Role of Calcium Chloride on the Eluting Process of Residual Ammonium from Weathered Crust Elution-Deposited Rare Earth Ore Tailings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Residual Ammonium Salt Leaching Test
2.3. Analytical Methods
3. Results
3.1. Effect of Calcium Concentration on the Eluting Process of RA
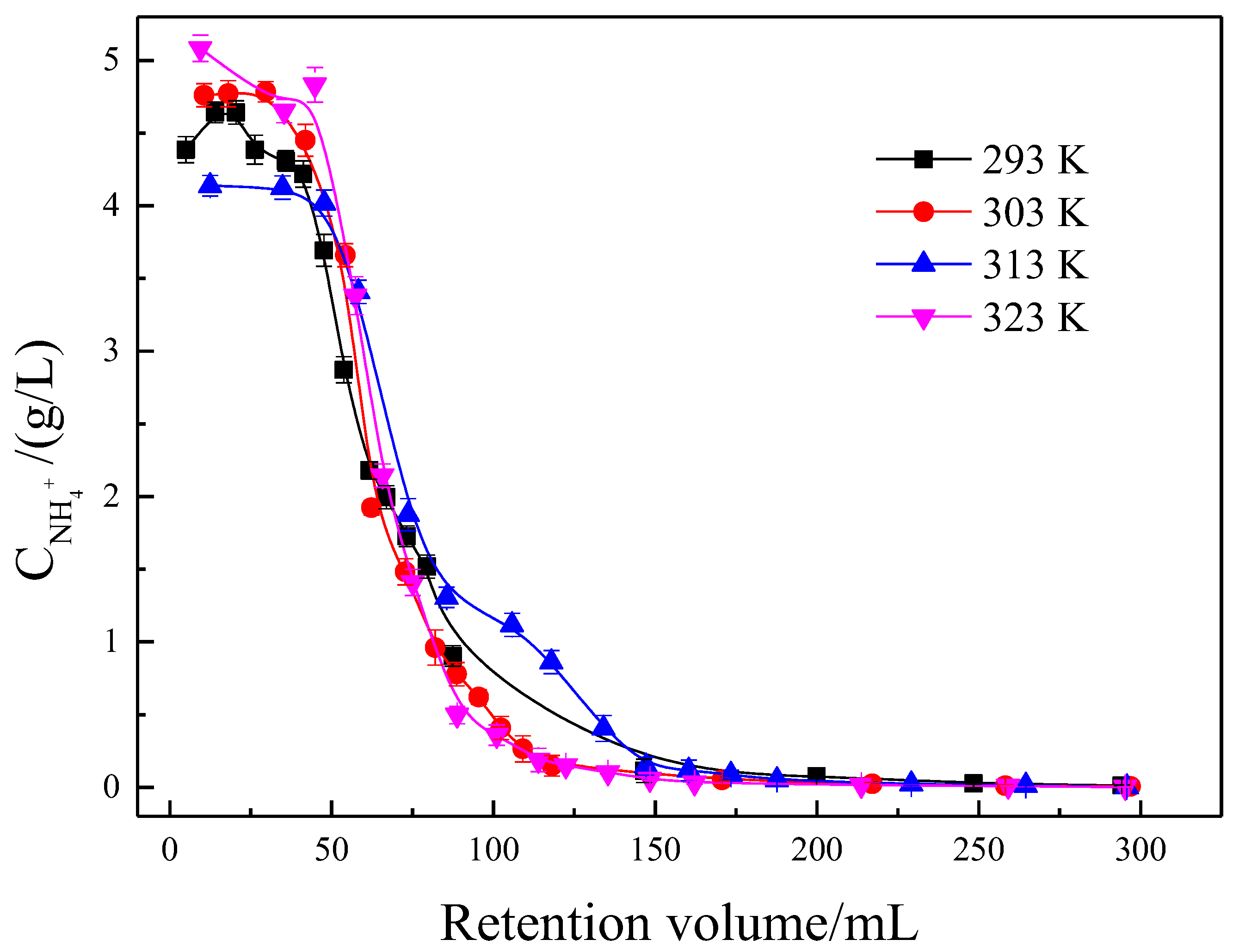
3.2. Effect of Temperature on the Eluting Process of RA
3.3. Effect of Liquid-Solid Ratio on the Elution Efficiency of RA
3.4. Effect of Flow Rate on the Eluting Process of RA
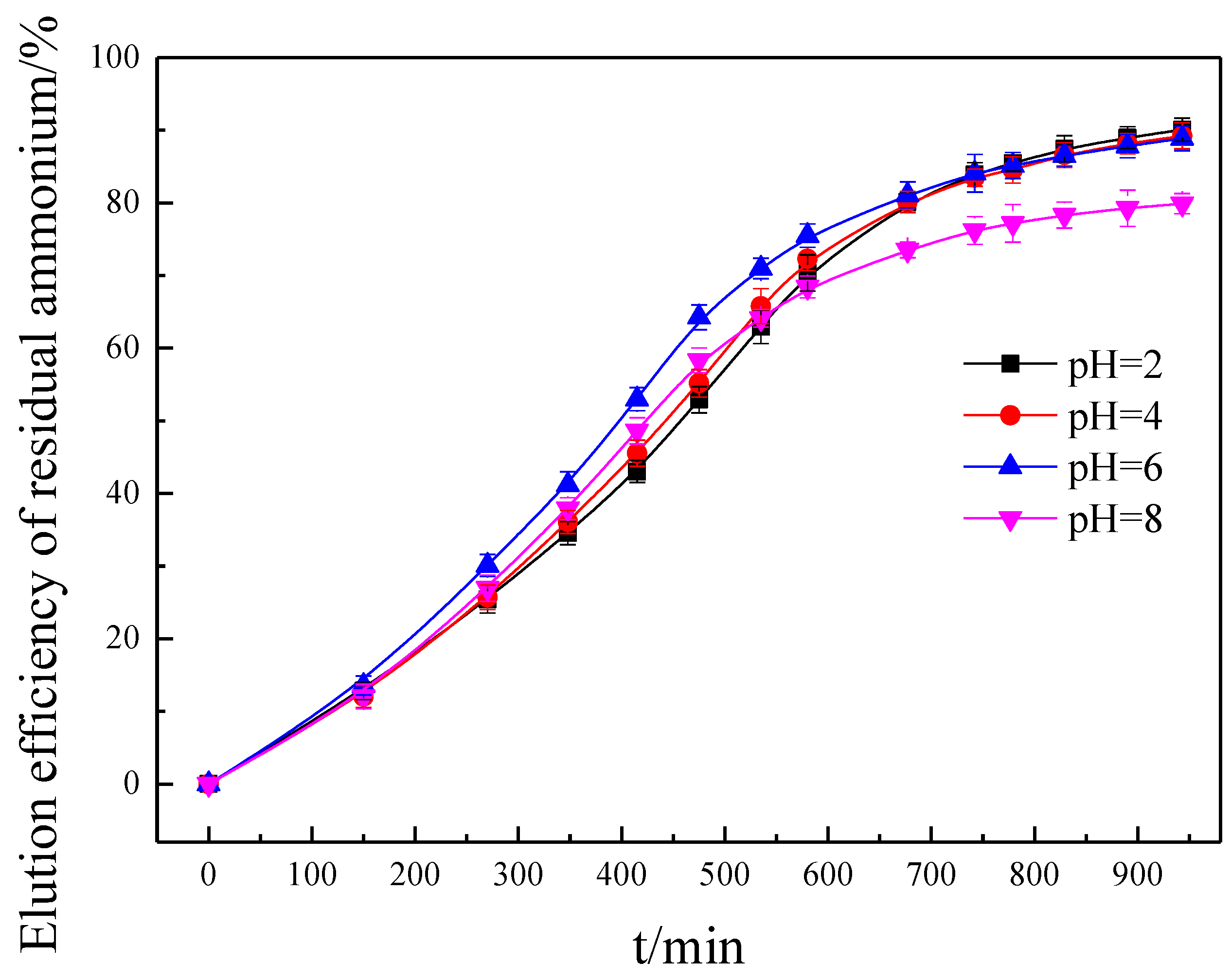
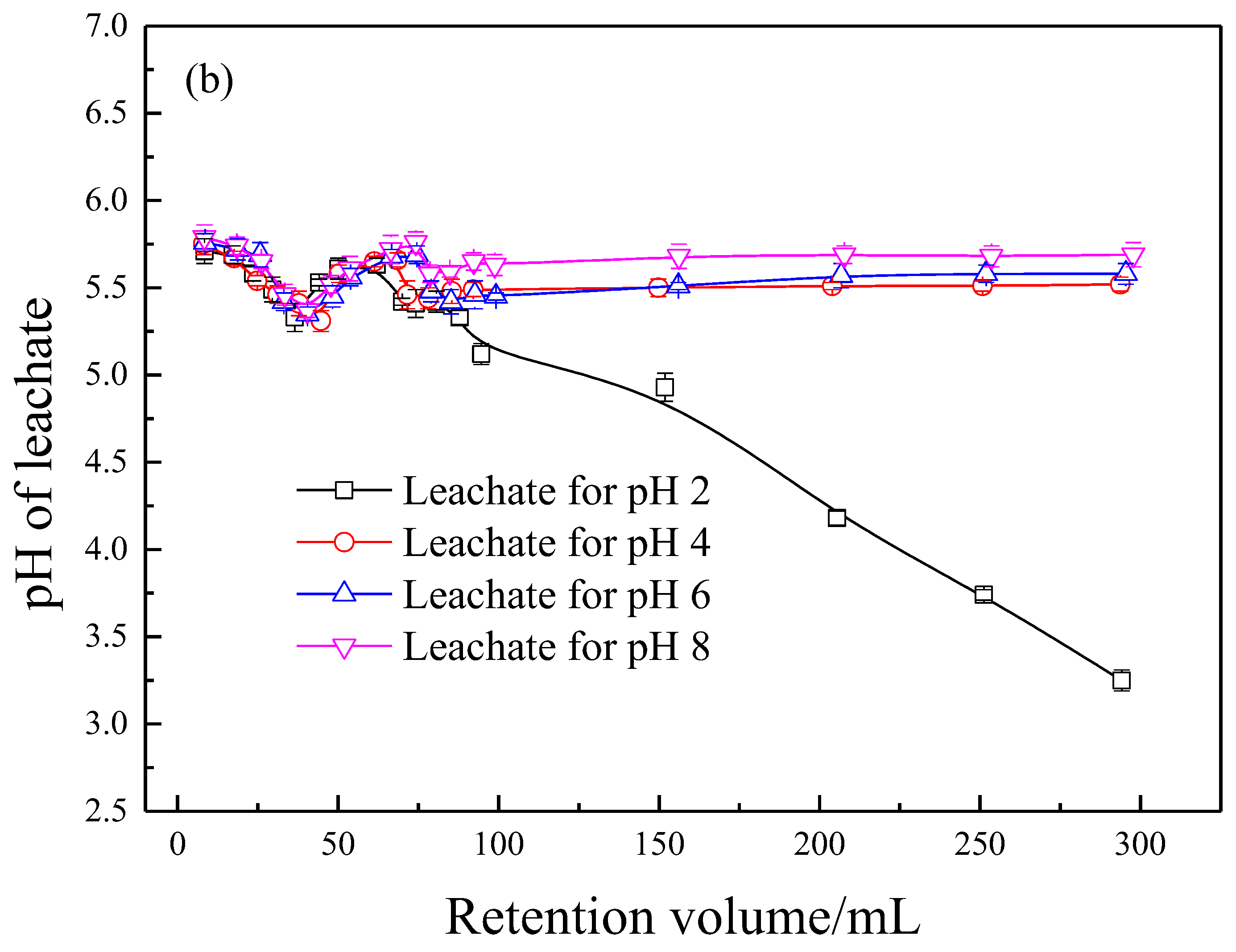
3.5. Effect of Solution pH on the Eluting Process of RA
3.6. Effect of Calcium Chloride on the Eluting Mass Transfer Process of RA
3.6.1. Effect of Calcium Chloride Concentration
3.6.2. Effect of Temperature
3.6.3. Effect of Flow Rate
3.6.4. Effect of Solution pH
3.7. Eluting Dynamics Analysis of Concentration
3.8. Reaction Activation Energy Analysis of Eluting Temperature
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, X.P.; Zhang, Y.B.; Zhou, H.P.; He, K.Z.; Luo, C.G.; Liu, Z.S.; Tang, X.K. Review on the Development and Utilization of Ionic Rare Earth Ore. Minerals 2022, 12, 554. [Google Scholar] [CrossRef]
- Zeng, X.G.; Zeng, B.; Huang, L.J.H.; Zhong, L.; Li, X.D.; Huang, W.F. Adsorption of Y (III) on the Interface of Kaolinite-H2O: A DFT Study. Minerals 2022, 12, 1128. [Google Scholar] [CrossRef]
