Tribological Behavior of Friction Materials Containing Aluminum Anodizing Waste Obtained by Different Industrial Drying Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pin-on-Disc and Emission Analysis
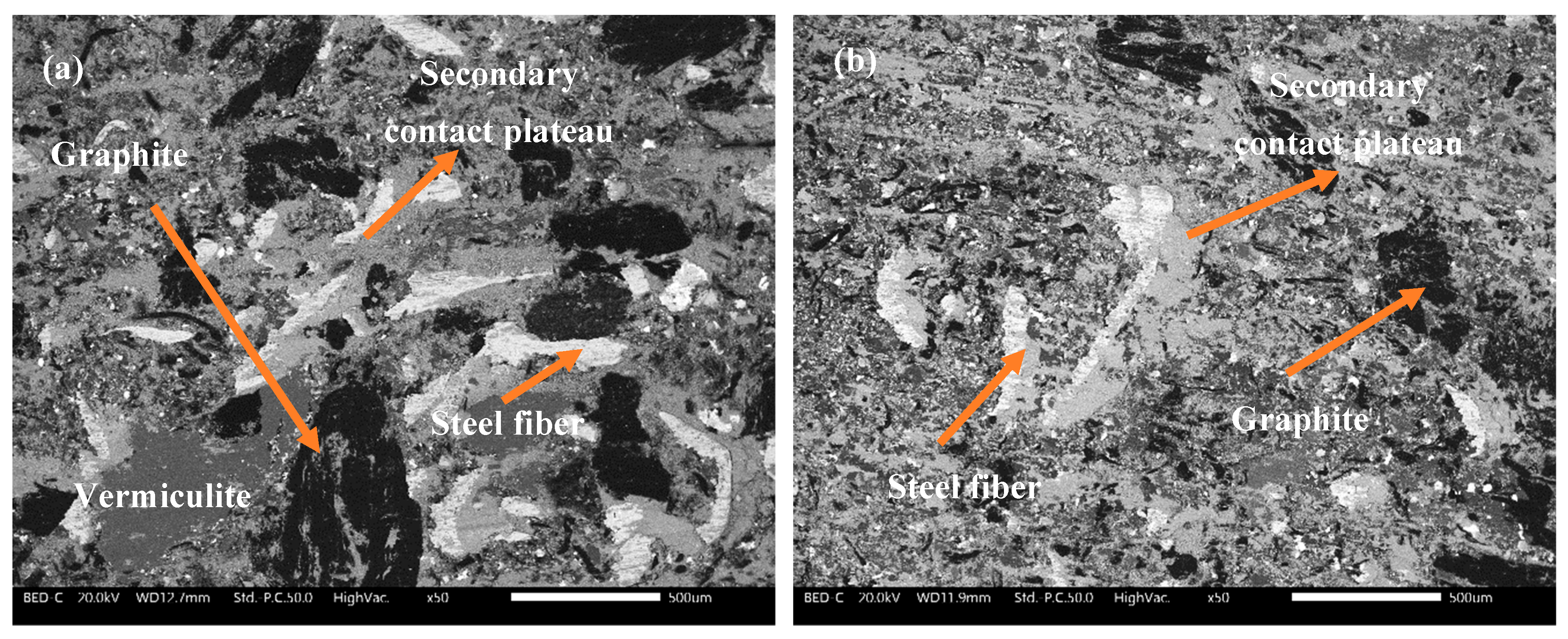
2.3. Characterization of the Worn Surfaces and Materials
3. Results and Discussion
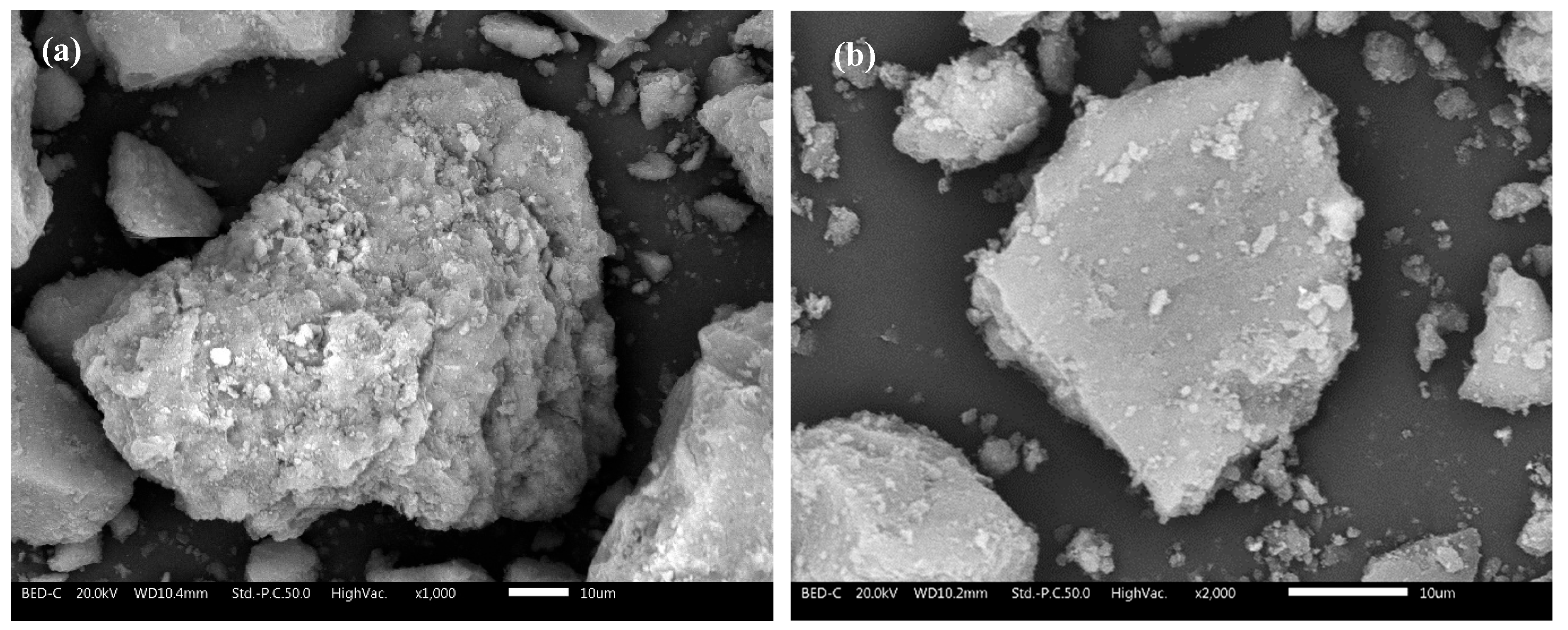
3.1. Various Industrially Dried AAW Characterization
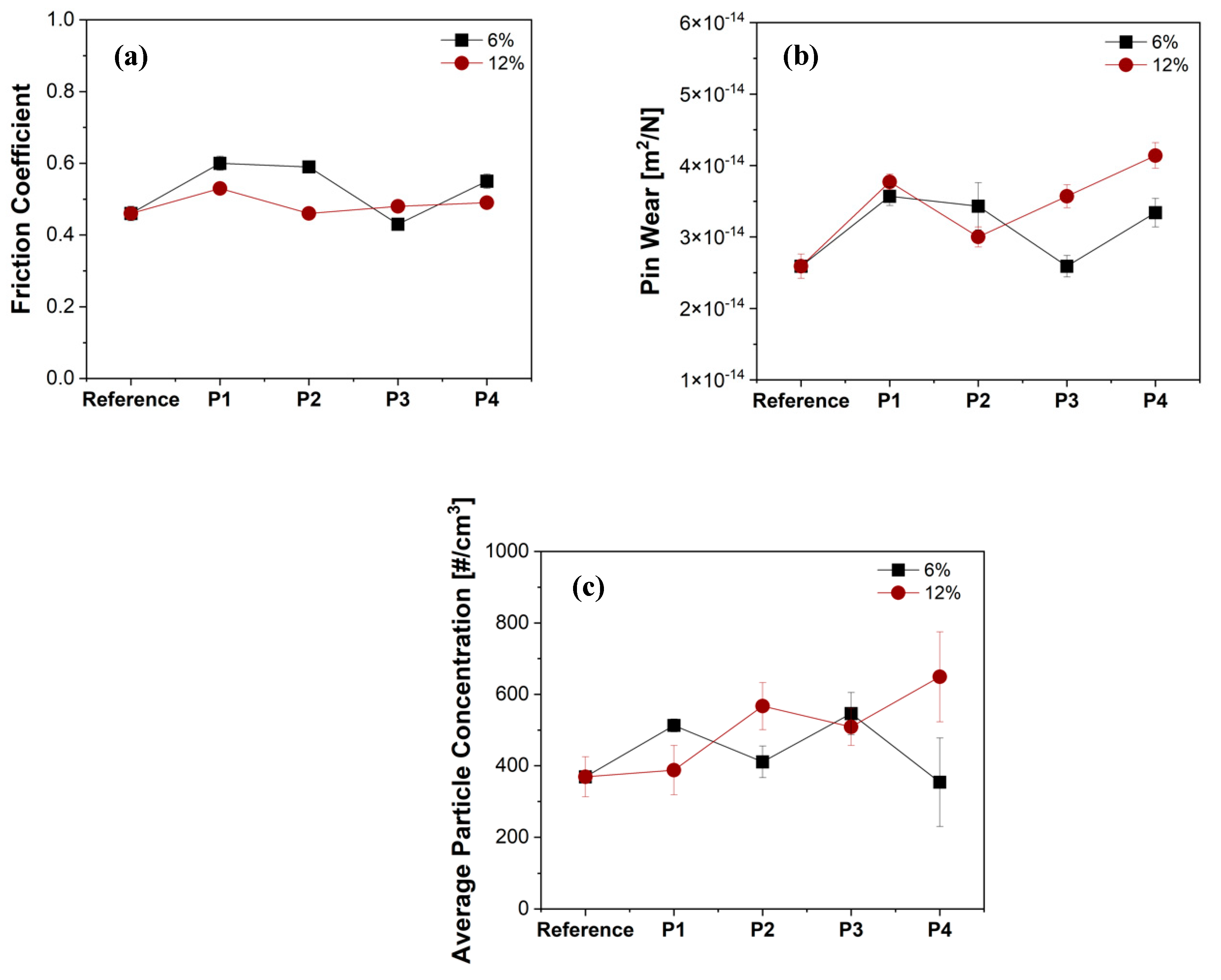
3.2. Friction, Wear, and Emission Analysis
4. Conclusions
- From the initial drying methods observations, P2 had the most feasible procedure, and the drying temperature was feasible but effective. Furthermore, the P1 results concerning the relative humidity and crushability index were also satisfactory with a simple drying procedure.
- The FT-IR and SEM/EDX analysis showed the presence of Al, O, and alumina for all the dried waste powders.
- Regarding the friction, wear, and emission characteristics, P1 showed a stable and allowable CoF, pin wear, and emission magnitude when compared to other drying methods. Through this, it was seen that there is a direct relationship between the drying method and tribological and emission characteristics.
- Amongst the alternatives, P1 and P4 had expansive secondary contact plateaus and P1 and P3 had the highest participation of AAW in the formation of the secondary contact plateaus. Hence, even from the worn surface analysis, the advantage of the P1 drying method utilization was observed.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miladinović, S.; Stojanović, B.; Gajević, S.; Vencl, A. Hypereutectic Aluminum Alloys and Composites: A Review. Silicon 2023, 15, 2507–2527. [Google Scholar] [CrossRef]
- Galindo, R.; Padilla, I.; Rodríguez, O.; Sánchez-Hernández, R.; López-Andrés, S.; López-Delgado, A. Characterization of Solid Wastes from Aluminum Tertiary Sector: The Current State of Spanish Industry. J. Miner. Mater. Charact. Eng. 2015, 3, 55–64. [Google Scholar] [CrossRef]
