Early Age Assessment of a New Course of Irish Fly Ash as a Cement Replacement
Abstract
:1. Introduction
2. Materials and Methods
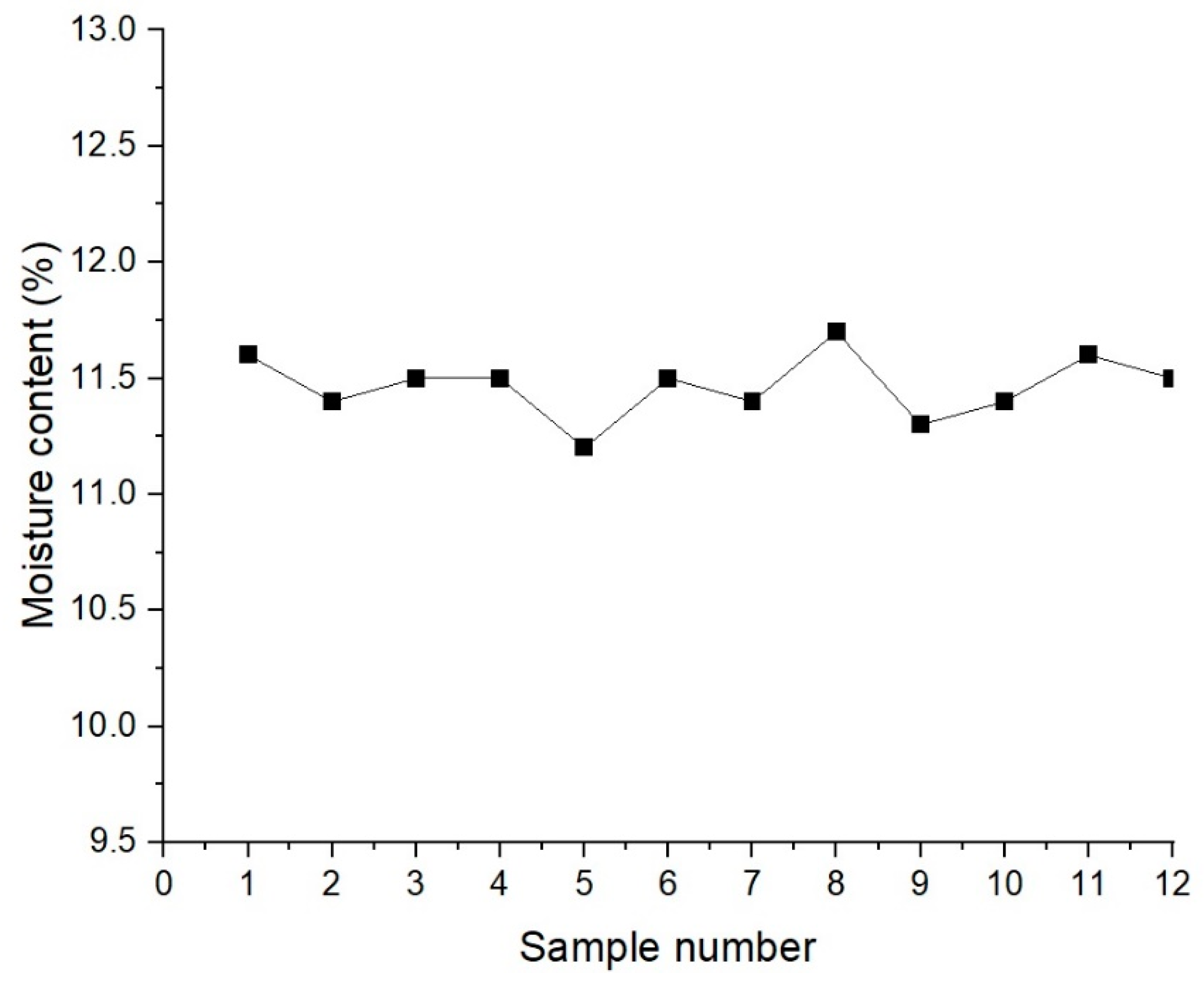
2.1. Moisture Content of Fly Ash
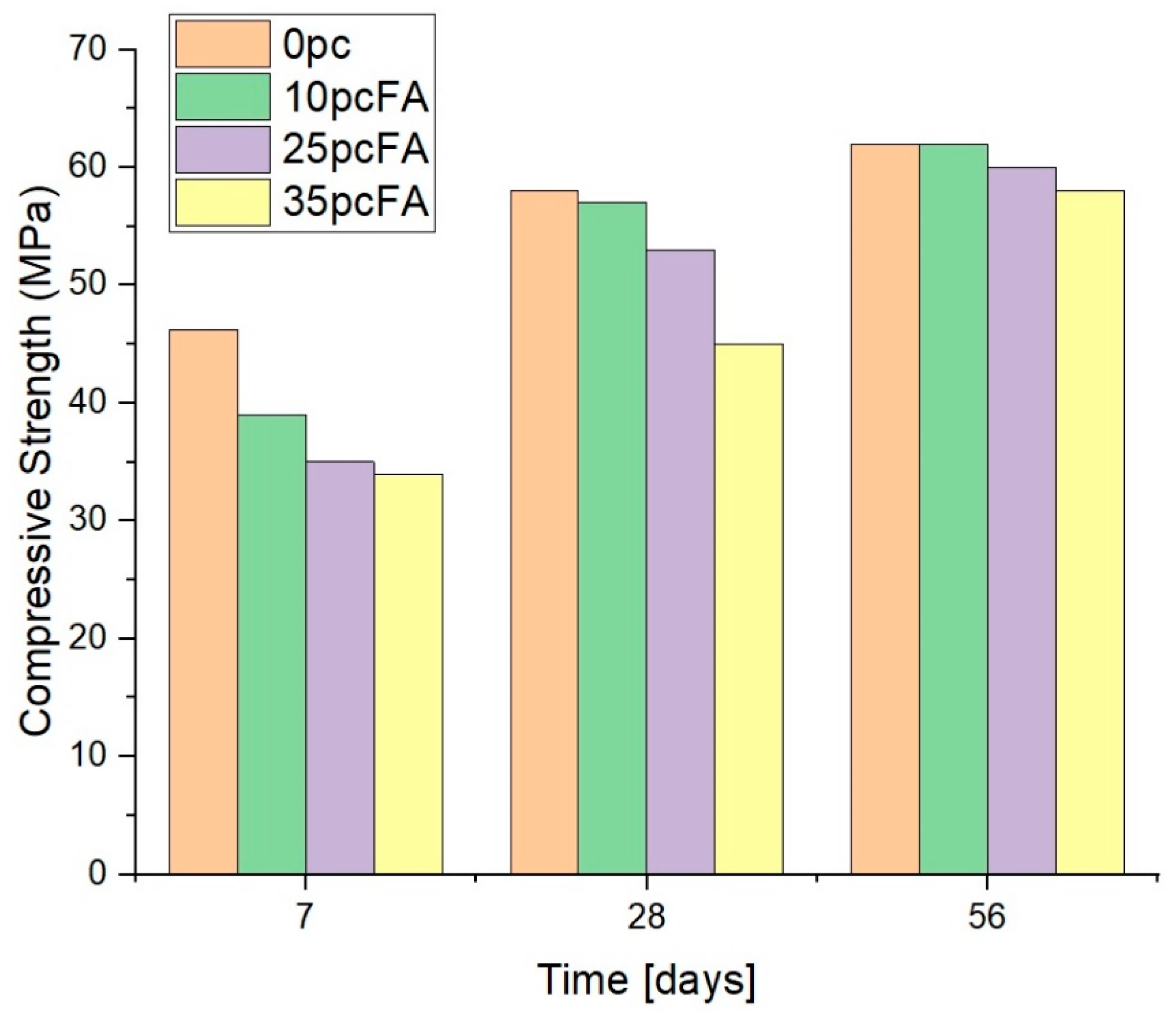
2.2. Concrete Compressive Strength Assessment
3. Thermodynamic Modelling
| FA % | FA amorphous/glass phase equations (log_k for the reactive and inactive phases are +999 and −999, respectively) |
| 5 | (SiO2)9.167(Al2O3)1.6678(Fe2O3)0.8449(CaO)1.3541(MgO)0.5311(K2O)0.2218(Na2O)0.0002(H2O)1.4427 = + 9.167 SiO2 + 3.3356 AlO2− + 1.6898 FeO2− + 1.3541 Ca+2 + 0.5311 Mg+2 + 0.4436 K+ + 0.0004 Na+ + 0.811 H+ + 1.0372 H2O |
| 10 | (SiO2)9.219(Al2O3)1.6537(Fe2O3)0.8299(CaO)1.3605(MgO)0.5359(K2O)0.2238(Na2O)0.0002(H2O)1.4416 = + 9.219 SiO2 + 3.3074 AlO2− + 1.6598 FeO2− + 1.3605 Ca+2 + 0.5359 Mg+2 + 0.4476 K+ + 0.0004 Na+ + 0.7264 H+ + 1.0784 H2O |
| 15 | (SiO2)9.2718(Al2O3)1.6394(Fe2O3)0.8145(CaO)1.3671(MgO)0.5407(K2O)0.2258(Na2O)0.0002(H2O)1.4405 = + 9.2718 SiO2 + 3.2788 AlO2− + 1.629 FeO2− + 1.3671 Ca+2 + 0.5407 Mg+2 + 0.4516 K+ + 0.0004 Na+ + 0.6402 H+ + 1.1204 H2O |
| 20 | (SiO2)9.3257(Al2O3)1.6248(Fe2O3)0.7989(CaO)1.3737(MgO)0.5457(K2O)0.2279(Na2O)0.0002(H2O)1.4393 = + 9.3257 SiO2 + 3.2496 AlO2− + 1.5978 FeO2− + 1.3737 Ca+2 + 0.5457 Mg+2 + 0.4558 K+ + 0.0004 Na+ + 0.5524 H+ + 1.1631 H2O |
