Ticks and Tick-Borne Pathogens Circulating in Peri-Domestic Areas in Mainland Portugal
Abstract
:1. Introduction
2. Materials and Methods
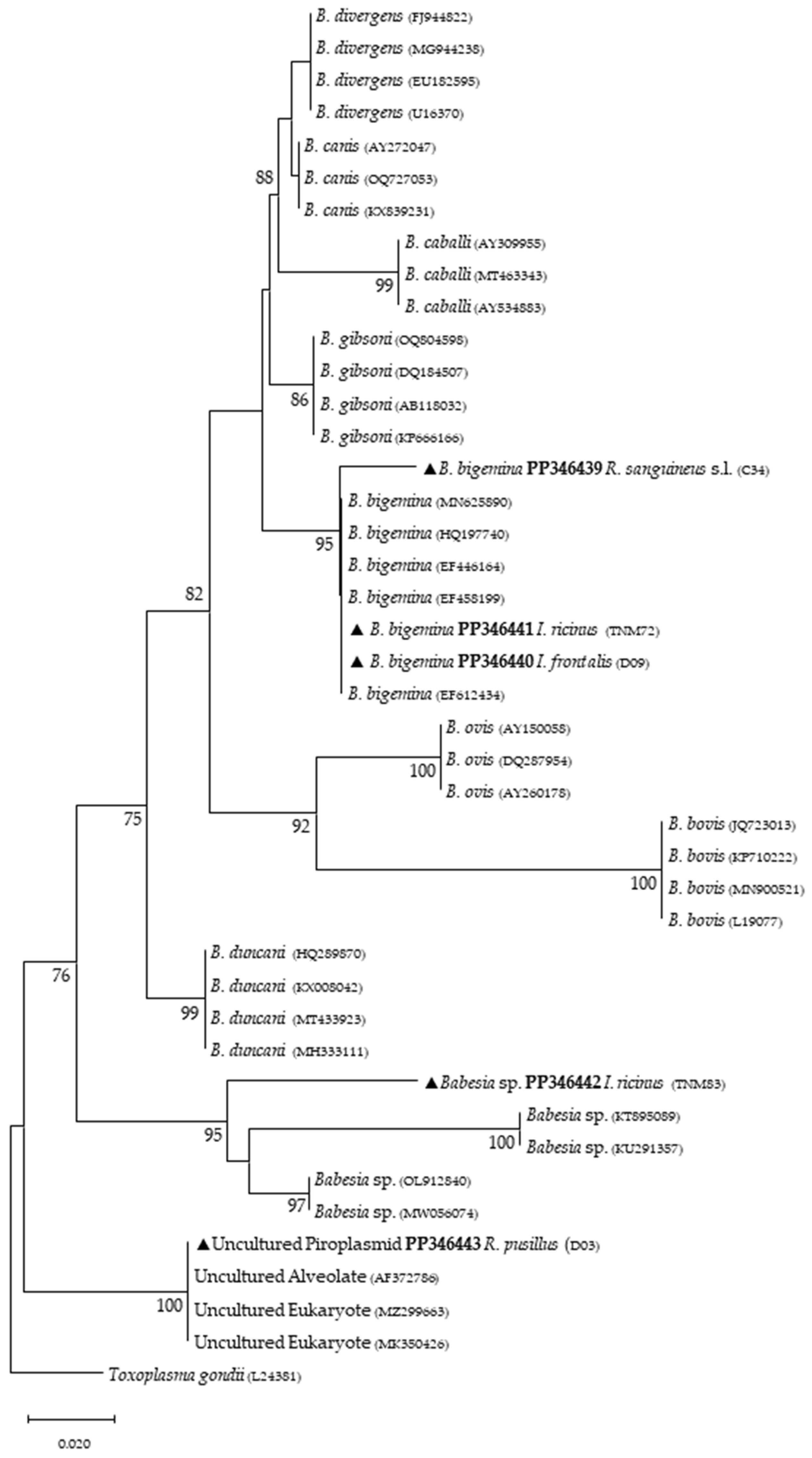
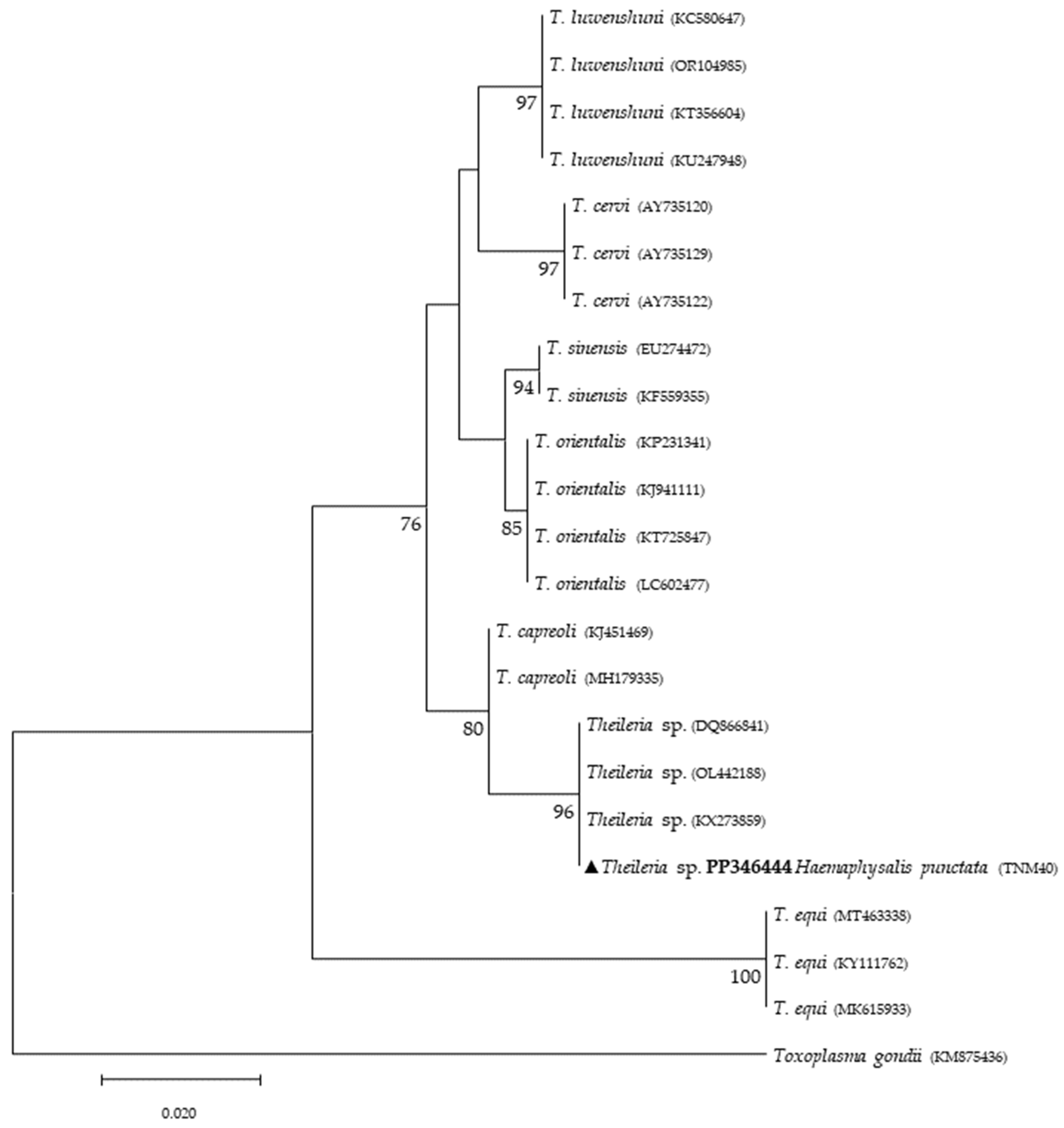
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madison-Antenucci, S.; Kramer, L.D.; Gebhardt, L.L.; Kauffman, E. Emerging Tick-Borne Diseases. Clin. Microbiol. Rev. 2020, 33, e00083-18. [Google Scholar] [CrossRef] [PubMed]
- Wimms, C.; Aljundi, E.; Halsey, S.J. Regional Dynamics of Tick Vectors of Human Disease. Curr. Opin. Insect Sci. 2023, 55, 101006. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.; Phipps, L.P.; Hansford, K.M.; Folly, A.J.; Fooks, A.R.; Medlock, J.M.; Mansfield, K.L. One Health Approach to Tick and Tick-Borne Disease Surveillance in the United Kingdom. Int. J. Environ. Res. Public Health 2022, 19, 5833. [Google Scholar] [CrossRef]
- Anderson, J.F.; Magnarelli, L.A. Biology of Ticks. Infect. Dis. Clin. N. Am. 2008, 22, 195–215. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Saúde Doutor Ricardo Jorge. Culicídeos e Ixodídeos Rede de Vigilância de Vetores; ISBN: 978-989-8794-78-9 (Online). Available online: http://hdl.handle.net/10400.18/8611 (accessed on 3 January 2024).