- Feng, J.; Zhou, F.; Chi, R.A.; Liu, X.; Xu, Y.L.; Liu, Q. Effect of a Novel Compound on Leaching Process of Weathered Crust Elution-Deposited Rare Earth Ore. Miner. Eng. 2018, 129, 63–70. [Google Scholar] [CrossRef]
- Ju, W.; Yang, J.H.; Yao, C.; Zhang, X.B.; Ye, Z.W.; Liu, D. Experimental Study on the Permeability of Rare Earths with Different Particle Composition for a Novel Heap Leaching Technology. Appl. Sci. 2022, 12, 11368. [Google Scholar] [CrossRef]
- Wang, G.F.; Xu, J.; Ran, L.Y.; Zhu, R.L.; Ling, B.W.; Liang, X.L.; Kang, S.C.; Wang, Y.Y.; Wei, J.M.; Ma, L.Y.; et al. A Green and Efficient Technology to Recover Rare Earth Elements from Weathering Crusts. Nat. Sustain. 2023, 6, 81–92. [Google Scholar] [CrossRef]
- Lai, F.G.; Huang, L.; Gao, G.H.; Yang, R.; Xiao, Y.F. Recovery of Rare Earths from Ion-Absorbed Rare Earths Ore with MgSO4- Ascorbic Acid Compound Leaching Agent. J. Rare Earths 2018, 36, 521–527. [Google Scholar] [CrossRef]
- Yaraghi, A.; Ariffin, K.S.; Baharun, N. A Short Review on REE Recovery from Ion-Adsorption Clays. Development 2019, 8, 131–136. [Google Scholar]
- Meng, X.Y.; Zhao, H.B.; Zhang, Y.S.; Shen, L.; Gu, G.H.; Qiu, G.Z.; Zhang, X.G.; Yu, H.; He, X.; Liu, C. Simulated Bioleaching of Ion-Adsorption Rare Earth Ore Using Metabolites of Biosynthetic Citrate: An Alternative to Cation Exchange Leaching. Miner. Eng. 2022, 189, 107900. [Google Scholar] [CrossRef]
- Xia, H.B.; Deng, P.; Wu, X.; Liu, D.B.; Qiu, S.; Liu, Y.Q.; Yu, Y.H. Simulation Research on Pilot Experimental of In-situ Leaching Ion-adsorption Rare Earth Ores with Plant Leaching Agent. Nonferrous Met. Eng. 2020, 10, 52–59. [Google Scholar]
