MRI Application and Challenges of Hyperpolarized Carbon-13 Pyruvate in Translational and Clinical Cardiovascular Studies: A Literature Review
Abstract
:1. Background
2. Brief Overview of Hyperpolarization and Dissolution–Dynamic Nuclear Polarization
3. Biological and Technical Considerations of Pyruvate Metabolism
4. 13C Radiofrequency Coils
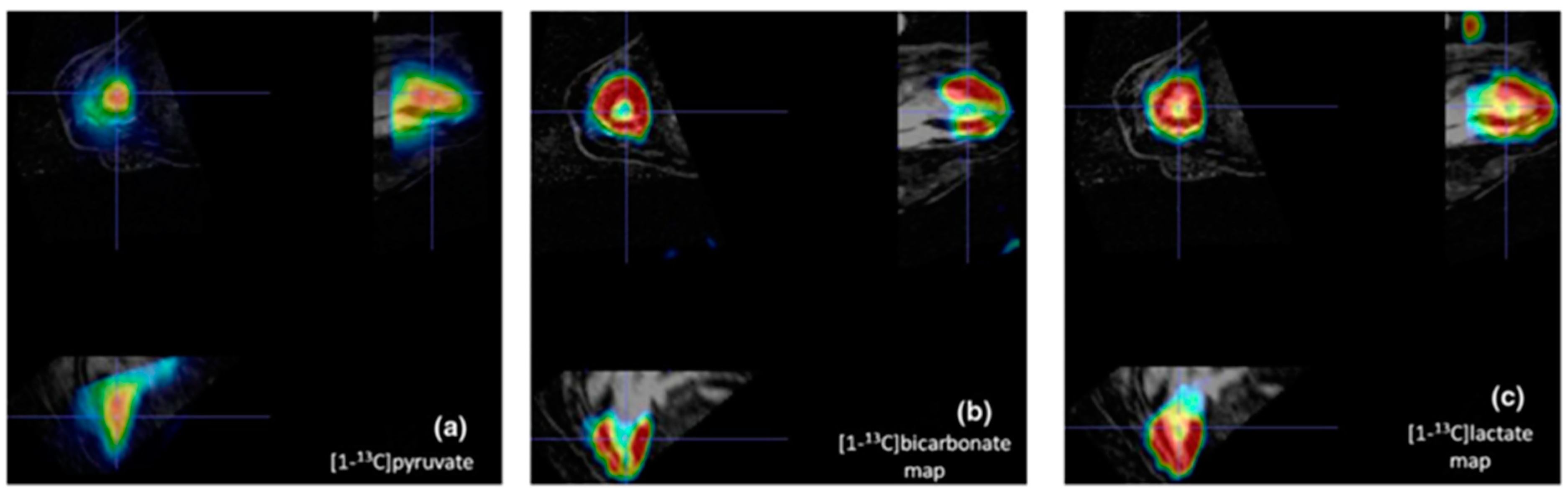
5. 13C-MRI Image Acquisition
6. Clinical Applications from Pre-Clinical to Human Studies
6.1. Pre-Clinical Cardiovascular Studies in Large Animal Models
6.2. Human Cardiovascular Studies
7. Current Limitations and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsampasian, V.; Cameron, D.; Sobhan, R.; Bazoukis, G.; Vassiliou, V.S. Phosphorus Magnetic Resonance Spectroscopy (31P MRS) and Cardiovascular Disease: The Importance of Energy. Medicina 2023, 59, 174. [Google Scholar] [CrossRef] [PubMed]
- Seelig, J.; Burlina, A.P. Carbon-13 magnetic resonance in biology and medicine. Clin. Chim. Acta 1992, 206, 125–136. [Google Scholar] [CrossRef]
- Madelin, G.; Lee, J.-S.; Regatte, R.R.; Jerschow, A. Sodium MRI: Methods and applications. Prog. Nucl. Magn. Reson. Spectrosc. 2014, 79, 14–47. [Google Scholar] [CrossRef]
- Marshall, H.; Stewart, N.J.; Chan, H.-F.; Rao, M.; Norquay, G.; Wild, J.M. In vivo methods and applications of xenon-129 magnetic resonance. Prog. Nucl. Magn. Reson. Spectrosc. 2021, 122, 42–62. [Google Scholar] [CrossRef]
- Harris, R.K.; Becker, E.D.; de Menezes, S.M.C.; Goodfellowd, R.; Grangere, P. NMR Nomenclature: Nuclear Spin Properties and Conventions for Chemical Shifts: IUPAC Recommendations 2001. Solid State Nucl. Magn. Reson. 2002, 22, 458–483. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, M.; Vettukattil, R. In Vivo Magnetic Resonance Spectroscopy Methods for Investigating Cardiac Metabolism. Metabolites 2022, 12, 189. [Google Scholar] [CrossRef]
- Josan, S.; Park, J.M.; Hurd, R.; Yen, Y.; Pfefferbaum, A.; Spielman, D.; Mayer, D. In vivo investigation of cardiac metabolism in the rat using MRS of hyperpolarized [1-13C] and [2-13C]pyruvate. NMR Biomed. 2013, 26, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- Vaeggemose, M.; Schulte, R.F.; Laustsen, C. Comprehensive Literature Review of Hyperpolarized Carbon-13 MRI: The Road to Clinical Application. Metabolites 2021, 11, 219. [Google Scholar] [CrossRef]
- Ardenkjaer-Larsen, J.H.; Fridlund, B.; Gram, A.; Hansson, G.; Hansson, L.; Lerche, M.H.; Servin, R.; Thaning, M.; Golman, K. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. USA 2003, 100, 10158–10163. [Google Scholar] [CrossRef]
- Chaumeil, M.M.; Bankson, J.A.; Brindle, K.M.; Epstein, S.; Gallagher, F.A.; Grashei, M.; Guglielmetti, C.; Kaggie, J.D.; Keshari, K.R.; Knecht, S.; et al. New Horizons in Hyperpolarized 13C MRI. Mol. Imaging Biol. 2023, 26, 222–232. [Google Scholar] [CrossRef]
- Deen, S.S.; Rooney, C.; Shinozaki, A.; McGing, J.; Grist, J.T.; Tyler, D.J.; Serrão, E.; Gallagher, F.A. Hyperpolarized Carbon 13 MRI: Clinical Applications and Future Directions in Oncology. Radiol. Imaging Cancer 2023, 5, e230005. [Google Scholar] [CrossRef] [PubMed]
- Apps, A.; Lau, J.Y.; Miller, J.J.; Tyler, A.; Young, L.A.; Lewis, A.J.; Barnes, G.; Trumper, C.; Neubauer, S.; Rider, O.J.; et al. Proof-of-Principle Demonstration of Direct Metabolic Imaging Following Myocardial Infarction Using Hyperpolarized 13C CMR. Cardiovasc. Imaging 2021, 14, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- Hunold, P.; Jakob, H.; Erbel, R.; Barkhausen, J.; Heilmaier, C. Accuracy of myocardial viability imaging by cardiac MRI and PET depending on left ventricular function. World J. Cardiol. 2018, 10, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Fuetterer, M.; Traechtler, J.; Busch, J.; Peereboom, S.M.; Dounas, A.; Manka, R.; Weisskopf, M.; Cesarovic, N.; Stoeck, C.T.; Kozerke, S. Hyperpolarized Metabolic and Parametric CMR Imaging of Longitudinal Metabolic-Structural Changes in Experimental Chronic Infarction. Cardiovasc. Imaging 2022, 15, 2051–2064. [Google Scholar] [CrossRef] [PubMed]
- Rider, O.J.; Apps, A.; Miller, J.J.; Lau, J.Y.; Lewis, A.J.; Peterzan, M.A.; Dodd, M.S.; Lau, A.Z.; Trumper, C.; Gallagher, F.A.; et al. Noninvasive In Vivo Assessment of Cardiac Metabolism in the Healthy and Diabetic Human Heart Using Hyperpolarized 13 C MRI. Circ. Res. 2020, 126, 725–736. [Google Scholar] [CrossRef]
- Golman, K.; Olsson, L.E.; Axelsson, O.; Månsson, S.; Karlsson, M.; Petersson, J.S. Molecular imaging using hyperpolarized13C. Br. J. Radiol. 2003, 76, S118–S127. [Google Scholar] [CrossRef] [PubMed]
- Levitt, M.H. Long live the singlet state! J. Magn. Reson. 2019, 306, 69–74. [Google Scholar] [CrossRef]
