to Aromatic Amino Acid Transporters and Beyond. Pflugers Arch. Eur. J. Physiol. 2004, 447, 619–628. [Google Scholar] [CrossRef] [PubMed]
- PARRISH, J.J.; SUSKO-PARRISH, J.L.; FIRST, N.L. Capacitation of Bovine Sperm by Heparin: Inhibitory Effect of Glucose and Role of Intracellular PH1. Biol. Reprod. 1989, 41, 683–699. [Google Scholar] [CrossRef]
- Mukai, C.; Okuno, M. Glycolysis Plays a Major Role for Adenosine Triphosphate Supplementation in Mouse Sperm Flagellar Movement. Biol. Reprod. 2004, 71, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Meizel, S.; Deamer, D.W. The PH of the Hamster Sperm Acrosome. J. Histochem. Cytochem. 1978, 26, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Working, P.K.; Meizel, S. Correlation of Increased Intraacrosomal PH with the Hamster Sperm Acrosome Reaction. J. Exp. Zool. 1983, 227, 97–107. [Google Scholar] [CrossRef]
- Nakanishi, T.; Ikawa, M.; Yamada, S.; Toshimori, K.; Okabe, M. Alkalinization of Acrosome Measured by GFP as a PH Indicator and Its Relation to Sperm Capacitation. Dev. Biol. 2001, 237, 222–231. [Google Scholar] [CrossRef]
- De Blas, G.; Michaut, M.; Treviño, C.L.; Tomes, C.N.; Yunes, R.; Darszon, A.; Mayorga, L.S. The Intraacrosomal Calcium Pool Plays a Direct Role in Acrosomal Exocytosis. J. Biol. Chem. 2002, 277, 49326–49331. [Google Scholar] [CrossRef] [PubMed]
- Costello, S.; Michelangeli, F.; Nash, K.; Lefievre, L.; Morris, J.; Machado-Oliveira, G.; Barratt, C.; Kirkman-Brown, J.; Publicover, S. Ca2+-Stores in Sperm: Their Identities and Functions. Reproduction 2009, 138, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Hirose, M.; Honda, A.; Fulka, H.; Tamura-Nakano, M.; Matoba, S.; Tomishima, T.; Mochida, K.; Hasegawa, A.; Nagashima, K.; Inoue, K.; et al. Acrosin Is Essential for Sperm Penetration through the Zona Pellucida in Hamsters. Proc. Natl. Acad. Sci. USA 2020, 117, 2513–2518. [Google Scholar] [CrossRef] [PubMed]
- Florman, H.M. Sequential Focal and Global Elevations of Sperm Intracellular Ca2+ Are Initiated by the Zona Pellucida during Acrosomal Exocytosis. Dev. Biol. 1994, 165, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Berruti, G.; Paiardi, C. Acrosome Biogenesis. Spermatogenesis 2011, 1, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Khawar, M.B.; Gao, H.; Li, W. Mechanism of Acrosome Biogenesis in Mammals. Front. Cell Dev. Biol. 2019, 8, 195. [Google Scholar] [CrossRef]
- Xiong, J.; Zhu, M.X. Regulation of Lysosomal Ion Homeostasis by Channels and Transporters. Sci. China Life Sci. 2016, 59, 777–791. [Google Scholar] [CrossRef]
- Forgac, M. Vacuolar ATPases: Rotary Proton Pumps in Physiology and Pathophysiology. Nat. Rev. Mol. Cell Biol. 2007, 8, 917–929. [Google Scholar] [CrossRef]
- Oot, R.A.; Couoh-Cardel, S.; Sharma, S.; Stam, N.J.; Wilkens, S. Breaking up and Making up: The Secret Life of the Vacuolar H+-ATPase. Protein Sci. 2017, 26, 896–909. [Google Scholar] [CrossRef]