- Zhu, D.M.; Qiu, T.S.; Zhong, J.F.; Zeng, Q.H.; Fang, X.H. Molecular Dynamics Simulation of Aluminum Inhibited Leaching during Ion-Adsorbed Type Rare Earth Ore Leaching Process. J. Rare Earths 2019, 37, 1334–1340. [Google Scholar] [CrossRef]
- Shi, Q.Y.; Zhao, Y.; Meng, X.Y.; Shen, L.; Qiu, G.Z.; Zhang, X.G.; Yu, H.; He, X.; He, H.J.; Zhao, H.B. Column Leaching of Ion Adsorption Rare Earth Ore at Low Ammonium Concentration. J. Mater. Res. Technol. 2022, 19, 2135–2145. [Google Scholar] [CrossRef]
- Yang, F.; Liao, H.; Jin, S. The environmental costs of the mining on rare earths in the south of Jiangxi province. Prices Mon. 2013, 6, 87–90. (In Chinese) [Google Scholar]
- Wei, J.; Wang, H.; Yan, J. Environmental damages and control measures in exploiting ion-absorbed rare earth of South China. Nonferrous Met. Sci. Eng. 2016, 7, 125–132. (In Chinese) [Google Scholar]
- Feng, J.; Yu, J.; Huang, S.; Wu, X.; Zhou, F.; Xiao, C.; Xu, Y.; Chi, R. Effect of potassium chloride on leaching process of residual ammonium from weathered crust elution-deposited rare earth ore tailings. Miner. Eng. 2021, 163, 106800. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Zhu, Q.; Xiao, Z.; Zhang, N. Distribution characteristics of nitrides in soil of south China ion-adsorption rare earth ore mining area. Chin. Rare Earths 2015, 36, 1–5. (In Chinese) [Google Scholar]