| 25 | (SiO2)9.3805(Al2O3)1.61(Fe2O3)0.783(CaO)1.3805(MgO)0.5507(K2O)0.23(Na2O)0.0002(H2O)1.4382 = + 9.3805 SiO2 + 3.22 AlO2− + 1.566 FeO2− + 1.3805 Ca+2 + 0.5507 Mg+2 + 0.46 K+ + 0.0004 Na+ + 0.4632 H+ + 1.2066 H2O |
| 30 | (SiO2)9.4364(Al2O3)1.5949(Fe2O3)0.7667(CaO)1.3874(MgO)0.5559(K2O)0.2321(Na2O)0.0002(H2O)1.437 = + 9.4364 SiO2 + 3.1898 AlO2− + 1.5334 FeO2− + 1.3874 Ca+2 + 0.5559 Mg+2 + 0.4642 K+ + 0.0004 Na+ + 0.372 H+ + 1.251 H2O |
| 35 | (SiO2)9.4933(Al2O3)1.5794(Fe2O3)0.7502(CaO)1.3944(MgO)0.5611(K2O)0.2343(Na2O)0.0002(H2O)1.4358 = + 9.4933 SiO2 + 3.1588 AlO2− + 1.5004 FeO2− + 1.3944 Ca+2 + 0.5611 Mg+2 + 0.4686 K+ + 0.0004 Na+ + 0.2792 H+ + 1.2962 H2O |
4. Results
4.1. Fly Ash Composition
4.2. Fly Ash Moisture Content
4.3. Concrete Compressive Strength
4.4. X-ray Diffraction
4.5. Thermodynamic Modelling Analysis
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ireland Energy Needs. Available online: https://www.seai.ie/about/irelands-energy-targets/ (accessed on 7 November 2023).
- Making Concrete Change. Available online: https://www.chathamhouse.org/2018/06/making-concrete-change-innovation-low-carbon-cement-and-concrete (accessed on 7 November 2023).