- Prasad, A. Developments of discontinuously reinforced aluminium matrix composites: Solving the needs for the matrix Developments of discontinuously reinforced aluminium matrix composites: Solving the needs for the matrix. J. Phys. Conf. Ser. 2022, 2212, 012029. [Google Scholar] [CrossRef]
- Prasad, D.S.; Shoba, C.; Ramanaiah, N. Investigations on mechanical properties of aluminum hybrid composites. J. Mater. Res. Technol. 2014, 3, 79–85. [Google Scholar] [CrossRef]
- Gregson, N.; Crang, M.; Fuller, S.; Holmes, H. Interrogating the circular economy: The moral economy of resource recovery in the EU. Econ. Soc. 2015, 44, 218–243. [Google Scholar] [CrossRef]
- Souza, M.T.; Simão, L.; Montedo, O.R.K.; Pereira, F.R.; de Oliveira, A.P.N. Aluminum anodizing waste and its uses: An overview of potential applications and market opportunities. Waste Manag. 2019, 84, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Jayashree, P.; Straffelini, G. The Influence of the Addition of Aluminum Anodizing Waste on the Friction and Emission Behavior of Different Kinds of Friction Material Formulations. Tribol. Int. 2022, 173, 107676. [Google Scholar] [CrossRef]
- Leonardi, M.; Alemani, M.; Straffelini, G.; Gialanella, S. A pin-on-disc study on the dry sliding behavior of a Cu-free friction material containing different types of natural graphite. Wear 2020, 442–443, 203157. [Google Scholar] [CrossRef]
- Jayashree, P.; Matějka, V.; Foniok, K.; Straffelini, G. Comparative Studies on the Dry Sliding Behavior of a Low-Metallic Friction Material with the Addition of Graphite and Exfoliated g-C3N4. Lubricants 2022, 10, 27. [Google Scholar] [CrossRef]
- Mathissen, M.; Grochowicz, J.; Schmidt, C.; Vogt, R.; Farwick zum Hagen, F.H.; Grabiec, T.; Steven, H.; Grigoratos, T. A novel real-world braking cycle for studying brake wear particle emissions. Wear 2018, 414–415, 219–226. [Google Scholar] [CrossRef]