- Pamarthy, S.; Kulshrestha, A.; Katara, G.K.; Beaman, K.D. The Curious Case of Vacuolar ATPase: Regulation of Signaling Pathways. Mol. Cancer 2018, 17, 41. [Google Scholar] [CrossRef]
- Sánchez-Tusie, A.A.; Vasudevan, S.R.; Churchill, G.C.; Nishigaki, T.; Treviño, C.L. Characterization of NAADP-Mediated Calcium Signaling in Human Spermatozoa. Biochem. Biophys. Res. Commun. 2014, 443, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, N.; Pisitkun, T.; Belleannée, C.; Miller, L.R.; Nelson, R.; Knepper, M.A.; Brown, D.; Breton, S. Proteomic Analysis of V-ATPase-Rich Cells Harvested from the Kidney and Epididymis by Fluorescence-Activated Cell Sorting. Am. J. Physiol. Physiol. 2010, 298, C1326–C1342. [Google Scholar] [CrossRef] [PubMed]
- DiCiccio, J.E.; Steinberg, B.E. Lysosomal PH and Analysis of the Counter Ion Pathways That Support Acidification. J. Gen. Physiol. 2011, 137, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, C.F.; Ullrich, F.; Leisle, L.; Stauber, T.; Jentsch, T.J. Common Gating of Both CLC Transporter Subunits Underlies Voltage-Dependent Activation of the 2Cl−/1H+ Exchanger ClC-7/Ostm1. J. Biol. Chem. 2013, 288, 28611–28619. [Google Scholar] [CrossRef] [PubMed]
- Wertheimer, E.V.; Salicioni, A.M.; Liu, W.; Trevino, C.L.; Chavez, J.; Hernández-González, E.O.; Darszon, A.; Visconti, P.E. Chloride Is Essential for Capacitation and for the Capacitation-Associated Increase in Tyrosine Phosphorylation. J. Biol. Chem. 2008, 283, 35539–35550. [Google Scholar] [CrossRef] [PubMed]
- Orta, G.; Ferreira, G.; José, O.; Treviño, C.L.; Beltrán, C.; Darszon, A. Human Spermatozoa Possess a Calcium-Dependent Chloride Channel That May Participate in the Acrosomal Reaction. J. Physiol. 2012, 590, 2659–2675. [Google Scholar] [CrossRef]
- SATO, Y.; SON, J.-H.; MEIZEL, S. The Mouse Sperm Glycine Receptor/Chloride Channel: Cellular Localization and Involvement in the Acrosome Reaction Initiated by Glycine. J. Androl. 2000, 21, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Puente, M.A.; Tartaglione, C.M.; Ritta, M.N. Bull Sperm Acrosome Reaction Induced by Gamma-Aminobutyric Acid (GABA) Is Mediated by GABAergic Receptors Type A. Anim. Reprod. Sci. 2011, 127, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Martínez, J.; Reyes-Miguel, T.; Rodríguez-Páez, L.; Garduño-Siciliano, L.; Maldonado-García, D.; Roa-Espitia, A.L.; Hernández-González, E.O. TMEM16A Inhibition Impedes Capacitation and Acquisition of Hyperactivated Motility in Guinea Pig Sperm. J. Cell. Biochem. 2018, 119, 5944–5959. [Google Scholar] [CrossRef]
- Jentsch, T.J. Discovery of CLC Transport Proteins: Cloning, Structure, Function and Pathophysiology. J. Physiol. 2015, 593, 4091–4109. [Google Scholar] [CrossRef]
- Patel, S.; Cai, X. Evolution of Acidic Ca2+ Stores and Their Resident Ca2+-Permeable Channels. Cell Calcium 2015, 57, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Oliver, E.I.; Jabloñski, M.; Buffone, M.G.; Darszon, A. Two-Pore Channel 1 and Ca2+ Release-Activated Ca2+ Channels Contribute to the Acrosomal PH-Dependent Intracellular Ca2+ Increase in Mouse Sperm. J. Physiol. 2023, 601, 2935–2958. [Google Scholar] [CrossRef]