- BS EN 197-1; Cement: Composition, Specification and Conformity Criteria for Common Cements. European Committee for Standardization (CEN): Brussels, Belgium, 2011.
- Snowdon, R.; Richardson, M. Influence of mix parameters on the permeability of PFA-concretes manufactured with PFA sourced in Ireland. In Proceedings of the Colloquium on Concrete Research in Ireland, Dublin, Ireland, 28 August 1997; pp. 3–9. [Google Scholar]
- Lenehan, J.; Richardson, M.; O’Kiely, P. Evaluation of pulverised fuel ash as a cement replacer in concretes used in silage storage structures. Irish J. Agric. Food Res. 2001, 39, 489. [Google Scholar]
- ASTM C618-12; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM International: West Conshohocken, PA, USA, 2012.
- ASTM C1365-06; Standard Test Method for Determination of the Proportion of Phases in Portland Cement and Portland-Cement Clinker Using X-ray Powder Diffraction Analysis. ASTM International: West Conshohocken, PA, USA, 2007.
- Holmes, N.; Tyrer, M.; West, R.P.; Lowe, A.; Kelliher, D. Using PHREEQC to model cement hydration. Constr. Build. Mater. 2022, 319, 126–129. [Google Scholar] [CrossRef]
- BS EN 933-1; Tests for Geometrical Properties of Aggregates. Determination of Particle Size Distribution. Sieving Method. European Committee for Standardization (CEN): Brussels, Belgium, 2012.