- Gietl, J.K.; Lawrence, R.; Thorpe, A.J.; Harrison, R.M. Identification of brake wear particles and derivation of a quantitative tracer for brake dust at a major road. Atmos. Environ. 2010, 44, 141–146. [Google Scholar] [CrossRef]
- Matějka, V.; Leonardi, M.; Praus, P.; Straffelini, G.; Gialanella, S. The Role of Graphitic Carbon Nitride in the Formulation of Copper-Free Friction Composites Designed for Automotive Brake Pads. Metals 2022, 12, 123. [Google Scholar] [CrossRef]
- Jayashree, P.; Matejka, V.; Sinha, A.; Gialanella, S.; Straffelini, G. A comprehensive study on the particulate matter characteristics of a friction material containing blast furnace slags. Tribol. Int. 2023, 186, 108567. [Google Scholar] [CrossRef]
- Jayashree, P.; Matějka, V.; Leonardi, M.; Straffelini, G. The influence of the addition of different kinds of slags on the friction and emission behavior of a commercially employed friction material formulation. Wear 2023, 522, 204705. [Google Scholar] [CrossRef]
- Nogueira, A.P.G.; Carlevaris, D.; Menapace, C.; Straffelini, G. Tribological and emission behavior of novel friction materials. Atmosphere 2020, 11, 1050. [Google Scholar] [CrossRef]
- Menapace, C.; Cipolloni, G.; Hebda, M.; Ischia, G. Spark plasma sintering behaviour of copper powders having different particle sizes and oxygen contents. Powder Technol. 2016, 291, 170–177. [Google Scholar] [CrossRef]
- Jayashree, P.; Sinha, A.; Gialanella, S.; Straffelini, G. Dry Sliding Behavior and Particulate Emissions of a SiC-Graphite Composite Friction Material Paired with HVOF-Coated Counterface. Atmosphere 2022, 13, 296. [Google Scholar] [CrossRef]
- Mat, V.; Jayashree, P.; Leonardi, M.; Vl, J.; Sabov, T. Utilization of Metallurgical Slags in Cu-Free Friction Material Formulations. Lubricants 2022, 10, 219. [Google Scholar] [CrossRef]