- Elliott, S.J.; Kadeřávek, P.; Brown, L.J.; Sabba, M.; Glöggler, S.; O’Leary, D.J.; Brown, R.C.D.; Ferrage, F.; Levitt, M.H. Field-cycling long-lived-state NMR of 15N2 spin pairs. Mol. Phys. 2019, 117, 861–867. [Google Scholar] [CrossRef]
- Stevanato, G.; Hill-Cousins, J.T.; Håkansson, P.; Roy, S.S.; Brown, L.J.; Brown, R.C.D.; Pileio, G.; Levitt, M.H. A Nuclear Singlet Lifetime of More than One Hour in Room-Temperature Solution. Angew. Chem. Int. Ed. 2015, 54, 3740–3743. [Google Scholar] [CrossRef]
- Hirsch, M.L.; Kalechofsky, N.; Belzer, A.; Rosay, M.; Kempf, J.G. Brute-Force Hyperpolarization for NMR and MRI. J. Am. Chem. Soc. 2015, 137, 8428–8434. [Google Scholar] [CrossRef]
- Frossati, G. Polarization of 3He, 2D and (eventually) 129Xe using low temperatures and high magnetic fields. J. Low Temp. Phys. 1998, 111, 521–532. [Google Scholar] [CrossRef]
- Krjukov, E.V.; O’neill, J.D.; Owers-Bradley, J.R. Brute Force Polarization of 129Xe. J. Low Temp. Phys. 2005, 140, 397–408. [Google Scholar] [CrossRef]
- Hirsch, M.L.; Smith, B.A.; Mattingly, M.; Goloshevsky, A.G.; Rosay, M.; Kempf, J.G. Transport and imaging of brute-force 13C hyperpolarization. J. Magn. Reson. 2015, 261, 87–94. [Google Scholar] [CrossRef]
- Khan, A.S.; Harvey, R.L.; Birchall, J.R.; Irwin, R.K.; Nikolaou, P.; Schrank, G.; Emami, K.; Dummer, A.; Barlow, M.J.; Goodson, B.M.; et al. Enabling Clinical Technologies for Hyperpolarized 129Xenon Magnetic Resonance Imaging and Spectroscopy. Angew. Chem. Int. Ed. 2021, 60, 22126–22147. [Google Scholar] [CrossRef]
- Shammi, U.A.; D’Alessandro, M.F.; Altes, T.; Hersman, F.W.; Ruset, I.C.; Mugler, J.; Meyer, C.; Mata, J.; Qing, K.; Thomen, R. Comparison of Hyperpolarized 3He and 129Xe MR Imaging in Cystic Fibrosis Patients. Acad. Radiol. 2021, 29 (Suppl. 2), S82–S90. [Google Scholar] [CrossRef]
- Wild, J.M.; Gleeson, F.V.; Svenningsen, S.; Grist, J.T.; Saunders, L.C.; Collier, G.J.; Sharma, M.; Tcherner, S.; Mozaffaripour, A.; Matheson, A.M.; et al. Review of Hyperpolarized Pulmonary Functional 129Xe MR for Long-COVID. J. Magn. Reson. Imaging 2024, 59, 1120–1134. [Google Scholar] [CrossRef] [PubMed]
- Shepelytskyi, Y.; Grynko, V.; Rao, M.R.; Li, T.; Agostino, M.; Wild, J.M.; Albert, M.S. Hyperpolarized 129 Xe imaging of the brain: Achievements and future challenges. Magn. Reason. Med. 2022, 88, 83–105. [Google Scholar] [CrossRef]
- Kimura, A.; Utsumi, S.; Shimokawa, A.; Nishimori, R.; Hosoi, R.; Stewart, N.J.; Imai, H.; Fujiwara, H. Targeted Imaging of Lung Cancer with Hyperpolarized 129Xe MRI Using Surface-Modified Iron Oxide Nanoparticles as Molecular Contrast Agents. Cancers 2022, 14, 6070. [Google Scholar] [CrossRef]
- Reineri, F.; Boi, T.; Aime, S. ParaHydrogen Induced Polarization of 13C carboxylate resonance in acetate and pyruvate. Nat. Commun. 2015, 6, 5858. [Google Scholar] [CrossRef]
- Cavallari, E.; Carrera, C.; Sorge, M.; Bonne, G.; Muchir, A.; Aime, S.; Reineri, F. The 13C hyperpolarized pyruvate generated by ParaHydrogen detects the response of the heart to altered metabolism in real time. Sci. Rep. 2018, 8, 8366. [Google Scholar] [CrossRef]
- Adams, R.W.; Aguilar, J.A.; Atkinson, K.D.; Cowley, M.J.; Elliott, P.I.P.; Duckett, S.B.; Green, G.G.R.; Khazal, I.G.; López-Serrano, J.; Williamson, D.C. Reversible interactions with para-hydrogen enhance nmr sensitivity by polarization transfer. Science 2009, 323, 1708–1711. [Google Scholar] [CrossRef] [PubMed]
- Wodtke, P.; Grashei, M.; Schilling, F. Quo Vadis Hyperpolarized 13C MRI? Z. Für Med. Phys. 2023. [Google Scholar] [CrossRef]
- Jähnig, F.; Kwiatkowski, G.; Ernst, M. Conceptual and instrumental progress in dissolution DNP. J. Magn. Reson. 2016, 264, 22–29. [Google Scholar] [CrossRef]
- Hurd, R.E.; Yen, Y.; Chen, A.; Ardenkjaer-Larsen, J.H. Hyperpolarized 13C metabolic imaging using dissolution dynamic nuclear polarization. J. Magn. Reson. Imaging 2012, 36, 1314–1328. [Google Scholar] [CrossRef] [PubMed]
- Comment, A.; Merritt, M.E. Hyperpolarized magnetic resonance as a sensitive detector of metabolic function. Biochemistry 2014, 53, 7333–7357. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, C.; Marin-Montesinos, I.; Saunders, M.G.; Günther, U.L. Optimizing the polarization matrix for ex situ dynamic nuclear polarization. J. Am. Chem. Soc. 2010, 132, 2508–2509. [Google Scholar] [CrossRef]
- Leavesley, A.; Wilson, C.B.; Sherwin, M.; Han, S. Effect of water/glycerol polymorphism on dynamic nuclear polarization. Phys. Chem. Chem. Phys. 2018, 20, 9897–9903. [Google Scholar] [CrossRef] [PubMed]
