Association between Obesity and Atrial Function in Patients with Non-Valvular Atrial Fibrillation: An Echocardiographic Study
Abstract
:1. Introduction
1.1. Role of Echocardiography in Atrial Fibrillation
1.2. Purpose of the Study
2. Materials and Methods
2.1. Study Population
2.2. Echocardiography
2.2.1. Atrial Dimension
2.2.2. Estimation of Left Atrial Function by Speckle Tracking Echocardiography
- -
- Peak atrial longitudinal strain (PALS), measured at the end of the atrial reservoir phase. Which in normal subjects is greater than 40%. In patients with atrial fibrillation it has been seen that the reduction in strain reflects structural alterations of the left atrium (wall fibrosis) [36] (Figure 2)
- -
- Peak atrial contraction strain (PACS) identified just before the onset of the active phase of atrial contraction [37], which indicates the contribution of the active contraction of the left atrium to the filling phase of the left ventricle [38] and which is lacking in patients with permanent atrial fibrillation.

2.3. CHA2DS2-VASc Score Assessment
2.4. Statistical Analysis
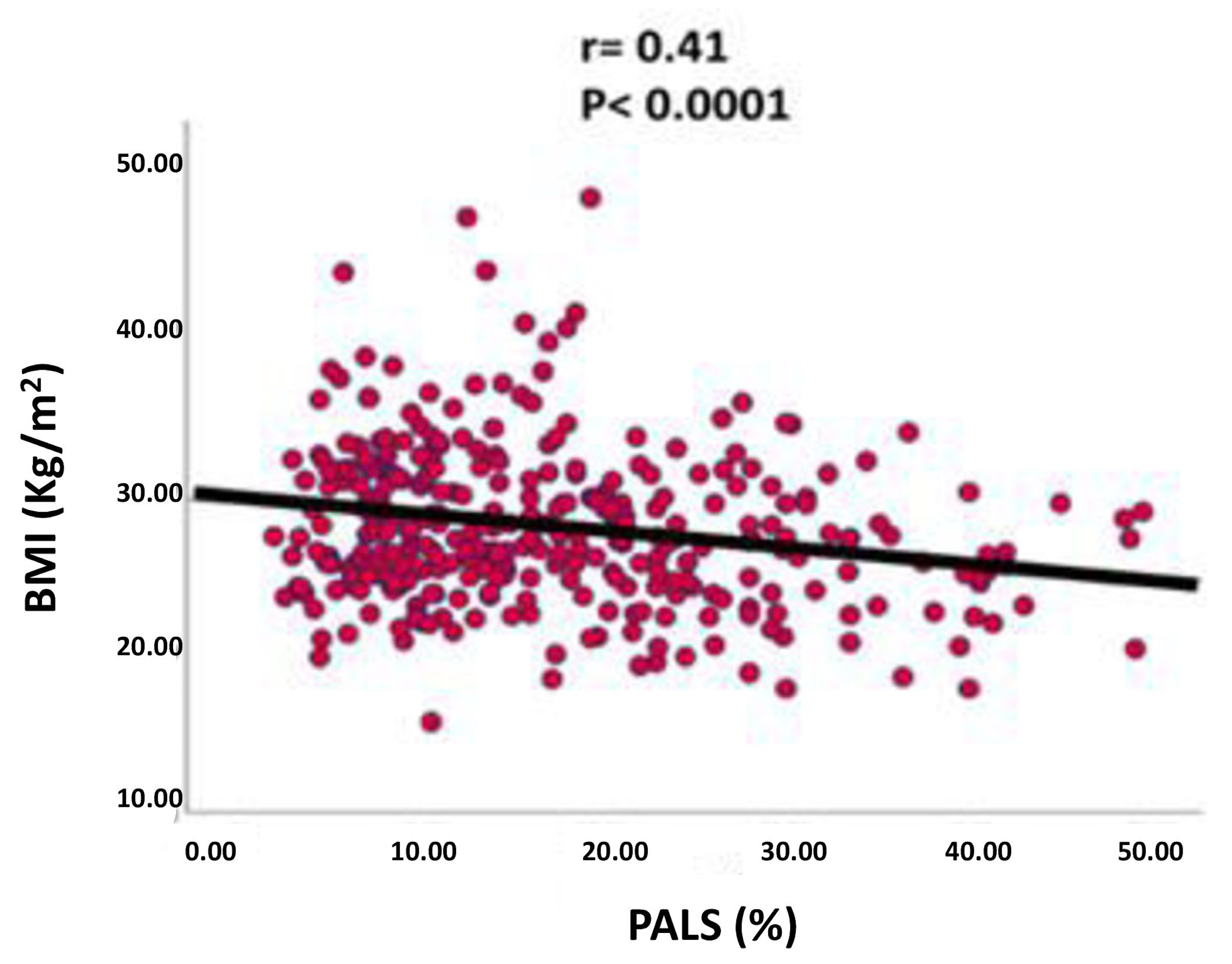
3. Results
- First tertile: patients with BMI less than 25.3 kg/m2 (n = 127).