- Jayashree, P.; Federici, M.; Bresciani, L.; Turani, S.; Sicigliano, R.; Straffelini, G. Effect of Steel Counterface on the Dry Sliding Behaviour of a Cu-Based Metal Matrix Composite. Tribol. Lett. 2018, 66, 123. [Google Scholar] [CrossRef]
- Kyu, W.; Wook, M.; Hwan, S.; Jang, H.; Hyung, M. The influence of humidity on the sliding friction of brake friction material. WEA 2013, 302, 1397–1403. [Google Scholar]









| Industrial Process | Method | Average Air Temperature |
|---|---|---|
| Process 1 (P1) | Moving belt, hot air flow, 2 h and 15 min as residence time | 70–80 °C |
| Process 2 (P2) | Moving belt, hot air flow, 2 h and 15 min as residence time under technological vacuum | 60 °C |
| Process 3 (P3) | Muffle furnace with air circulation, duration: 32 h | 50 °C |
| Process 4 (P4) | Moving belt, hot air flow, 2 h and 15 min as residence time | 40–50 °C |
| Constituents | Function | Content |
|---|---|---|
| Phenolic resin | Binder | 8 |
| Steel | Reinforcing fibers | 30 |
| Vermiculite, others | Fillers | 24 |
| Silicon Carbide, magnesium oxide, aluminum oxide | Abrasives | 25 |
| Graphite, tin sulfide, zinc oxide | Lubricants | 13 |
| Disc | Chemical Composition, wt.% | Hardness [HV 30] | Thermal Conductivity (W/mK) | Specific Heat (J/gK) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | Mn | Si | Sn | P | S | Fe | ||||
| Pearlitic Grey Cast Iron | 3.40 | 0.50 | 2.00 | 0.11 | 0.15 | 0.05 | Rest | 245 ± 6 | 52 | 0.447 |
| Element | P1 | P2 | P3 | P4 |
|---|---|---|---|---|
| Oxygen | 58 | 47 | 52 | 53 |
| Aluminum | 34 | 45 | 38 | 33 |
| Copper | 1.23 | - | - | - |
| Silicon | 0.68 | - | 1.18 | 1.62 |
| Calcium | 3.22 | 3.20 | 2.61 | 4.16 |
| Iron | 1.05 | 1.43 | 0.86 | 1.13 |
| Tin | 1.88 | 2.7 | 2.29 | 3.34 |
| Sulfur | - | - | 3.81 | 3.23 |
| Element | P1 | P2 | P3 | P4 |
|---|---|---|---|---|
| Oxygen | 41 ± 7 | 35 ± 6 | 38 ± 7 | 43 ± 3 |
| Aluminum | 29 ± 7 | 38 ± 4 | 40 ± 6 | 32 ± 2 |
| Carbon | 17 ± 3 | 19 ± 1 | 11 ± 3 | 13 ± 4 |
| Copper | 0.5 ± 0.3 | - | - | - |