- Brender, J.R.; Kishimoto, S.; Eaton, G.R.; Eaton, S.S.; Saida, Y.; Mitchell, J.; Krishna, M.C. Trehalose as an alternative to glycerol as a glassing agent for in vivo DNP MRI. Magn. Reson. Med. 2021, 85, 42–48. [Google Scholar] [CrossRef]
- Ravera, E.; Shimon, D.; Feintuch, A.; Goldfarb, D.; Vega, S.; Flori, A.; Luchinat, C.; Menichetti, L.; Parigi, G. The effect of Gd on trityl-based dynamic nuclear polarization in solids. Phys. Chem. Chem. Phys. 2015, 17, 26969. [Google Scholar] [CrossRef]
- Wenckebach, W. Dynamic nuclear polarization via thermal mixing: Beyond the high temperature approximation. J. Magn. Reson. 2017, 277, 68–78. [Google Scholar] [CrossRef]
- Banerjee, D.; Shimon, D.; Feintuch, A.; Vega, S.; Goldfarb, D. The interplay between the solid effect and the cross effect mechanisms in solid state 13C DNP at 95 GHz using trityl radicals. J. Magn. Reson. 2013, 230, 212–219. [Google Scholar] [CrossRef]
- Günther, U.L. Dynamic nuclear hyperpolarization in liquids. Top Curr. Chem. 2013, 335, 23–69. [Google Scholar] [CrossRef] [PubMed]
- Hovav, Y.; Feintuch, A.; Vega, S. Theoretical aspects of dynamic nuclear polarization in the solid state—Spin temperature and thermal mixing. Phys. Chem. Chem. Phys. 2013, 15, 188–203. [Google Scholar] [CrossRef] [PubMed]
- Hovav, Y.; Feintuch, A.; Vega, S. Theoretical aspects of dynamic nuclear polarization in the solid state—The cross effect. J. Magn. Reson. 2012, 214, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Hovav, Y.; Feintuch, A.; Vega, S. Theoretical aspects of dynamic nuclear polarization in the solid state—The solid effect. J. Magn. Reson. 2010, 207, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Lumata, L.; Chen, W.; Zhang, S.; Kovacs, Z.; Sherry, A.D.; Khemtong, C. Hyperpolarized 15N-pyridine derivatives as pH-sensitive MRI agents. Sci. Rep. 2015, 5, 9104. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jo, D.; Yang, S.-H.; Joo, C.-G.; Whiting, N.; Pudakalakatti, S.; Seo, H.; Son, H.Y.; Min, S.-J.; Bhattacharya, P.; et al. 29Si Isotope-Enriched Silicon Nanoparticles for an Efficient Hyperpolarized Magnetic Resonance Imaging Probe. ACS Appl. Mater. Interfaces 2021, 13, 56923–56930. [Google Scholar] [CrossRef] [PubMed]
- Lipsø, K.W.; Hansen, E.S.S.; Tougaard, R.S.; Laustsen, C.; Ardenkjaer-Larsen, J.H. Dynamic coronary MR angiography in a pig model with hyperpolarized water. Magn. Reson. Med. 2018, 80, 1165–1169. [Google Scholar] [CrossRef] [PubMed]
- Ardenkjaer-Larsen, J.H.; Leach, A.M.; Clarke, N.; Urbahn, J.; Anderson, D.; Skloss, T.W. Dynamic nuclear polarization polarizer for sterile use intent. NMR Biomed. 2011, 24, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Ardenkjaer-Larsen, J.H. On the present and future of dissolution-DNP. J. Magn. Reson. 2016, 264, 3–12. [Google Scholar] [CrossRef]
- Larson, P.E.; Bernard, J.M.; Bankson, J.A.; Bøgh, N.; Bok, R.A.; Chen, A.P.; Cunningham, C.H.; Gordon, J.; Hövener, J.-B.; Laustsen, C.; et al. Current Methods for Hyperpolarized [1-13C]pyruvate MRI Human Studies. arXiv 2023, arXiv:2309.04040v2. [Google Scholar] [CrossRef]
- Pinon, A.C.; Capozzi, A.; Ardenkjær-Larsen, J.H. Hyperpolarization via dissolution dynamic nuclear polarization: New technological and methodological advances. Magn. Reson. Mater. Physics, Biol. Med. 2021, 34, 5–23. [Google Scholar] [CrossRef]
- Lama, B.; Collins, J.H.P.; Downes, D.; Smith, A.N.; Long, J.R. Expeditious dissolution dynamic nuclear polarization without glassing agents. NMR Biomed. 2016, 29, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Gajan, D.; Bornet, A.; Vuichoud, B.; Milani, J.; Melzi, R.; van Kalkeren, H.A.; Veyre, L.; Thieuleux, C.; Conley, M.P.; Grüning, W.R.; et al. Hybrid polarizing solids for pure hyperpolarized liquids through dissolution dynamic nuclear polarization. Proc. Natl. Acad. Sci. USA 2014, 111, 14693–14697. [Google Scholar] [CrossRef] [PubMed]
- Zanella, C.C.; Capozzi, A.; Yoshihara, H.A.; Radaelli, A.; Mackowiak, A.L.C.; Arn, L.P.; Gruetter, R.; Bastiaansen, J.A.M. Radical-free hyperpolarized MRI using endogenously occurring pyruvate analogues and UV-induced nonpersistent radicals. NMR Biomed. 2021, 34, e4584. [Google Scholar] [CrossRef] [PubMed]
- Ardenkjær-Larsen, J.H.; Bowen, S.; Petersen, J.R.; Rybalko, O.; Vinding, M.S.; Ullisch, M.; Nielsen, N.C. Cryogen-free dissolution dynamic nuclear polarization polarizer operating at 3.35 T, 6.70 T, and 10.1 T. Magn. Reson. Med. 2019, 81, 2184–2194. [Google Scholar] [CrossRef] [PubMed]