- Liu, S.; Huang, Y.; Han, Z.; Liu, X. Practices of the soil ecological remediation in ion-absorbed rare earth mine. Environ. Eng. 2015, 33, 160–165. (In Chinese) [Google Scholar]
- Yang, S. Study on the Adsorption and Desorption Behavior of Ammonium in the Mining Process of Ion-Absorbed Rare-Earth Mineral. Master’s Thesis, China University of Geosciences, Beijing, China, 2015. (In Chinese). [Google Scholar]
- Osborne, G. The extraction and definition of non-exchangeable or fixed ammonium in some soils from southern New South Wales. Soil Res. 1976, 14, 373–380. [Google Scholar] [CrossRef]
- Wang, F.L.; Alva, A.K. Ammonium adsorption and desorption in sandy soils. Soil Sci. Soc. Am. J. 2000, 64, 1669–1674. [Google Scholar] [CrossRef]
- Aydogan, S.; Ucar, G.; Canbazoglu, M. Dissolution kinetics of chalcopyrite in acidic potassium dichromate solution. J. Hydrometall. 2006, 81, 45–51. [Google Scholar] [CrossRef]
- Ghosh, A.; Ray, H. Principles of Extractive Metallurgy, 2nd ed.; Wiley Eastern Ltd.: New Delhi, India, 1991. [Google Scholar]
- Sohn, H.; Wadsworth, M. Rate Processes in Extractive Metallurgy; Plenum: New York, NY, USA, 1979. [Google Scholar]
- Li, M.; Zhang, X.W.; Liu, Z.G.; Hu, Y.H.; Wang, M.T.; Liu, J.; Yang, J.P. Kinetics of leaching fluoride from mixed rare earth concentrate with hydrochloric acid and aluminum chloride. Hydrometallurgy 2013, 140, 71–76. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, X.J.; Li, K.F.; Qian, G.R.; Zhou, M. Kinetics of leaching refractory gold ores by ultrasonic-assisted electro-chlorination. Int. J. Miner. Metall. Mater. 2012, 19, 473–477. [Google Scholar] [CrossRef]