- Santos, A.S.; Santos-Silva, M.M.; Almeida, V.C.; Bacellar, F.; Dumler, J.S. Detection of Anaplasma phagocytophilum DNA in Ixodes Ticks (Acari: Ixodidae) from Madeira Island and Setubal District, Mainland Portugal. Emerg. Infect. Dis. 2004, 10, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Silaghi, C.; Santos, A.S.; Gomes, J.; Christova, I.; Matei, I.A.; Walder, G.; Domingos, A.; Bell-Sakyi, L.; Sprong, H.; Von Loewenich, F.D.; et al. Guidelines for the Direct Detection of Anaplasma spp. in Diagnosis and Epidemiological Studies. Vector Borne Zoonotic Dis. 2017, 17, 12–22. [Google Scholar] [CrossRef] [PubMed]
- MacQueen, D.; Centellas, F. Human Granulocytic Anaplasmosis. Infect. Dis. Clin. N. Am. 2022, 36, 639–654. [Google Scholar] [CrossRef] [PubMed]
- Maggi, R.G.; Mascarelli, P.E.; Havenga, L.N.; Naidoo, V.; Breitschwerdt, E.B. Co-Infection with Anaplasma platys, Bartonella henselae and Candidatus Mycoplasma haematoparvum in a Veterinarian. Parasit. Vectors 2013, 6, 103. [Google Scholar] [CrossRef] [PubMed]
- Paddock, C.D.; Childs, J.E. Ehrlichia chaffeensis: A Prototypical Emerging Pathogen. Clin. Microbiol. Rev. 2003, 16, 37–64. [Google Scholar] [CrossRef] [PubMed]
- David Morais, J.; Dawson, J.E.; Greene, C.; Filipe, A.R.; Galhardas, L.C.; Bacellar, F. First European Case of Ehrlichiosis. Lancet 1991, 338, 633–634. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.E.; Greene, C.E.; Jones, D.C.; Dawson, J.E. Ehrlichia ewingii sp. nov., the Etiologic Agent of Canine Granulocytic Ehrlichiosis. Int. J. Syst. Bacteriol. 1992, 42, 299–302. [Google Scholar] [CrossRef]
- Buller, R.S.; Arens, M.; Hmiel, S.P.; Paddock, C.D.; Sumner, J.W.; Rikihisa, Y.; Unver, A.; Gaudreault-Keener, M.; Manian, F.A.; Liddell, A.M.; et al. Ehrlichia ewingii, a Newly Recognized Agent of Human Ehrlichiosis. N. Engl. J. Med. 1999, 341, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Heitman, N.; Scott Dahlgren, F.; Drexler, N.A.; Massung, R.F.; Behravesh, C.B. Increasing Incidence of Ehrlichiosis in the United States: A Summary of National Surveillance of Ehrlichia chaffeensis and Ehrlichia ewingii Infections in the United States, 2008–2012. Am. J. Trop. Med. Hyg. 2016, 94, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Bodor, M.; Zhang, C.; Xiong, Q.; Rikihisa, Y. Human infection with Ehrlichia canis accompanied by clinical signs in Venezuela. Ann. N. Y. Acad. Sci. 2006, 1078, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Reeves, W.K.; Loftis, A.D.; Nicholson, W.L.; Czarkowski, A.G. The First Report of Human Illness Associated with the Panola Mountain Ehrlichia Species: A Case Report. J. Med. Case Rep. 2008, 2, 139. [Google Scholar] [CrossRef] [PubMed]
- Pritt, B.S.; Sloan, L.M.; Johnson, D.K.H.; Munderloh, U.G.; Paskewitz, S.M.; McElroy, K.M.; McFadden, J.D.; Binnicker, M.J.; Neitzel, D.F.; Liu, G.; et al. Emergence of a New Pathogenic Ehrlichia Species, Wisconsin and Minnesota, 2009. N. Engl. J. Med. 2011, 365, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, M.; Rikihisa, Y.; Isogai, E.; Takahashi, M.; Misumi, H.; Suto, C.; Shibata, S.; Zhang, C.; Tsuji, M. Ultrastructure and Phylogenetic Analysis of “Candidatus Neoehrlichia mikurensis” in the Family Anaplasmataceae, Isolated from Wild Rats and Found in Ixodes Ovatus Ticks. Int. J. Syst. Evol. Microbiol. 2004, 54, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Welinder-Olsson, C.; Kjellin, E.; Vaht, K.; Jacobsson, S.; Wennerås, C. First Case of Human “Candidatus Neoehrlichia mikurensis” Infection in a Febrile Patient with Chronic Lymphocytic Leukemia. J. Clin. Microbiol. 2010, 48, 1956–1959. [Google Scholar] [CrossRef] [PubMed]