- Topping, G.J.; Hundshammer, C.; Nagel, L.; Grashei, M.; Aigner, M.; Skinner, J.G.; Schulte, R.F.; Schilling, F. Acquisition strategies for spatially resolved magnetic resonance detection of hyperpolarized nuclei. Magn. Reson. Mater. Physics, Biol. Med. 2020, 33, 221–256. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.W.; Chen, H.-Y.; Dwork, N.; Tang, S.; Larson, P.E.Z. Fast Imaging for Hyperpolarized MR Metabolic Imaging. J. Magn. Reson. Imaging 2021, 53, 686–702. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.L.; Jaswal, J.S.; Stanley, W.C. Myocardial Fatty Acid Metabolism in Health and Disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef]
- Moreno, K.X.; Sabelhaus, S.M.; Merritt, M.E.; Sherry, A.D.; Malloy, C.R. Competition of pyruvate with physiological substrates for oxidation by the heart: Implications for studies with hyperpolarized [1-13C]pyruvate. Am. J. Physiol. Circ. Physiol. 2010, 298, H1556–H1564. [Google Scholar] [CrossRef]
- Lionetti, V.; Stanley, W.C.; Recchia, F.A. Modulating fatty acid oxidation in heart failure. Cardiovasc. Res. 2011, 90, 202–209. [Google Scholar] [CrossRef]
- Yoshihara, H.A.; Bastiaansen, J.A.; Karlsson, M.; Lerche, M.H.; Comment, A.; Schwitter, J. Detection of myocardial medium-chain fatty acid oxidation and tricarboxylic acid cycle activity with hyperpolarized [1–13C]octanoate. NMR Biomed. 2020, 33, e4243. [Google Scholar] [CrossRef] [PubMed]
- Bastiaansen, J.A.; Cheng, T.; Lei, H.; Gruetter, R.; Comment, A. Direct noninvasive estimation of myocardial tricarboxylic acid cycle flux in vivo using hyperpolarized 13C magnetic resonance. J. Mol. Cell. Cardiol. 2015, 87, 129–137. [Google Scholar] [CrossRef]
- Koellisch, U.; Gringeri, C.V.; Rancan, G.; Farell, E.V.; Menzel, M.I.; Haase, A.; Schwaiger, M.; Schulte, R.F. Metabolic imaging of hyperpolarized [1-13C]acetate and [1-13C]acetylcarnitine—Investigation of the influence of dobutamine induced stress. Magn. Reson. Med. 2015, 74, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Ball, D.R.; Rowlands, B.; Dodd, M.S.; Le Page, L.; Ball, V.; Carr, C.A.; Clarke, K.; Tyler, D.J. Hyperpolarized Butyrate: A Metabolic Probe of Short Chain Fatty Acid Metabolism in the Heart. Magn. Reson. Med. 2014, 71, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Bastiaansen, J.A.M.; Merritt, M.E.; Comment, A. Measuring changes in substrate utilization in the myocardium in response to fasting using hyperpolarized [1-13C]butyrate and [1-13C]pyruvate. Sci. Rep. 2016, 6, 25573. [Google Scholar] [CrossRef] [PubMed]
- Fuetterer, M.; Busch, J.; Peereboom, S.M.; von Deuster, C.; Wissmann, L.; Lipiski, M.; Fleischmann, T.; Cesarovic, N.; Stoeck, C.T.; Kozerke, S. Hyperpolarized 13C urea myocardial first-pass perfusion imaging using velocity-selective excitation. J. Cardiovasc. Magn. Reson. 2017, 19, 46. [Google Scholar] [CrossRef]
- Lau, A.Z.; Miller, J.J.; Robson, M.D.; Tyler, D.J. Simultaneous assessment of cardiac metabolism and perfusion using copolarized [1-13C]pyruvate and 13C-urea. Magn. Reson. Med. 2017, 77, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Jin, J. Electromagnetic Analysis and Design in Magnetic Resonance Imaging; CRC: Boca Raton, FL, USA, 1999. [Google Scholar]
- Mispelter, J.; Lupu, M.; Briguet, A. NMR Probeheads for Biophysical and Biomedical Experiments: Theoretical Principles and Practical Guidelines, 2nd ed.; Imperial College Press: London, UK, 2015. [Google Scholar]
- Hasse, A.; Odoj, F.; Kienline, M.V.; Warnking, J.; Fidler, F.; Weisser, A.; Nittka, M.; Rommel, E.; Lanz, T.; Kalusher, B.; et al. NMR probeheads for in vivo applications. Concepts Magn. Reson. 2000, 12, 361–388. [Google Scholar] [CrossRef]
- Roemer, P.B.; Edelstein, W.A.; Hayes, C.E.; Souza, S.P.; Mueller, O.M. The NMR phased array. Magn. Reson. Med. 1990, 16, 192–225. [Google Scholar] [CrossRef]
- Ohliger, M.A.; Sodickson, D.K. An introduction to coil array design for parallel MRI. NMR Biomed. 2006, 19, 300–315. [Google Scholar] [CrossRef]
- Cunningham, C.H.; Lau, J.Y.; Chen, A.P.; Geraghty, B.J.; Perks, W.J.; Roifman, I.; Wright, G.A.; Connelly, K.A. Hyperpolarized 13C Metabolic MRI of the Human Heart: Initial Experience. Circ. Res. 2016, 119, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gordon, J.W.; Dwork, N.; Chung, B.T.; Riselli, A.; Sivalokanathan, S.; Bok, R.A.; Slater, J.B.; Vigneron, D.B.; Abraham, M.R.; et al. Probing human heart TCA cycle metabolism and response to glucose load using hyperpolarized [2-13C]pyruvate MRS. NMR Biomed. 2024, 37, e5074. [Google Scholar] [CrossRef] [PubMed]