- Treviño, C.L.; Santi, C.M.; Beltrán, C.; Hernández-Cruz, A.; Darszon, A.; Lomeli, H. Localisation of Inositol Trisphosphate and Ryanodine Receptors during Mouse Spermatogenesis: Possible Functional Implications. Zygote 1998, 6, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Lawson, C.; Dorval, V.; Goupil, S.; Leclerc, P. Identification and Localisation of SERCA 2 Isoforms in Mammalian Sperm. Mol. Hum. Reprod. 2007, 13, 307–316. [Google Scholar] [CrossRef]
- Arndt, L.; Castonguay, J.; Arlt, E.; Meyer, D.; Hassan, S.; Borth, H.; Zierler, S.; Wennemuth, G.; Breit, A.; Biel, M.; et al. NAADP and the Two-Pore Channel Protein 1 Participate in the Acrosome Reaction in Mammalian Spermatozoa. Mol. Biol. Cell 2014, 25, 948–964. [Google Scholar] [CrossRef]
- Chávez, J.C.; De la Vega-Beltrán, J.L.; José, O.; Torres, P.; Nishigaki, T.; Treviño, C.L.; Darszon, A. Acrosomal Alkalization Triggers Ca2+ Release and Acrosome Reaction in Mammalian Spermatozoa. J. Cell. Physiol. 2018, 233, 4735–4747. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Inositol Trisphosphate and Calcium Signalling. Nature 1993, 361, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, R.; Zhou, Y.; Zhou, W.; Zeng, X. Ip3r Channels in Male Reproduction. Int. J. Mol. Sci. 2020, 21, 9179. [Google Scholar] [CrossRef]
- Pizzo, P.; Lissandron, V.; Capitanio, P.; Pozzan, T. Ca2+ Signalling in the Golgi Apparatus. Cell Calcium 2011, 50, 184–192. [Google Scholar] [CrossRef]
- Walensky, L.D.; Snyder, S.H. Inositol 1,4,5-Trisphosphate Receptors Selectively Localized to the Acrosomes of Mammalian Sperm. J. Cell Biol. 1995, 130, 857–869. [Google Scholar] [CrossRef]
- Mata-Martínez, E.; Sánchez-Cárdenas, C.; Chávez, J.C.; Guerrero, A.; Treviño, C.L.; Corkidi, G.; Montoya, F.; Hernandez-Herrera, P.; Buffone, M.G.; Balestrini, P.A.; et al. Role of Calcium Oscillations in Sperm Physiology. Biosystems 2021, 209, 104524. [Google Scholar] [CrossRef]
- Sánchez-Cárdenas, C.; Servín-Vences, M.R.; José, O.; Treviño, C.L.; Hernández-Cruz, A.; Darszon, A. Acrosome Reaction and Ca2+ Imaging in Single Human Spermatozoa: New Regulatory Roles of [Ca2+]I. Biol. Reprod. 2014, 91, 1–13. [Google Scholar] [CrossRef]
- Mata-Martínez, E.; Darszon, A.; Treviño, C.L. PH-Dependent Ca+2 Oscillations Prevent Untimely Acrosome Reaction in Human Sperm. Biochem. Biophys. Res. Commun. 2018, 497, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Otčenášková, T.; Macíčková, E.; Vondráková, J.; Frolíková, M.; Komrskova, K.; Stopková, R.; Stopka, P. Proteomic Analysis of the Mouse Sperm Acrosome - towards an Understanding of an Organelle with Diverse Functionality. Eur. J. Cell Biol. 2023, 102, 151296. [Google Scholar] [CrossRef] [PubMed]
- De Duve, C.; De Barsy, T.; Poole, B.; Trouet, A.; Tulkens, P.; Van Hoof, F. Lysosomotropic Agents. Biochem. Pharmacol. 1974, 23, 2495–2531. [Google Scholar] [CrossRef] [PubMed]