- Wass, L.; Grankvist, A.; Bell-Sakyi, L.; Bergström, M.; Ulfhammer, E.; Lingblom, C.; Wennerås, C. Cultivation of the Causative Agent of Human Neoehrlichiosis from Clinical Isolates Identifies Vascular Endothelium as a Target of Infection. Emerg. Microbes Infect. 2019, 8, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Portillo, A.; Santibáñez, P.; Palomar, A.M.; Santibáñez, S.; Oteo, J.A. ‘Candidatus Neoehrlichia mikurensis’ in Europe. New Microbes New Infect. 2018, 22, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.S.; de Bruin, A.; Veloso, A.R.; Marques, C.; Pereira da Fonseca, I.; de Sousa, R.; Sprong, H.; Santos-Silva, M.M. Detection of Anaplasma phagocytophilum, Candidatus Neoehrlichia sp., Coxiella burnetii and Rickettsia spp. in Questing Ticks from a Recreational Park, Portugal. Ticks Tick. Borne Dis. 2018, 9, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, A.; Zintl, A.; Montero, E.; Hunfeld, K.P.; Gray, J. Human Babesiosis in Europe. Pathogens 2021, 10, 1165. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.S.; Bacellar, F.; França, A. Medicina Interna Revista Da Sociedade Portuguesa De Medicina Interna Febre Q: Revisão de Conceitos Q Fever: A Revision of Concepts. Revista SPMFR 2007, 14, 90–99. Available online: https://www.spmi.pt/revista/vol14/vol14_n2_2007_090_099.pdf (accessed on 3 January 2024).
- Anastácio, S.; Tavares, N.; Carolino, N.; Sidi-Boumedine, K.; Da Silva, G.J. Serological Evidence of Exposure to Coxiella burnetii in Sheep and Goats in Central Portugal. Vet. Microbiol. 2013, 167, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Cumbassá, A.; Barahona, M.J.; Cunha, M.V.; Azórin, B.; Fonseca, C.; Rosalino, L.M.; Tilburg, J.; Hagen, F.; Santos, A.S.; Botelho, A. Coxiella burnetii DNA Detected in Domestic Ruminants and Wildlife from Portugal. Vet. Microbiol. 2015, 180, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.; Esteves, F.; Vasconcelos-Nóbrega, C.; Santos, C.; Ferreira, A.S.; Mega, C.; Coelho, A.C.; Vala, H.; Mesquita, J.R. A Nationwide Seroepidemiologic Study on Q Fever Antibodies in Sheep of Portugal. Vector Borne Zoonotic Dis. 2018, 18, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Sormunen, J.J.; Mänttäri, J.; Vesterinen, E.J.; Klemola, T. Blood Meal Analysis Reveals Sources of Tick-Borne Pathogens and Differences in Host Utilization of Juvenile Ixodes ricinus across Urban and Sylvatic Habitats. Zoonoses Public Health 2024, 71, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Chomel, B.B.; Otranto, D. Ticks and Tick-Borne Diseases: A One Health Perspective. Trends Parasitol. 2012, 28, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Zając, Z.; Kulisz, J.; Bartosik, K.; Woźniak, A.; Dzierżak, M.; Khan, A. Environmental Determinants of the Occurrence and Activity of Ixodes ricinus Ticks and the Prevalence of Tick-Borne Diseases in Eastern Poland. Sci. Rep. 2021, 11, 15472. [Google Scholar] [CrossRef] [PubMed]
- Santos-Silva, M.M.; Beati, L.; Santos, A.S.; De Sousa, R.; Núncio, M.S.; Melo, P.; Santos-Reis, M.; Fonseca, C.; Formosinho, P.; Vilela, C.; et al. The Hard-Tick Fauna of Mainland Portugal (Acari: Ixodidae): An Update on Geographical Distribution and Known Associations with Hosts and Pathogens. Exp. Appl. Acarol. 2011, 55, 85–121. [Google Scholar] [CrossRef] [PubMed]
- EEM_Relatorio.pdf (cm-grandola.pt). Available online: http://planeamento.cm-grandola.pt/planos_vigor/PDM/02.REVISAO%20-%20DEZEMBRO%202017/TOMO_II_ACOMPANHAMENTO/1505_PDMREV_ANEXOS/1505_PDMREV_ANEXO_VI/EEM_Relatorio.pdf (accessed on 7 May 2024).
- ICNB, IP-Instituto da Conservação e da Biodiversidade. Plano de gestão florestal e orientação da utilização pública da Mata Nacional do Choupal, Reserva Natural do Paúl de Arzila. Available online: https://www.icnf.pt/api/file/doc/5df9343734da0d02 (accessed on 7 May 2024).