- Ashraf, M.; Zafar, Z.; Ansari, T. Selective leaching kinetics and upgrading of low-grade calcareous phosphate rock in succinic acid. J. Hydrometall. 2005, 80, 286–292. [Google Scholar] [CrossRef]









| Calcium Concentration (mol/L) | k (min−1) | R2 |
|---|---|---|
| 0.008 | 0.000105 | 0.971 |
| 0.01 | 0.000133 | 0.971 |
| 0.02 | 0.000156 | 0.965 |
| 0.06 | 0.000211 | 0.990 |
| 0.10 | 0.000266 | 0.981 |
| 0.15 | 0.000347 | 0.969 |
| 0.20 | 0.000376 | 0.995 |
| Temperature (K) | k (min−1) | R2 |
|---|---|---|
| 293 | 0.000291 | 0.995 |
| 303 | 0.000346 | 0.984 |
| 313 | 0.000393 | 0.992 |
| 323 | 0.000473 | 0.971 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Wu, X.; Zhou, F.; Chi, R. Role of Calcium Chloride on the Eluting Process of Residual Ammonium from Weathered Crust Elution-Deposited Rare Earth Ore Tailings. Minerals 2024, 14, 521. https://doi.org/10.3390/min14050521
Feng J, Wu X, Zhou F, Chi R. Role of Calcium Chloride on the Eluting Process of Residual Ammonium from Weathered Crust Elution-Deposited Rare Earth Ore Tailings. Minerals. 2024; 14(5):521. https://doi.org/10.3390/min14050521
Chicago/Turabian StyleFeng, Jian, Xiaoyan Wu, Fang Zhou, and Ruan Chi. 2024. "Role of Calcium Chloride on the Eluting Process of Residual Ammonium from Weathered Crust Elution-Deposited Rare Earth Ore Tailings" Minerals 14, no. 5: 521. https://doi.org/10.3390/min14050521