- Guyonnet, B.; Egge, N.; Cornwall, G.A. Functional Amyloids in the Mouse Sperm Acrosome. Mol. Cell. Biol. 2014, 34, 2624–2634. [Google Scholar] [CrossRef] [PubMed]
- Buffone, M.; Foster, J.; Gerton, G. The Role of the Acrosomal Matrix in Fertilization. Int. J. Dev. Biol. 2008, 52, 511–522. [Google Scholar] [CrossRef]
- Gunderson, S.J.; Puga Molina, L.C.; Spies, N.; Balestrini, P.A.; Buffone, M.G.; Jungheim, E.S.; Riley, J.; Santi, C.M. Machine-Learning Algorithm Incorporating Capacitated Sperm Intracellular PH Predicts Conventional in Vitro Fertilization Success in Normospermic Patients. Fertil. Steril. 2021, 115, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Guo, Y.; Zhou, T.; Shi, X.; Yu, J.; Yang, Y.; Wu, Y.; Wang, J.; Liu, M.; Chen, X.; et al. In-Depth Proteomic Analysis of the Human Sperm Reveals Complex Protein Compositions. J. Proteomics 2013, 79, 114–122. [Google Scholar] [CrossRef]
- Schork, K.; Turewicz, M.; Uszkoreit, J.; Rahnenführer, J.; Eisenacher, M. Characterization of Peptide-Protein Relationships in Protein Ambiguity Groups via Bipartite Graphs. PLoS One 2022, 17, e0276401. [Google Scholar] [CrossRef]
- Momenbeitollahi, N.; Cloet, T.; Li, H. Pushing the Detection Limits: Strategies towards Highly Sensitive Optical-Based Protein Detection. Anal. Bioanal. Chem. 2021, 413, 5995–6011. [Google Scholar] [CrossRef]
- De-la-Rosa, V.; Suárez-Delgado, E.; Rangel-Yescas, G.E.; Islas, L.D. Currents through Hv1 Channels Deplete Protons in Their Vicinity. J. Gen. Physiol. 2016, 147, 127–136. [Google Scholar] [CrossRef]
- Chung, J.-J.; Shim, S.-H.; Everley, R.A.; Gygi, S.P.; Zhuang, X.; Clapham, D.E. Structurally Distinct Ca(2+) Signaling Domains of Sperm Flagella Orchestrate Tyrosine Phosphorylation and Motility. Cell 2014, 157, 808–822. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Ke, M.; Zhang, Y.; Yan, Z.; Wu, J. Structure of a Mammalian Sperm Cation Channel Complex. Nature 2021, 595, 746–750. [Google Scholar] [CrossRef]
- Lin, J.; Nicastro, D. Asymmetric Distribution and Spatial Switching of Dynein Activity Generates Ciliary Motility. Science 2018, 360, aar1968. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, H.; Wiesehoefer, C.; Shah, N.B.; Reetz, E.; Hwang, J.Y.; Huang, X.; Wang, T.; Lishko, P.V.; Davies, K.M.; et al. 3D Structure and in Situ Arrangements of CatSper Channel in the Sperm Flagellum. Nat. Commun. 2022, 13, 3439. [Google Scholar] [CrossRef] [PubMed]
- Bleil, J.D.; Wassarman, P.M. Mammalian Sperm-Egg Interaction: Identification of a Glycoprotein in Mouse Egg Zonae Pellucidae Possessing Receptor Activity for Sperm. Cell 1980, 20, 873–882. [Google Scholar] [CrossRef]
- Okabe, M. Sperm–Egg Interaction and Fertilization: Past, Present, and Future. Biol. Reprod. 2018, 99, 134–146. [Google Scholar] [CrossRef]




| Molecule | Species | Cellular Localization | Related Function | Reference |
|---|---|---|---|---|
| CAI | Hs | PP | Control of motility, participation in the RA, HCO3−/CO2 balance and pH regulation | [34,35] |
| CAII | Hs; Mm | PP | [34,35,36,41] | |
| CAIV | Hs; Mm | MP | [35,37] | |