- Zuleger, A.M.; Perino, A.; Wolf, F.; Wheeler, H.C.; Pereira, H.M. Long-Term Monitoring of Mammal Communities in the Peneda-Gerês National Park Using Camera-Trap Data. Biodivers. Data J. 2023, 11, e99588. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Vasques, A.; Maia, P.; Ramos Pereira, M.J.; Fonseca, C.; Matos, M. Native and Exotic Seed Dispersal by the Stone Marten (Martes Foina): Implications for the Regeneration of a Relict Climactic Forest in Central Portugal. Integr. Zool. 2019, 14, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Fernández, N.; Revuelta, B.; Aguilar, I.; Soares, J.F.; Zintl, A.; Gray, J.; Montero, E.; Gonzalez, L.M. Babesia and Theileria Identification in Adult Ixodid Ticks from Tapada Nature Reserve, Portugal. Pathogens 2022, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Mihalca, A.D.; Petney, T.N. Ticks of Europe and North Africa, 1st ed.; Springer: Cham, Switzerland, 2017; p. 404. ISBN 978-3-319-63760-0. [Google Scholar] [CrossRef]
- Mangold, A.; Bargues, M.; Mas-Coma, S. 18S RRNA Gene Sequences and Phylogenetic Relationships of European Hard-Tick Species (Acari: Ixodidae). Parasitol. Res. 1998, 84, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Olmeda, A.S.; Armstrong, P.M.; Rosenthal, B.M.; Valladares, B.; Del Castillo, A.; De Armas, F.; Miguelez, M.; González, A.; Rodríguez Rodríguez, J.A.; Spielman, A.; et al. Short Report: A Subtropical Case of Human Babesiosis. Acta Trop. 1997, 67, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Roux, V.; Camicas, J.L.; Baradji, I.; Brouqui, P.; Raoult, D. Detection of Ehrlichiae in African Ticks by Polymerase Chain Reaction. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 707–708. [Google Scholar] [CrossRef] [PubMed]
- Klee, S.R.; Tyczka, J.; Ellerbrok, H.; Franz, T.; Linke, S.; Baljer, G.; Appel, B. Highly Sensitive Real-Time PCR for Specific Detection and Quantification of Coxiella burnetii. BMC Microbiol. 2006, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Sandelin, L.L.; Tolf, C.; Larsson, S.; Wilhelmsson, P.; Salaneck, E.; Jaenson, T.G.T.; Lindgren, P.E.; Olsen, B.; Waldenström, J. Candidatus Neoehrlichia mikurensis in Ticks from Migrating Birds in Sweden. PLoS ONE 2015, 10, e0133250. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Homer, M.J.; Aguilar-Delfin, I.; Telford, S.R.; Krause, P.J.; Persing, D.H. Babesiosis. Clin. Microbiol. Rev. 2000, 13, 451–469. [Google Scholar] [CrossRef] [PubMed]
- Springer, A.; Höltershinken, M.; Lienhart, F.; Ermel, S.; Rehage, J.; Hülskötter, K.; Lehmbecker, A.; Wohlsein, P.; Barutzki, D.; Gietl, C.; et al. Emergence and Epidemiology of Bovine Babesiosis Due to Babesia divergens on a Northern German Beef Production Farm. Front. Vet. Sci. 2020, 7, 649. [Google Scholar] [CrossRef] [PubMed]
- Calvopiña, M.; Montesdeoca-Andrade, M.; Bastidas-Caldes, C.; Enriquez, S.; Rodríguez-Hidalgo, R.; Aguilar-Rodríguez, D.; Cooper, P. Case Report: First Report on Human Infection by Tick-Borne Babesia bigemina in the Amazon Region of Ecuador. Front. Public Health 2023, 11, 1079042. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.; Lihou, K.; Lait, P.; Wall, R. Prevalence of Babesia spp. Pathogens in the Ticks Dermacentor reticulatus and Ixodes ricinus in the UK. Acta Trop. 2022, 236, 106692. [Google Scholar] [CrossRef] [PubMed]