- Joergensen, S.H.; Hansen, E.S.S.; Bã¸gh, N.; Bertelsen, L.B.; Staehr, P.B.; Schulte, R.F.; Malloy, C.; Wiggers, H.; Laustsen, C.; Bøgh, N. Detection of increased pyruvate dehydrogenase flux in the human heart during adenosine stress test using hyperpolarized [1-13C]pyruvate cardiovascular magnetic resonance imaging. J. Cardiovasc. Magn. Reson. 2022, 24, 34. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.P.; Hurd, R.E.; Schroeder, M.A.; Lau, A.Z.; Gu, Y.-P.; Lam, W.W.; Barry, J.; Tropp, J.; Cunningham, C.H. Simultaneous investigation of cardiac pyruvate dehydrogenase flux, Krebs cycle metabolism and pH, using hyperpolarized [1,2-13C2]pyruvate in vivo. NMR Biomed. 2012, 25, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, M.A.; Lau, A.Z.; Chen, A.P.; Gu, Y.; Nagendran, J.; Barry, J.; Hu, X.; Dyck, J.R.; Tyler, D.J.; Clarke, K.; et al. Hyperpolarized 13C magnetic resonance reveals early- and late-onset changes to in vivo pyruvate metabolism in the failing heart. Eur. J. Heart Fail. 2013, 15, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.Z.; Chen, A.P.; Hurd, R.E.; Cunningham, C.H. Spectral–spatial excitation for rapid imaging of DNP compounds. NMR Biomed. 2011, 24, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Frijia, F.; Santarelli, M.F.; Koellisch, U.; Giovannetti, G.; Lanz, T.; Flori, A.; Durst, M.; Aquaro, G.D.; Schulte, R.F.; De Marchi, D.; et al. 16-Channel Surface Coil for 13C-Hyperpolarized Spectroscopic Imaging of Cardiac Metabolism in Pig Heart. J. Med. Biol. Eng. 2016, 36, 53–61. [Google Scholar] [CrossRef]
- Jørgensen, S.; Bøgh, N.; Hansen, E.; Væggemose, M.; Wiggers, H.; Laustsen, C. Hyperpolarized MRI—An Update and Future Perspectives. Semin. Nucl. Med. 2022, 52, 374–381. [Google Scholar] [CrossRef]
- Lau, A.Z.; Chen, A.P.; Ghugre, N.R.; Ramanan, V.; Lam, W.W.; Connelly, K.A.; Wright, G.A.; Cunningham, C.H. Rapid multislice imaging of hyperpolarized 13C pyruvate and bicarbonate in the heart. Magn. Reson. Med. 2010, 64, 1323–1331. [Google Scholar] [CrossRef]
- Golman, K.; Petersson, J.S.; Magnusson, P.; Johansson, E.; Åkeson, P.; Chai, C.; Hansson, G.; Månsson, S. Cardiac metabolism measured noninvasively by hyperpolarized 13C MRI. Magn. Reson. Med. 2008, 59, 1005–1013. [Google Scholar] [CrossRef]
- Olsson, L.E.; Chai, C.; Axelsson, O.; Karlsson, M.; Golman, K.; Petersson, J.S. MR coronary angiography in pigs with intraarterial injections of a hyperpolarized 13C substance. Magn. Reson. Med. 2006, 55, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Heredia, J.D.; Hansen, E.S.S.; Laustsen, C.; Zhurbenko, V.; Ardenkjær-Larsen, J.H. Low-Noise Active Decoupling Circuit and its Application to 13C Cryogenic RF Coils at 3 T. Tomography 2017, 3, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, V.; Vanello, N.; Giovannetti, G.; De Marchi, D.; Lombardi, M.; Landini, L.; Santarelli, M.F. B1+/actual flip angle and reception sensitivity mapping methods: Simulation and comparison. Magn. Reson. Imaging 2011, 29, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, V.; Giovannetti, G.; Vanello, N.; Landini, L.; Santarelli, M.F. Numerical Calculation of Peak-to-Average Specific Absorption Rate on Different Human Thorax Models for Magnetic Resonance Safety Considerations. Appl. Magn. Reson. 2010, 38, 337–348. [Google Scholar] [CrossRef]
- Giovannetti, G.; Flori, A.; Santarelli, M.F.; Positano, V.; Martini, N.; Francischello, R.; Schulte, R.F.; Ardenkjaer-Larsen, J.H.; Menichetti, L.; Aquaro, G.D.; et al. Radio Frequency Coils for Hyperpolarized 13C Magnetic Resonance Experiments with a 3T MR Clinical Scanner: Experience from a Cardiovascular Lab. Electronics 2021, 10, 366. [Google Scholar] [CrossRef]
- Giovannetti, G.; Frijia, F.; Menichetti, L.; Milanesi, M.; Ardenkjaer-Larsen, J.H.; De Marchi, D.; Hartwig, V.; Positano, V.; Landini, L.; Lombardi, M.; et al. Hyperpolarized C13 MRS surface coil: Design and signal-to-noise ratio estimation. Med. Phys. 2010, 37, 5361–5369. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, G.; Hartwig, V.; Frijia, F.; Menichetti, L.; Positano, V.; Ardenkjaer-Larsen, J.H.; Lionetti, V.; Aquaro, G.D.; De Marchi, D.; Flori, A.; et al. Hyperpolarized 13C MRS Cardiac Metabolism Studies in Pigs: Comparison Between Surface and Volume Radiofrequency Coils. Appl. Magn. Reson. 2012, 42, 413–428. [Google Scholar] [CrossRef]
- Giovannetti, G.; Frijia, F.; Hartwig, V.; Menichetti, L.; Positano, V.; Ardenkjaer-Larsen, J.H.; Lionetti, V.; Aquaro, G.D.; De Marchi, D.; Schulte, R.F.; et al. Transmit-Only/Receive-Only Radiofrequency System for Hyperpolarized 13C MRS Cardiac Metabolism Studies in Pigs. Appl. Magn. Reson. 2013, 44, 1125–1138. [Google Scholar] [CrossRef]
- Giovannetti, G.; Frijia, F.; Hartwig, V.; Attanasio, S.; Menichetti, L.; Vanello, N.; Positano, V.; Ardenkjaer-Larsen, J.; Lionetti, V.; Aquaro, G.; et al. Design of a quadrature surface coil for hyperpolarized 13C MRS cardiac metabolism studies in pigs. Concepts Magn. Reson. Part B Magn. Reson. Eng. 2013, 43, 69–77. [Google Scholar] [CrossRef]