- Second tertile: patients with BMI between 25.3 kg/m2 and 29.3 kg/m2 (n = 137).
- Third tertile: patients with BMI greater than 29.3 kg/m2 (n = 131).
4. Discussion
5. Limitations of the Study and Future Prospects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bastien, M.; Poirier, P.; Lemieux, I.; Després, J.P. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog. Cardiovasc. Dis. 2014, 56, 369–381. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016, 387, 1377–1396, Erratum in Lancet 2016, 387, 1998. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Swinburn, B.; Sacks, G.; Ravussin, E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am. J. Clin. Nutr. 2009, 90, 1453–1456. [Google Scholar] [CrossRef] [PubMed]
- Church, T.S.; Thomas, D.M.; Tudor-Locke, C.; Katzmarzyk, P.T.; Earnest, C.P.; Rodarte, R.Q.; Martin, C.K.; Blair, S.N.; Bouchard, C. Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PLoS ONE 2011, 6, e19657. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Faienza, M.F.; Wang, D.Q.; Frühbeck, G.; Garruti, G.; Portincasa, P. The dangerous link between childhood and adulthood predictors of obesity and metabolic syndrome. Intern. Emerg. Med. 2016, 11, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; McAuley, P.A.; Church, T.S.; Milani, R.V.; Blair, S.N. Obesity and cardiovascular diseases: Implications regarding fitness, fatness, and severity in the obesity paradox. J. Am. Coll. Cardiol. 2014, 63, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.; Petraglia, L.; Cabaro, S.; Valerio, V.; Poggio, P.; Pilato, E.; Attena, E.; Russo, V.; Ferro, A.; Formisano, P.; et al. Epicardial Adipose Tissue and Cardiac Arrhythmias: Focus on Atrial Fibrillation. Front. Cardiovasc. Med. 2022, 9, 932262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taegtmeyer, H.; Young, M.E.; Lopaschuk, G.D.; Abel, E.D.; Brunengraber, H.; Darley-Usmar, V.; Des Rosiers, C.; Gerszten, R.; Glatz, J.F.; Griffin, J.L.; et al. Assessing Cardiac Metabolism: A Scientific Statement from the American Heart Association. Circ. Res. 2016, 118, 1659–1701, Erratum in Circ. Res. 2016, 118, e35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scheuermann-Freestone, M.; Madsen, P.L.; Manners, D.; Blamire, A.M.; Buckingham, R.E.; Styles, P.; Radda, G.K.; Neubauer, S.; Clarke, K. Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation 2003, 107, 3040–3046. [Google Scholar] [CrossRef] [PubMed]
- Alpert, M.A.; Lavie, C.J.; Agrawal, H.; Kumar, A.; Kumar, S.A. Cardiac Effects of Obesity: Pathophysiologic, Clinical, and Prognostic Consequences—A Review. J. Cardiopulm. Rehabil. Prev. 2016, 36, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.M.; Beleigoli, A.M.; de Fatima Diniz, M.; Freire, C.V.; Ribeiro, A.L.; Nunes, M.C. Strain imaging in morbid obesity: Insights into subclinical ventricular dysfunction. Clin. Cardiol. 2011, 34, 288–293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wong, C.Y.; O’Moore-Sullivan, T.; Leano, R.; Hukins, C.; Jenkins, C.; Marwick, T.H. Association of subclinical right ventricular dysfunction with obesity. J. Am. Coll. Cardiol. 2006, 47, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Obokata, M.; Reddy, Y.N.V.; Pislaru, S.V.; Melenovsky, V.; Borlaug, B.A. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure with Preserved Ejection Fraction. Circulation 2017, 136, 6–19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kosmala, W.; Sanders, P.; Marwick, T.H. Subclinical Myocardial Impairment in Metabolic Diseases. JACC Cardiovasc. Imaging 2017, 10, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Ayer, J.G.; Almafragy, H.S.; Patel, A.A.; Hellyer, R.L.; Celermajer, D.S. Body mass index is an independent determinant of left atrial size. Heart Lung Circ. 2008, 17, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Aga, Y.; Acardag, Y.; Chin, J.F.; Kroon, D.; Snelder, S.M.; De Groot-De Laat, L.; Biter, U.; Zijlstra, F.; Brugts, J.; van Dalen, B. Improved identification of left atrial enlargement in patients with obesity. Int. J. Cardiovasc. Imaging 2024, 40, 65–72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aiad, N.N.; Hearon, C., Jr.; Hieda, M.; Dias, K.; Levine, B.D.; Sarma, S. Mechanisms of Left Atrial Enlargement in Obesity. Am. J. Cardiol. 