- Azagi, T.; Jaarsma, R.I.; van Leeuwen, A.D.; Fonville, M.; Maas, M.; Franssen, F.F.J.; Kik, M.; Rijks, J.M.; Montizaan, M.G.; Groenevelt, M.; et al. Circulation of Babesia Species and Their Exposure to Humans through Ixodes ricinus. Pathogens 2021, 10, 386. [Google Scholar] [CrossRef]
- Wilhelmsson, P.; Pawełczyk, O.; Jaenson, T.G.T.; Waldenström, J.; Olsen, B.; Forsberg, P.; Lindgren, P.E. Three Babesia Species in Ixodes ricinus Ticks from Migratory Birds in Sweden. Parasit. Vectors 2021, 14, 183. [Google Scholar] [CrossRef] [PubMed]
- Hornok, S.; Takács, N.; Kontschán, J.; György, Z.; Micsutka, A.; Iceton, S.; Flaisz, B.; Farkas, R.; Hofmann-Lehmann, R. Diversity of Haemaphysalis-Associated Piroplasms of Ruminants in Central-Eastern Europe, Hungary. Parasit. Vectors 2015, 8, 627. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Salgueiro, P.; Inácio, J.; Amaro, A.; Pinto, J.; Tait, A.; Shiels, B.; Pereira da Fonseca, I.; Santos-Gomes, G.; Weir, W. Population Diversity of Theileria annulata in Portugal. Infect. Genet. Evol. 2016, 42, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Man, S.Q.; Qiao, K.; Cui, J.; Feng, M.; Fu, Y.F.; Cheng, X.J. A Case of Human Infection with a Novel Babesia Species in China. Infect. Dis. Poverty 2016, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.B.; Jeong, D.H.; Cho, B.C.; Park, J.S. The Diversity Patterns of Rare to Abundant Microbial Eukaryotes Across a Broad Range of Salinities in a Solar Saltern. Microb. Ecol. 2022, 84, 1103–1121. [Google Scholar] [CrossRef] [PubMed]
- Belasen, A.M.; Bletz, M.C.; Leite, D.S.; Toledo, L.F.; James, T.Y. Long-Term Habitat Fragmentation Is Associated with Reduced MHC IIB Diversity and Increased Infections in Amphibian Hosts. Front. Ecol. Evol. 2019, 6, 422063. [Google Scholar] [CrossRef]
- Dawson, S.C.; Pace, N.R. Novel Kingdom-Level Eukaryotic Diversity in Anoxic Environments. Proc. Natl. Acad. Sci. USA 2002, 99, 8324–8329. [Google Scholar] [CrossRef] [PubMed]
- Szczotko, M.; Kubiak, K.; Michalski, M.M.; Moerbeck, L.; Antunes, S.; Domingos, A.; Dmitryjuk, M. Neoehrlichia mikurensis-A New Emerging Tick-Borne Pathogen in North-Eastern Poland? Pathogens 2023, 12, 307. [Google Scholar] [CrossRef] [PubMed]
- Boyer, P.H.; Baldinger, L.; Degeilh, B.; Wirth, X.; Kamdem, C.M.; Hansmann, Y.; Zilliox, L.; Boulanger, N.; Jaulhac, B. The Emerging Tick-Borne Pathogen Neoehrlichia mikurensis: First French Case Series and Vector Epidemiology. Emerg. Microbes Infect. 2021, 10, 1731–1738. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Geller, J.; Katargina, O.; Värv, K.; Lundkvist, Å.; Golovljova, I. Detection of Candidatus Neoehrlichia mikurensis and Ehrlichia Muris in Estonian Ticks. Ticks Tick. Borne Dis. 2017, 8, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Cafiso, A.; Bazzocchi, C.; De Marco, L.; Opara, M.N.; Sassera, D.; Plantard, O. Molecular Screening for Midichloria in Hard and Soft Ticks Reveals Variable Prevalence Levels and Bacterial Loads in Different Tick Species. Ticks Tick. Borne Dis. 2016, 7, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Sassera, D.; Beninati, T.; Bandi, C.; Bouman, E.A.P.; Sacchi, L.; Fabbi, M.; Lo, N. “Candidatus Midichloria mitochondrii”, an Endosymbiont of the Tick Ixodes ricinus with a Unique Intramitochondrial Lifestyle. Int. J. Syst. Evol. Microbiol. 2006, 56, 2535–2540. [Google Scholar] [CrossRef] [PubMed]