| Silicon | 0.8 ± 0.28 | 0.78 ± 0.3 | 0.84 ± 0.4 | 1.5 ± 0.65 |
| Calcium | 3.81 ± 1.3 | 2.78 ± 0.29 | 2.91 ± 0.61 | 3.02 ± 0.18 |
| Iron | 1.7 ± 1.1 | 1.36 ± 0.53 | 0.93 ± 0.14 | 0.93 ± 0.21 |
| Tin | 1.4 ± 0.8 | 2.96 ± 1 | 2.71 ± 0.44 | 3.4 ± 0.21 |
| Sulfur | 2.5 ± 1.64 | - | 3.86 ± 0.9 | 3 ± 0.22 |
| Element | Reference | P1 | P2 | P3 | P4 |
|---|---|---|---|---|---|
| Oxygen | 24 ± 4 | 24 ± 3 | 23 ± 1 | 20 ± 5 | 20 ± 3 |
| Magnesium | 3 ± 0.5 | 2.5 ± 0.4 | 2.2 ± 0.7 | 3 ± 0.9 | 2 ± 0.2 |
| Aluminum | 2 ± 0.08 | 4 ± 0.9 | 3 ± 0.5 | 4 ± 0.6 | 3 ± 0.05 |
| Silicon | 1.5 ± 0.02 | 2 ± 0.8 | 1.6 ± 0.2 | 1.3 ± 0.3 | 1.5 ± 0.09 |
| Sulfur | 1.6 ± 0.2 | 2 ± 1.1 | 1.4 ± 0.5 | 1.7 ± 0.3 | 1.4 ± 0.05 |
| Calcium | 0.6 ± 0.05 | 0.7 ± 0.09 | 0.6 ± 0.05 | 0.8 ± 0.09 | 0.8 ± 0.1 |
| Chromium | 0.7 ± 0.05 | 0.6 ± 0.03 | 0.5 ± 0.03 | 0.9 ± 0.01 | 0.7 ± 0.04 |
| Iron | 61 ± 4 | 60 ± 5 | 62 ± 3 | 64 ± 1 | 65 ± 3 |
| Zinc | 3 ± 1.2 | 3 ± 0.7 | 3 ± 0.4 | 3 ± 1.3 | 3 ± 0.8 |
| Tin | 1.7 ± 0.3 | 1.7 ± 0.8 | 1.6 ± 0.05 | 1.3 ± 0.9 | 1.2 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Straffelini, G.; Jayashree, P.; Barbieri, A.; Masciocchi, R. Tribological Behavior of Friction Materials Containing Aluminum Anodizing Waste Obtained by Different Industrial Drying Processes. Lubricants 2024, 12, 173. https://doi.org/10.3390/lubricants12050173
Straffelini G, Jayashree P, Barbieri A, Masciocchi R. Tribological Behavior of Friction Materials Containing Aluminum Anodizing Waste Obtained by Different Industrial Drying Processes. Lubricants. 2024; 12(5):173. https://doi.org/10.3390/lubricants12050173
Chicago/Turabian StyleStraffelini, Giovanni, Priyadarshini Jayashree, Andrea Barbieri, and Roberto Masciocchi. 2024. "Tribological Behavior of Friction Materials Containing Aluminum Anodizing Waste Obtained by Different Industrial Drying Processes" Lubricants 12, no. 5: 173. https://doi.org/10.3390/lubricants12050173