- Parkhust, D.L.; Appelo, C.A.J. A Computer Program for Speciation, Batch—Reaction, One—Dimensional Transport and Inverse Geochemical Calculations; U.S. Geological Survey: Reston, VA, USA, 1999.
- Parkhurst, D.J.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport and Inverse Geochemical Calculations; U.S. Geological Survey: Reston, VA, USA, 2013.
- Lothenbach, B.; Kulik, D.A.; Matschei, T.; Balonis, M.; Baquerizo, L.; Dilnesa, B.; Miron, G.D.; Myers, R.J. Cemdata18: A chemical thermodynamic database for hydrated Portland cements and alkali-activated materials. Cem. Concr. Res. 2019, 115, 472–506. [Google Scholar] [CrossRef]
- Shaji, N.; Holmes, N.; Tyrer, M. Derivation of fly-ash glass phase equations for use in thermodynamic models. In Proceedings of the Civil Engineering Research in Ireland, Galway, Ireland, 29–30 August 2024. [Google Scholar]
- Walker, C.; Sutou, S.; Oda, C.; Mihara, M.; Honda, A. Calcium silicate hydrate (C-S-H) gel solubility data and a discrete solid phase model at 25 °C based on two binary non-ideal solid solutions. Cem. Concr. Res. 2016, 79, 1–30. [Google Scholar] [CrossRef]
- Holmes, N.; Walker, C.; Tyrer, M.; Kelliher, D. Deriving discrete solid phases from CSH-3T and CSHQ end-members to model cement hydration in PHREEQC. In Proceedings of the Civil Engineering Research in Ireland (CERI) Conference, Dublin, Ireland, 25–26 August 2022; pp. 28–33. [Google Scholar]
- Alelweet, O.; Pavia, S. Potential of a low-calcium fly ash (FA) for the production of alkali-activated materials. In Proceedings of the Civil Engineering Research in Ireland, Bishopstown, Ireland, 27–28 August 2020; pp. 162–167. [Google Scholar]
- Xu, G.; Shi, X. Characteristics and applications of fly ash as a sustainable construction material: A state-of-the-art review. Resour. Conserv. Recycl. 2018, 136, 95–109. [Google Scholar] [CrossRef]
- Shi, C.; Roy, D.; Krivenko, P. Alkali-Activated Cements and Concretes; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Walker, R.; Pavía, S. Physical properties and reactivity of pozzolans, and their influence on the properties of lime–pozzolan pastes. Mater. Struct. 2011, 44, 1139–1150. [Google Scholar] [CrossRef]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. The role of calcium carbonate in cement hydration. Cem. Concr. Res. 2007, 37, 551–558. [Google Scholar] [CrossRef]
- Holmes, N.; Russell, M.; Davis, G.; Tyrer, M. Comparing the Measured and Thermodynamically Predicted AFm Phases in a Hydrating Cement. Appl. Sci. 2022, 12, 10147. [Google Scholar] [CrossRef]
- De Weerdt, K.; Haha, M.B.; Le Saout, G.; Kjellsen, K.O.; Justnes, H.; Lothenbach, B. Hydration mechanisms of ternary Portland cements containing limestone powder and fly ash. Cem. Concr. Res. 2011, 41, 279–291. [Google Scholar] [CrossRef]
- Lothenbach, B.; Saout, G.L.; Gallucci, E.; Scrivener, K. Influence of limestone on the hydration of Portland cements. Cem. Concr. Res. 2008, 38, 848–860. [Google Scholar] [CrossRef]
- Schöler, A.; Lothenbach, B.; Winnefeld, F.; Zajac, M. Hydration of quaternary Portland cement blends containing blast-furnace slag, siliceous fly ash and limestone powder. Cem. Concr. Compos. 2015, 55, 374–382. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Luxán, M.P.; Madruga, F.; Saavedra, J. Rapid evaluation of pozzolanic activity of natural products by conductivity measurement. Cem. Concr. Res. 1989, 19, 63–68. [Google Scholar] [CrossRef]
- McCarter, W.J.; Tran, D. Monitoring pozzolanic activity by direct activation with calcium hydroxide. Constr. Build. Mater. 1996, 10, 179–184. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Q.; Zhang, H. Combined effect of polypropylene fiber and silica fume on mechanical properties of concrete composite containing fly ash. J. Reinf. Plast. Compos. 2011, 30, 1349–1358. [Google Scholar] [CrossRef]
- Sun, J.; Shen, X.; Tan, G.; Tanner, J.E. Compressive strength and hydration characteristics of high-volume fly ash concrete prepared from fly ash. J. Therm. Anal. Calorim. 2019, 136, 565–580. [Google Scholar] [CrossRef]
- Upadhyay, R.; Srivastava, V.; Arpan, H.; Mehta, P. Effect of Fly Ash on Flexural Strength of Portland Pozzolona Cement Concrete. J. Acad. Ind. Res. 2014, 3, 218–220. [Google Scholar]
- Kuzel, H.-J.; Pöllmann, H. Hydration of C3A in the presence of Ca(OH)2, CaSO4·2H2O and CaCO3. Cem. Concr. Res. 1991, 21, 885–895. [Google Scholar] [CrossRef]
- EN 450-1:2012; Fly Ash for Concrete—Part 1: Definition, Specifications and Conformity Criteria. European Committee for Standardization (CEN): Brussels, Belgium, 2012.