- Ahantarig, A.; Trinachartvanit, W.; Baimai, V.; Grubhoffer, L. Hard Ticks and Their Bacterial Endosymbionts (or Would Be Pathogens). Folia Microbiol. 2013, 58, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Sgroi, G.; Iatta, R.; Lovreglio, P.; Stufano, A.; Laidoudi, Y.; Mendoza-Roldan, J.A.; Bezerra-Santos, M.A.; Veneziano, V.; Di Gennaro, F.; Saracino, A.; et al. Detection of Endosymbiont Candidatus Midichloria mitochondrii and Tickborne Pathogens in Humans Exposed to Tick Bites, Italy. Emerg. Infect. Dis. 2022, 28, 1824–1832. [Google Scholar] [CrossRef]
- Guizzo, M.G.; Hatalová, T.; Frantová, H.; Zurek, L.; Kopáček, P.; Perner, J. Ixodes ricinus Ticks Have a Functional Association with Midichloria Mitochondrii. Front. Cell Infect. Microbiol. 2023, 12, 1081666. [Google Scholar] [CrossRef] [PubMed]
- Cafiso, A.; Sassera, D.; Romeo, C.; Serra, V.; Hervet, C.; Bandi, C.; Plantard, O.; Bazzocchi, C. Midichloria Mitochondrii, Endosymbiont of Ixodes ricinus: Evidence for the Transmission to the Vertebrate Host during the Tick Blood Meal. Ticks Tick. Borne Dis. 2019, 10, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Epis, S.; Sassera, D.; Beninati, T.; Lo, N.; Beati, L.; Piesman, J.; Rinaldi, L.; McCoy, K.D.; Torina, A.; Sacchi, L.; et al. Midichloria mitochondrii Is Widespread in Hard Ticks (Ixodidae) and Resides in the Mitochondria of Phylogenetically Diverse Species. Parasitology 2008, 135, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D. The Detection of Rickettsia-like Microorganisms within the Ovaries of Female Ixodes ricinus Ticks. Z. Parasitenkd. 1979, 59, 295–298. [Google Scholar] [CrossRef]
- Palomar, A.M.; Portillo, A.; Santibáñez, P.; Mazuelas, D.; Roncero, L.; García-Álvarez, L.; Santibáñez, S.; Gutiérrez, O.; Oteo, J.A. Detection of Tick-Borne Anaplasma bovis, Anaplasma phagocytophilum and Anaplasma centrale in Spain. Med. Vet. Entomol. 2015, 29, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Hornok, S.; Földvári, G.; Elek, V.; Naranjo, V.; Farkas, R.; de la Fuente, J. Molecular Identification of Anaplasma marginale and Rickettsial Endosymbionts in Blood-Sucking Flies (Diptera: Tabanidae, Muscidae) and Hard Ticks (Acari: Ixodidae). Vet. Parasitol. 2008, 154, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Uzum, Z.; Ershov, D.; Pavia, M.J.; Mallet, A.; Gorgette, O.; Plantard, O.; Sassera, D.; Stavru, F. Three-Dimensional Images Reveal the Impact of the Endosymbiont Midichloria mitochondrii on the Host Mitochondria. Nat. Commun. 2023, 14, 4133. [Google Scholar] [CrossRef] [PubMed]
- Duron, O.; Binetruy, F.; Noël, V.; Cremaschi, J.; McCoy, K.D.; Arnathau, C.; Plantard, O.; Goolsby, J.; Pérez de León, A.A.; Heylen, D.J.A.; et al. Evolutionary Changes in Symbiont Community Structure in Ticks. Mol. Ecol. 2017, 26, 2905–2921. [Google Scholar] [CrossRef] [PubMed]
- Plantard, O.; Bouju-Albert, A.; Malard, M.A.; Hermouet, A.; Capron, G.; Verheyden, H. Detection of Wolbachia in the Tick Ixodes ricinus Is Due to the Presence of the Hymenoptera Endoparasitoid Ixodiphagus hookeri. PLoS ONE 2012, 7, e30692. [Google Scholar] [CrossRef]
- Bonnet, S.I.; Pollet, T. Update on the Intricate Tango between Tick Microbiomes and Tick-Borne Pathogens. Parasite Immunol. 2021, 43, e12813. [Google Scholar] [CrossRef]
- Thomas, S.; Verma, J.; Woolfit, M.; O’Neill, S.L. Wolbachia-Mediated Virus Blocking in Mosquito Cells Is Dependent on XRN1-Mediated Viral RNA Degradation and Influenced by Viral Replication Rate. PLoS Pathog. 2018, 14, e1006879. [Google Scholar] [CrossRef] [PubMed]