- Giovannetti, G.; Frijia, F.; Attanasio, S.; Menichetti, L.; Hartwig, V.; Vanello, N.; Ardenkjaer-Larsen, J.H.; De Marchi, D.; Positano, V.; Schulte, R.; et al. Magnetic resonance butterfly coils: Design and application for hyperpolarized 13C studies. Measurement 2013, 46, 3282–3290. [Google Scholar] [CrossRef]
- Durst, M.; Koellisch, U.; Frank, A.; Rancan, G.; Gringeri, C.V.; Karas, V.; Wiesinger, F.; Menzel, M.I.; Schwaiger, M.; Haase, A.; et al. Comparison of acquisition schemes for hyperpolarised 13 C imaging. NMR Biomed. 2015, 28, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, M.A.; Cochlin, L.E.; Heather, L.C.; Clarke, K.; Radda, G.K.; Tyler, D.J. In vivo assessment of pyruvate dehydrogenase flux in the heart using hyperpolarized carbon-13 magnetic resonance. Proc. Natl. Acad. Sci. USA 2008, 105, 12051–12056. [Google Scholar] [CrossRef] [PubMed]
- Schulte, R.F.; Sacolick, L.; Deppe, M.H.; Janich, M.A.; Schwaiger, M.; Wild, J.M.; Wiesinger, F. Transmit gain calibration for nonproton MR using the Bloch–Siegert shift. NMR Biomed. 2011, 24, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Wiesinger, F.; Weidl, E.; Menzel, M.I.; Janich, M.A.; Khegai, O.; Glaser, S.J.; Haase, A.; Schwaiger, M.; Schulte, R.F. IDEAL spiral CSI for dynamic metabolic MR imaging of hyperpolarized [1-13C]pyruvate. Magn. Reson. Med. 2011, 68, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Flori, A.; Frijia, F.; Lionetti, V.; Ardenkjaer-Larsen, J.H.; Positano, V.; Giovannetti, G.; Schulte, R.F.; Wiesinger, F.; Recchia, F.A.; Landini, L.; et al. DNP Methods for Cardiac Metabolic Imaging with Hyperpolarized [1-13C]pyruvate Large Dose Injection in Pigs. Appl. Magn. Reson. 2012, 43, 299–310. [Google Scholar] [CrossRef]
- Kurhanewicz, J.; Vigneron, D.B.; Ardenkjaer-Larsen, J.H.; Bankson, J.A.; Brindle, K.; Cunningham, C.H.; Gallagher, F.A.; Keshari, K.R.; Kjaer, A.; Laustsen, C.; et al. Hyperpolarized 13C MRI: Path to Clinical Translation in Oncology. Neoplasia 2019, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ardenkjaer-Larsen, J.H. Hyperpolarized MR—What’s up Doc? J. Magn. Reson. 2019, 306, 124–127. [Google Scholar] [CrossRef]
- Nelson, S.J.; Kurhanewicz, J.; Vigneron, D.B.; Larson, P.E.Z.; Harzstark, A.L.; Ferrone, M.; van Criekinge, M.; Chang, J.W.; Bok, R.; Park, I.; et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C]pyruvate. Sci. Transl. Med. 2013, 5, 198ra108. [Google Scholar] [CrossRef] [PubMed]
- Arponen, O.; Wodtke, P.; Gallagher, F.A.; Woitek, R. Hyperpolarised 13C-MRI using 13C-pyruvate in breast cancer: A review. Eur. J. Radiol. 2023, 167, 111058. [Google Scholar] [CrossRef]
- Mair, R.; Wright, A.J.; Ros, S.; Hu, D.E.; Booth, T.; Kreis, F.; Rao, J.; Watts, C.; Brindle, K.M. Metabolic Imaging Detects Low Levels of Glycolytic Activity That Vary with Levels of c-Myc Expression in Patient-Derived Xenograft Models of Glioblastoma. Cancer Res. 2018, 78, 5408–5418. [Google Scholar] [CrossRef]
- Zaccagna, F.; McLean, M.A.; Grist, J.T.; Kaggie, J.; Mair, R.; Riemer, F.; Woitek, R.; Gill, A.B.; Deen, S.; Daniels, C.J.; et al. Imaging Glioblastoma Metabolism by Using Hyperpolarized [1-13C]Pyruvate Demonstrates Heterogeneity in Lactate Labeling: A Proof of Principle Study. Radiol. Imaging Cancer 2022, 4, e210076. [Google Scholar] [CrossRef] [PubMed]
- Grist, J.T.; McLean, M.A.; Riemer, F.; Schulte, R.F.; Deen, S.S.; Zaccagna, F.; Woitek, R.; Daniels, C.J.; Kaggie, J.D.; Matys, T.; et al. Quantifying normal human brain metabolism using hyperpolarized [1–13C]pyruvate and magnetic resonance imaging. NeuroImage 2019, 189, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.T.; Chen, H.-Y.; Gordon, J.; Mammoli, D.; Sriram, R.; Autry, A.W.; Le Page, L.M.; Chaumeil, M.M.; Shin, P.; Slater, J.; et al. First hyperpolarized [2-13C]pyruvate MR studies of human brain metabolism. J. Magn. Reson. 2019, 309, 106617. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.T.; Kim, Y.; Gordon, J.W.; Chen, H.-Y.; Autry, A.W.; Lee, P.M.; Hu, J.Y.; Tan, C.T.; Suszczynski, C.; Chang, S.M.; et al. Hyperpolarized [2–13C]pyruvate MR molecular imaging with whole brain coverage. NeuroImage 2023, 280, 120350. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.; Ursprung, S.; Jensen, J.D.; Jespersen, B.; Gallagher, F.; Laustsen, C. Hyperpolarised 13C-MRI metabolic and functional imaging: An emerging renal MR diagnostic modality. Magn. Reson. Mater. Physics, Biol. Med. 2020, 33, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Laustsen, C.; Østergaard, J.A.; Lauritzen, M.H.; Nørregaard, R.; Bowen, S.; Søgaard, L.V.; Flyvbjerg, A.; Pedersen, M.; Ardenkjær-Larsen, J.H. Assessment of early diabetic renal changes with hyperpolarized [1-13C]pyruvate. Diabetes/Metabolism Res. Rev. 2013, 29, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Agger, P.; Hyldebrandt, J.A.; Hansen, E.S.S.; Omann, C.; Bøgh, N.; Waziri, F.; Nielsen, P.M.; Laustsen, C. Magnetic resonance hyperpolarization imaging detects early myocardial dysfunction in a porcine model of right ventricular heart failure. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.J.M.; Miller, J.J.; Lau, A.Z.; Curtis, M.K.; Rider, O.J.; Choudhury, R.P.; Neubauer, S.; Cunningham, C.H.; Carr, C.A.; Tyler, D.J. Noninvasive Immunometabolic Cardiac Inflammation Imaging Using Hyperpolarized Magnetic Resonance. Circ. Res. 2018, 122, 1084–1093. [Google Scholar] [CrossRef]
- Schroeder, M.A.; Swietach, P.; Atherton, H.J.; Gallagher, F.A.; Lee, P.; Radda, G.K.; Clarke, K.; Tyler, D.J. Measuring intracellular pH in the heart using hyperpolarized carbon dioxide and bicarbonate: A 13C and 31P magnetic resonance spectroscopy study. Cardiovasc. Res. 2010, 86, 82–91. [Google Scholar] [CrossRef]
- Aquaro, G.D.; Frijia, F.; Positano, V.; Menichetti, L.; Santarelli, M.F.; Lionetti, V.; Giovannetti, G.; Recchia, F.A.; Landini, L. Cardiac Metabolism in a Pig Model of Ischemia–Reperfusion by Cardiac Magnetic Resonance with Hyperpolarized 13C-Pyruvate. IJC Metab. Endocr. 2015, 6, 17–23. [Google Scholar] [CrossRef]
- Santarelli, M.F.; Positano, V.; Giovannetti, G.; Frijia, F.; Menichetti, L.; Ardenkjaer-Larsen, J.-H.; De Marchi, D.; Lionetti, V.; Aquaro, G.; Lombardi, M.; et al. How the signal-to-noise ratio influences hyperpolarized 13C dynamic MRS data fitting and parameter estimation. NMR Biomed. 2012, 25, 925–934. [Google Scholar] [CrossRef]
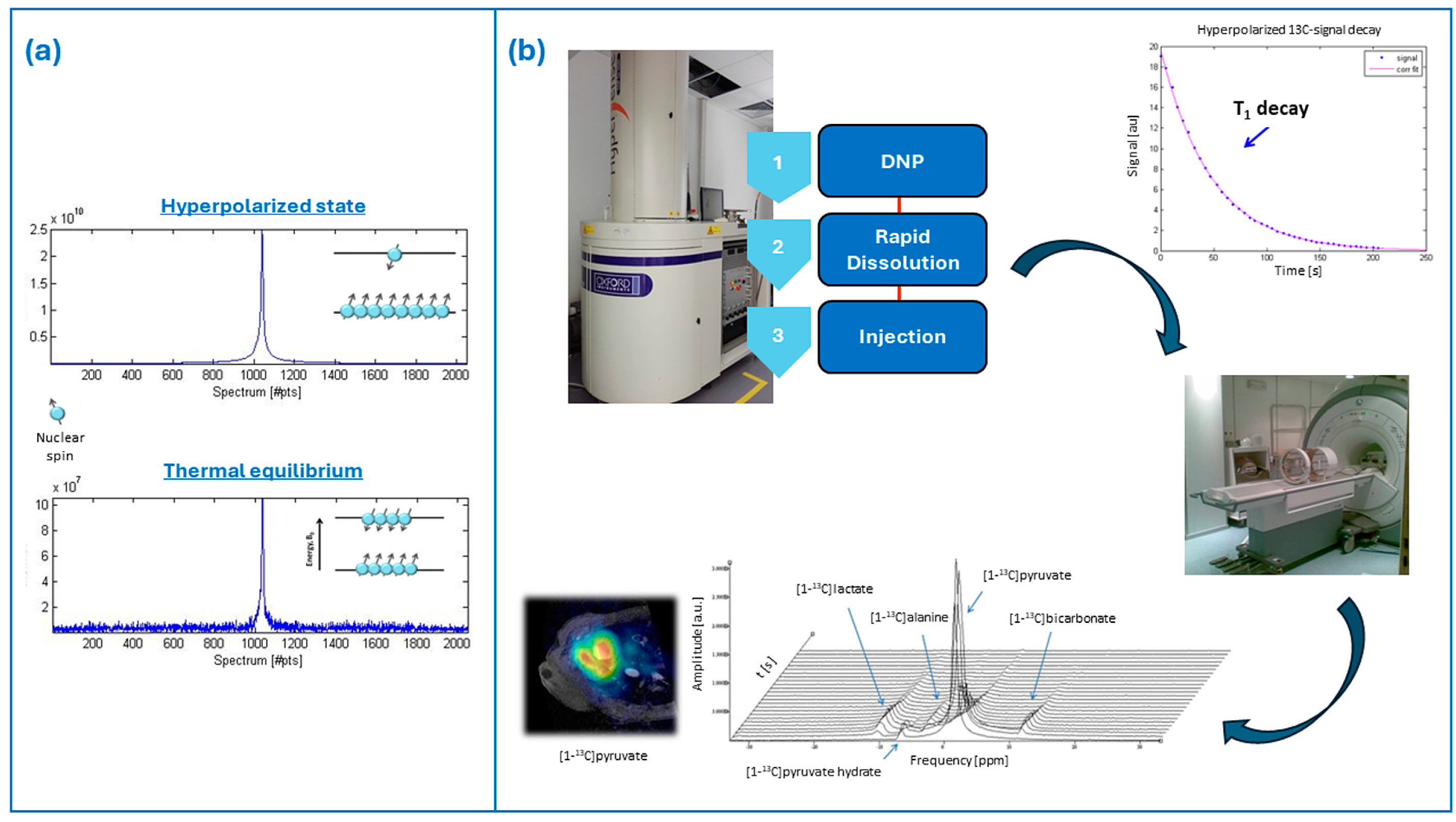





| Element | Gyromagnetic Ratios γ (MHz/T) | Natural Abundance (%) |
|---|---|---|
| 1H | 42.57 | 99.9885% |
| 31P | 11.26 | 100% |
| 13C | 10.70 | 1.07% |
| 23Na | 17.24 | 100% |
| 129Xe | −11.86 | 26.44% |
| Metabolite | Pathway | Significance |
|---|---|---|
| 1-13C-pyruvate | Glycolitic pathway | Product of glycolysis, it can be converted to 13C-lactate (anerobic conditions) or to acetyl-coA with production of 13C-bicarbonate in the mitochondria (oxidation) |
| 13C-lactate | Lactate dehydrogenase (LDH) | Derived from 13C-pyruvate from LDH (anerobic conditions); increased in cancer cells |
| 13C-CO2 | Pyruvate dehydrogenase (PDH) | Derived as a byproduct of 13C-pyruvate conversion to Acetil-CoA |
| 13C-bicarbonate | Extracellular pH | Derived from 13CO2, through extracellular carbonic anhydrase activity |
| 2-13C-pyruvate | Tricarboxylic acid cycle (TCA) | The labelled carbon is carried over to acetyl-CoA |
| 13C-butyrate | Fatty acid metabolism | |
| 13C-acetate | Tricarboxylic acid cycle (TCA) and fatty acid oxidation | Converted to acetyl-CoA by acetyl-CoA synthase |
| 13C-alanine | Muscle and liver metabolism | Pyruvate is transaminated to alanine in skeletal muscle; while alanine is deaminated to pyruvate in the liver |
| 13C-glucose | pentose phosphate pathway, glycolysis, lactate production | |
| 2-13C-dihydroxyacetone | Hepatic gluconeogenesis | |
| 13C-glutamine | Mutated isocitrate dehydrogenase (IDH) | In cancer cells, mutated isocitrate dehydrogenase (IDH) converts glutamine to oncometabolite 2-hydroxyglutarate |
| 13C-alpha ketoglutarate (αKG) | Mutated isocitrate dehydrogenase (IDH) | In cancer cells, mutated isocitrate dehydrogenase (IDH) converts αKG to oncometabolite 2-hydroxyglutarate and glutamate |
| 13C-dehydroascorbate | Redox potential | It is the oxidized form of Vitamin C; it is rapidly converted to [1-13C] vitamin C within the liver, kidneys, brain and tumors |
| 13C-acetoacetate | Mitochondrial redox status | |
| 13C-glutathione | Antioxidant and redox status | Antioxidant synthesized from glutamate (glu), cysteine (cys) and glycine (gly) |
| 13C-cystine | Antioxidant and redox status | Component of glutathione |
| 13C-urea | Perfusion | Inert metabolic probe |
| 13C-fumarate | Necrosis | In case of cell death, exogenous 13C-fumarate is converted to 13C-malate by intracellular fumarase (released in the extracellular space) |
| 13C-malate | Necrosis | Absent in healthy cells, while produced from 13C-fumarate by extracellular fumarase released by necrotic cells |
| Author | Animals | Scanner | Spatial Resolution | Sequence | Post-Processing | Disease | Results |
|---|---|---|---|---|---|---|---|
| Agger et al., 2020 [110] | 5 pigs | 3 T HDx (GE Healthcare, Waukesha, WI, USA) | 1.01 × 1.45 mm2 | Cardiac triggered 2D 13C IDEAL spiral | Not reported | Pulmonary banding | Increase in the lactate/bicarbonate ratio compared with healthy control |