| CEM I-FA | CEM I | FA |
|---|---|---|
| 100-0 | 150 | 0 |
| 90-10 | 135 | 15 |
| 80-20 | 120 | 30 |
| 65-35 | 97.5 | 52.5 |
| Oxide (g/100 g) | CEM I a | FA |
|---|---|---|
| SiO2 | 19.04 | 53.70 |
| Al2O3 | 5.01 | 16.81 |
| Fe2O3 | 2.83 | 13.46 |
| CaO | 63.4 | 7.41 |
| MgO | 2.31 | 2.08 |
| K2O | 0.54 | 2.03 |
| Na2O | 0.28 | -- |
| SO3 | 2.65 | 1.11 |
| CaO (free) | 1.71 | -- |
| CO2 | 2.2 | -- |
| Periclase | 1.0 | -- |
| P2O5 | 0.41 | |
| TiO2 | 1.82 | |
| MnO | 0.13 | |
| LOI | 3.20 | 2.55 |
| Density (g/cm3) | 2.95 | 2.24 |
| Blaine fineness (m2/kg) | 386 | 450 |
| CEM I a | FA b | ||
|---|---|---|---|
| C3S | 55.86 | Quartz | 1.29 |
| C2S | 10.07 | Calcite | 1.11 |
| C3A | 8.12 | Hematite | 4.65 |
| C4AF | 8.24 | Mullite | 7.95 |
| Lime | 1.64 | Magnetite | 3.37 |
| Calcite | 4.79 | Amorphous | 81.65 |
| Gypsum | 4.27 | ||
| Periclase | 0.96 | ||
| K2SO4 | 0.86 | ||
| Na2SO4 | 0.28 | ||
| K2O | 0.05 | ||
| Na2O | 0.15 | ||
| MgO | 2.21 | ||
| SO3 | 2.53 | ||
| Mix ID | % PFA | Mass (kg/m3) | ||||
|---|---|---|---|---|---|---|
| Water | CEM I | FA | CA | |||
| 10 mm | 20 mm | |||||
| 1 | 0 | 200 | 400 | 0 | 350 | 700 |
| 2 | 10 | 200 | 360 | 40 | 350 | 700 |
| 3 | 25 | 200 | 300 | 100 | 350 | 700 |
| 4 | 35 | 200 | 260 | 140 | 350 | 700 |
| Material | % Glass | % Crystalline | Mineral Composition |
|---|---|---|---|
| FA °C | 40–60 | 60–40 | Glass Quartz (SiO2) Mullite (2Al2O3.2SiO2) |
| FA 500 °C | 10–30 | 70–90 | Glass Quartz (SiO2) Mullite (2Al2O3.2SiO2) |
| FA 800 °C | 10–30 | 70–90 | Glass Quartz (SiO2) Mullite (2Al2O3.2SiO2) |
| FA 1000 °C | 10–30 | 70–90 | Glass Quartz (SiO2) Mullite (2Al2O3.2SiO2) Hematite (Fe2O3) Possible Ca3Al2O6 and alkali sulphate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaji, N.; Holmes, N.; Tyrer, M. Early Age Assessment of a New Course of Irish Fly Ash as a Cement Replacement. Appl. Sci. 2024, 14, 4128. https://doi.org/10.3390/app14104128
Shaji N, Holmes N, Tyrer M. Early Age Assessment of a New Course of Irish Fly Ash as a Cement Replacement. Applied Sciences. 2024; 14(10):4128. https://doi.org/10.3390/app14104128
Chicago/Turabian StyleShaji, Nikki, Niall Holmes, and Mark Tyrer. 2024. "Early Age Assessment of a New Course of Irish Fly Ash as a Cement Replacement" Applied Sciences 14, no. 10: 4128. https://doi.org/10.3390/app14104128