- Kolo, A.O.; Raghavan, R. Impact of Endosymbionts on Tick Physiology and Fitness. Parasitology 2023, 150, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Hirunkanokpun, S.; Ahantarig, A.; Baimai, V.; Trinachartvanit, W. A New Record of Wolbachia in the Elephant Ticks from Thailand. ScienceAsia 2018, 44S, 44–47. [Google Scholar] [CrossRef]
- Zhang, X.; Norris, D.E.; Rasgon, J.L. Distribution and Molecular Characterization of Wolbachia Endosymbionts and Filarial Nematodes in Maryland Populations of the Lone Star Tick (Amblyomma americanum). FEMS Microbiol. Ecol. 2011, 77, 50–56. [Google Scholar] [CrossRef] [PubMed]


| Method | Target Gene | Primer/Probe | Sequence 5′-3′ | Product Size (bp) | Reference |
|---|---|---|---|---|---|
| PCR | 18S rRNA | PIRO-A | AATACCCAATCCTGACACAGGG | 408 | [39] |
| PIRO-B | TTAAATACGAATGCCCCCAAC | ||||
| PCR | 16S rRNA | EHR16SD | GGTACCYACAGAAGAAGTCC | 345 | [40] |
| EHR16SR | TAGCACTCATCGTTTACAGC | ||||
| qPCR | IS1111 | Cox-F | GTCTTAAGGTGGGCTGCGTG | 295 | [41] |
| Cox-R | CCCCGAATCTCATTGATCAGC | ||||
| Cox-TM | FAM-AGCGAACCATTGGTATCGGACGTTTATGG-TAMRA | ||||
| qPCR | 16S rRNA | Neo_16S_F | GTAAAGGGCATGTAGGCGGTTTAA | 107 | [42] |
| Neo_16S_R | TCCACTATCCTCTCTCGATCTCTAGTTTAA |
| Collection Site | Tick Species (Number of Specimens/Stage *) | No. of Infected Samples/No. of Tested Samples (%) | ||
|---|---|---|---|---|
| Piroplasmida | Anaplasma spp./Ehrlichia spp. | N. mikurensis | ||
| Grândola | D. marginatus (39A) | 10/39 (25.6) | 4/39 (10.3) | - |
| I. ricinus (19A) | 4/19 (21.1) | 8/19 (42.1) | 2/14 (14.3) | |
| Parque Nacional Gerês | D. marginatus (2A) | 0/2 (0) | 0/2 (0) | 0/2 (0) |
| Serra do Bussaco | D. marginatus (7A) | 1/7 (14.3) | 3/7 (42.9) | 4/7 (57.1) |
| I. frontalis (1A; 2N) | 1/3 (33.3) | 2/3 (66.7) | 0/3 (0) | |
| R. pusillus (8A) | 1/8 (12.5) | 8/8 (100) | 0/8 (0) | |
| R. sanguineus s. l. (15A) | 1/15 (6.7) | 3/15 (20) | 4/15 (26.7) | |
| Mata do Choupal | D. marginatus (1A) | 1/1 (100) | 1/1 (100) | 0/1 (0) |
| R. pusillus (17A) | 5/17 (29.4) | 6/17 (35.3) | 3/17 (17.6) | |
| R. sanguineus s. l. (3A) | 0/3 (0) | 0/3 (0) | 3/3 (100) | |
| Tapada Nacional de Mafra | Haemaphysalis spp. (4N) | 1/1 (100) | 1/1 (100) | - |
| H. inermis (31A) | 3/31 (9.7) | 0/31 (0) | 0/4 (0) | |
| H. punctata (19A; 16N) | 3/22 (13.6) | 5/22 (22.7) | 0/7 (0) | |
| H. lusitanicum (17A) | 0/17 (0) | 13/17 (76.5) | - | |
| I. ricinus (82A; 517N; 1L) | 37/179 (20.67) | 52/179 (29.05) | 9/97 (9.3) | |
| R. sanguineus s. l. (1A) | 0/1 (0) | 0/1 (0) | - | |
| Total | 802 (262A; 118M, 144F); 539N; 1L | 68/365 (18.6) | 106/365 (29.0) | 25/178 (14) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moerbeck, L.; Parreira, R.; Szczotko, M.; Seixas, G.; Velez, R.; Dmitryjuk, M.; Santos, A.S.; Domingos, A.; Antunes, S. Ticks and Tick-Borne Pathogens Circulating in Peri-Domestic Areas in Mainland Portugal. Microorganisms 2024, 12, 1006. https://doi.org/10.3390/microorganisms12051006
Moerbeck L, Parreira R, Szczotko M, Seixas G, Velez R, Dmitryjuk M, Santos AS, Domingos A, Antunes S. Ticks and Tick-Borne Pathogens Circulating in Peri-Domestic Areas in Mainland Portugal. Microorganisms. 2024; 12(5):1006. https://doi.org/10.3390/microorganisms12051006
Chicago/Turabian StyleMoerbeck, Leonardo, Ricardo Parreira, Magdalena Szczotko, Gonçalo Seixas, Rita Velez, Małgorzata Dmitryjuk, Ana Sofia Santos, Ana Domingos, and Sandra Antunes. 2024. "Ticks and Tick-Borne Pathogens Circulating in Peri-Domestic Areas in Mainland Portugal" Microorganisms 12, no. 5: 1006. https://doi.org/10.3390/microorganisms12051006