| Schroeder et al., 2013 [78] | 5 pigs | 3 T MR750 (GE Healthcare, Waukesha, WI, USA) | 9 mm | SAGE™ software (GE Healthcare) MATLAB (MathWorks, Natick, MA, USA) | Dilated cardiomyopathy | Reduced pyruvate oxidation | |
| Golman et al., 2008 [83] | 10 pigs (5 with 15 min occlusion, 5 with 45 min occlusion) | 1.5T Magnetom Sonata (Siemens Medical Solutions, Erlangen, Germany) | 7.5 mm | 13C CSI | in house developed software | Effect of coronary artery occlusion | 15-min occlusion: bicarbonate reduces in diseased area; 45-min occlusion: 13C-bicarbonate and 13C-alanine signal reduced in the diseased area |
| Lewis et al., 2018 [111] | 7 pigs | 3 T MR750 3 T MR750 (GE Healthcare, Waukesha, WI, USA) | 10.7 mm | Spiral sequence | Not reported | Myocardial infarction after coronary artery balloon-occlusion | Increase 13C-lactate signal in infarct. No significant difference in 13C-bicarbonate signal |
| Aquaro et al., 2015 [113] | 7 pigs | 3 T HDx TWINSPEE 3 T MR750 (GE Healthcare, Waukesha, WI, USA) | 15 mm | 3D-IDEAL spiral CSI | MATLAB (MathWorks, Natick, MA, USA) | Ischemic myocardium after pneumatic occlusion | Increase 13C-lactate signal; reduced 13C-bicarbonate within the area at risk |
| Fuetterer et al., 2022 [14] | 8 pigs | 3 T (Philips Medical Systems, Best, The Netherlands) | 1 mm | Customized spatial-spectral excitation (IDEAL approach) | MRecon (GyroTools LLC, Zurich, Swizerland) | Catheter-based 90-min occlusion | Elevated lactate-to-bicarbonate ratios at day 6 after infarction |
| Chen et al., 2012 [77] | Not reported | 3 T MR750 (GE Healthcare, Waukesha, WI, USA) | Not reported | Pulse-acquire sequence | SAGE™ software (GE Healthcare) | Healthy pig | Feasibility of using dual-labeled hyperpolarized [1,2-13C2]pyruvate as a substrate for dynamic cardiac metabolic MRS studies |
| Fuetterer et al., 2016 [67] | 6 pigs | 3 T Ingenia wide-bore scanner (Philips, Best, The Netherlands) | 3 mm | Velocity-selective binomial excitation scheme | MRecon (GyroTools LLC, Zurich, Switzerland) | Healthy pig | Potential of hyperpolarized 13C-urea imaging for diagnostic purposes. |
| Author | Subjects | Scanner | Spatial Resolution | Sequence | Post-Processing | Disease | Results |
|---|---|---|---|---|---|---|---|
| Cunningham et al., 2016 [74] | 4 | 3 T MR750 (GE Healthcare, Waukesha, WI, USA) | 8.8 mm | Slice-selective spectral-spatial excitation | Not reported | Healthy subjects | 13C-bicarbonate in this healthy cohort |
| Apps et al., 2021 [12] | 2 | 3 T Tim Trio (Siemens Medical Solutions, Erlangen, Germany) | Not reported | Hybrid-shot spiral | AMARES algorithm | Myocardial Infarction | Reduced PDH-mediated aerobic conversion to 13C-bicarbonate |
| Rider et al., 2020 [15] | 13 (Diabetes) 12 (healthy group) | 3 T Tim Trio (Siemens Medical Solutions, Erlangen, Germany) | 8 mm | Pulse-acquire spectroscopy | Not reported | Diabetes mellitus | 13C-bicarbonate reduced |
| Joergensen et al., 2022 [76] | 6 | Not reported | 13.3 mm | Spectral-spatial (SPSP) excitation with spiral read-out | MATLAB (MathWorks, Natick, MA, USA) | Healthy subjects | Increased pyruvate oxidation during low to moderate cardiac stress |
| Chen et al., 2024 [75] | 3 | 3 T MR750 (GE Healthcare, Waukesha, WI, USA) | Not reported | Dynamic slab spectroscopy | MATLAB (MathWorks, Natick, MA, USA) | Healthy subjects | Cardiac metabolite measurement in the fasting/fed states provides information on cardiac metabolic flexibility and the acetylcarnitine pool. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frijia, F.; Flori, A.; Giovannetti, G.; Barison, A.; Menichetti, L.; Santarelli, M.F.; Positano, V. MRI Application and Challenges of Hyperpolarized Carbon-13 Pyruvate in Translational and Clinical Cardiovascular Studies: A Literature Review. Diagnostics 2024, 14, 1035. https://doi.org/10.3390/diagnostics14101035
Frijia F, Flori A, Giovannetti G, Barison A, Menichetti L, Santarelli MF, Positano V. MRI Application and Challenges of Hyperpolarized Carbon-13 Pyruvate in Translational and Clinical Cardiovascular Studies: A Literature Review. Diagnostics. 2024; 14(10):1035. https://doi.org/10.3390/diagnostics14101035
Chicago/Turabian StyleFrijia, Francesca, Alessandra Flori, Giulio Giovannetti, Andrea Barison, Luca Menichetti, Maria Filomena Santarelli, and Vincenzo Positano. 2024. "MRI Application and Challenges of Hyperpolarized Carbon-13 Pyruvate in Translational and Clinical Cardiovascular Studies: A Literature Review" Diagnostics 14, no. 10: 1035. https://doi.org/10.3390/diagnostics14101035