| CAXIII | Hs | MP | [35,42] | |
| AE1 (SLC4A2) | Hs | H; PP | Its participation in pH regulation has not been determined. | [43] |
| AE2 (SLC4A2) | Mm | N.D. | Mice lacking the expression of this transporter present infertility problems, due to a failure in spermatogenesis. Its participation in pH regulation has not been determined. | [44] |
| NBCe1 (SLC4A4) | Hs; Mm | N.D. | Functional experiments suggested a role in membrane hyperpolarization and HCO3− transport. | [45] |
| NBCe2 (SLC4A5) | Hs; Mm | N.D. | ||
| DRA (SLC26A3) | Hs; Mm | MP | Role in membrane hyperpolarization, HCO3− transport and pH regulation of mouse sperm | [39,46,47,48] |
| PAT1 (SLC26A6) | Hs; Mm | MP | ||
| CFTR | Hs; Mm | Eq; MP | Heterozygous CFTR mutant mice showed lowered fertility rates. Pharmacological inhibition affects capacitation related events including intracellular alkalization | [46,49,50,51,52,53] |
| NHE1 (SLC9A1) | Hs; Mm | MP | Possible participation in plasma membrane hyperpolarization | [54,55] |
| NHE5 (SLC9A5) | Hs; Mm | MP | N.D. | [55] |
| NHA1 (SLC9B1) | Mm | PP | A double knock-out (KO) of NHA1 and NHA2 results in an infertile male phenotype with a deficiency in cAMP signaling and flagellar motility | [56,57] |
| NHA2 (SLC9B2) | Mm | PP | ||
| sNHE/NHE10 (SLC9C1) | Hs; Mm | PP | Essential for male fertility in both humans and mice. | [16,17,18,22,56,57,58] |
| sNHE/NHE11 (SLC9C2) | Hs | PP | N.D. | |
| Hv1 | Hs | PP | Regulates human sperm pH. Participation in hyperactivation and in CatSper-dependent intracellular calcium increase. | [20,50,59,60,61] |
| PMCA4 (ATP2B4) | Mm | PP | Knockout mouse is infertile. PMCA pump functions as a Ca2+/H+ exchanger powered by ATP | [62,63] |
| MCT1 (SLC16A1) | Mm | MP; PP | Facilitate lactate uptake and evokes pHi acidification. | [64,65] |
| MCT2 (SLC16A7) | Mm | |||
| LDHC | Mm | PP | Converting pyruvate into lactate and producing NAD+ and consuming protons. Knockout mouse is infertile. | [66] |
| v-ATPase | Hs; Mm | Ac | Regulates intra acrosomal pH. Essential for acrosome reaction | [67,68] |
| ClC3 | Hs | Ac | Volume modulation. Possible participation in intra acrosomal pH regulation | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez, J.C.; Carrasquel-Martínez, G.; Hernández-Garduño, S.; Matamoros Volante, A.; Treviño, C.L.; Nishigaki, T.; Darszon, A. Cytosolic and Acrosomal pH Regulation in Mammalian Sperm. Cells 2024, 13, 865. https://doi.org/10.3390/cells13100865
Chávez JC, Carrasquel-Martínez G, Hernández-Garduño S, Matamoros Volante A, Treviño CL, Nishigaki T, Darszon A. Cytosolic and Acrosomal pH Regulation in Mammalian Sperm. Cells. 2024; 13(10):865. https://doi.org/10.3390/cells13100865
Chicago/Turabian StyleChávez, Julio C., Gabriela Carrasquel-Martínez, Sandra Hernández-Garduño, Arturo Matamoros Volante, Claudia L. Treviño, Takuya Nishigaki, and Alberto Darszon. 2024. "Cytosolic and Acrosomal pH Regulation in Mammalian Sperm" Cells 13, no. 10: 865. https://doi.org/10.3390/cells13100865