2019, 124, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Stritzke, J.; Markus, M.R.; Duderstadt, S.; Lieb, W.; Luchner, A.; Döring, A.; Keil, U.; Hense, H.W.; Schunkert, H.; MONICA/KORA Investigators. The aging process of the heart: Obesity is the main risk factor for left atrial enlargement during aging the MONICA/KORA (monitoring of trends and determinations in cardiovascular disease/cooperative research in the region of Augsburg) study. J. Am. Coll. Cardiol. 2009, 54, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Munger, T.M.; Dong, Y.X.; Masaki, M.; Oh, J.K.; Mankad, S.V.; Borlaug, B.A.; Asirvatham, S.J.; Shen, W.K.; Lee, H.C.; Bielinski, S.J.; et al. Electrophysiological and hemodynamic characteristics associated with obesity in patients with atrial fibrillation. J. Am. Coll. Cardiol. 2012, 60, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, R.; Nelson, A.; Pathak, R.K.; Middeldorp, M.E.; Wong, C.X.; Twomey, D.J.; Carbone, A.; Teo, K.; Agbaedeng, T.; Linz, D.; et al. Electroanatomical Remodeling of the Atria in Obesity: Impact of Adjacent Epicardial Fat. JACC Clin. Electrophysiol. 2018, 4, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, X.; Jiang, Y.; Cao, Z.; Wu, M.; Chen, Z.; Sun, R.; Yu, P.; Ma, J.; Zhu, W.; et al. Association of Body Mass Index and Abdominal Obesity with Incidence of Atrial Fibrillation in Heart Failure with Preserved Ejection Fraction. Curr. Med. Chem. 2023. [Google Scholar] [CrossRef] [PubMed]
- Lembo, M.; Strisciuglio, T.; Fonderico, C.; Mancusi, C.; Izzo, R.; Trimarco, V.; Bellis, A.; Barbato, E.; Esposito, G.; Morisco, C.; et al. Obesity: The perfect storm for heart failure. ESC Heart Fail. 2024, 15. [Google Scholar] [CrossRef] [PubMed]
- Hurd, H.P., 2nd; Starling, M.R.; Crawford, M.H.; Dlabal, P.W.; O’Rourke, R.A. Comparative accuracy of electrocardiographic and vectorcardiographic criteria for inferior myocardial infarction. Circulation 1981, 63, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart. J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Tops, L.F.; Schalij, M.J.; Bax, J.J. Imaging and atrial fibrillation: The role of multimodality imaging in patient evaluation and management of atrial fibrillation. Eur. Heart J. 2010, 31, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.A.; Lavie, C.J.; Milani, R.V.; Shah, S.; Gilliland, Y. Clinical implications of left atrial enlargement: A review. Ochsner J. 2009, 9, 191–196. [Google Scholar] [PubMed]
- Kou, S.; Caballero, L.; Dulgheru, R.; Voilliot, D.; De Sousa, C.; Kacharava, G.; Athanassopoulos, G.D.; Barone, D.; Baroni, M.; Cardim, N.; et al. Echocardiographic reference ranges for normal cardiac chamber size: Results from the NORRE study. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 680–690. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pritchett, A.M.; Jacobsen, S.J.; Mahoney, D.W.; Rodeheffer, R.J.; Bailey, K.R.; Redfield, M.M. Left atrial volume as an index of left atrial size: A population-based study. J. Am. Coll. Cardiol. 2003, 41, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.K.; Dahl, J.S.; Henriksen, J.E.; Hey, T.M.; Høilund-Carlsen, P.F.; Beck-Nielsen, H.; Møller, J.E. Left atrial volume index: Relation to long-term clinical outcome in type 2 diabetes. J. Am. Coll. Cardiol. 2013, 62, 2416–2421. [Google Scholar] [CrossRef] [PubMed]
- Prioli, A.; Marino, P.; Lanzoni, L.; Zardini, P. Increasing degrees of left ventricular filling impairment modulate left atrial function in humans. Am. J. Cardiol. 1998, 82, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Barbier, P.; Solomon, S.B.; Schiller, N.B.; Glantz, S.A. Left atrial relaxation and left ventricular systolic function determine left atrial reservoir function. Circulation 1999, 100, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Mondillo, S.; Galderisi, M.; Mele, D.; Cameli, M.; Lomoriello, V.S.; Zacà, V.; Ballo, P.; D’Andrea, A.; Muraru, D.; Losi, M.; et al. Speckle-tracking echocardiography: A new technique for assessing myocardial function. J. Ultrasound Med. 2011, 30, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Mor-Avi, V.; Lang, R.M.; Badano, L.P.; Belohlavek, M.; Cardim, N.M.; Derumeaux, G.; Galderisi, M.; Marwick, T.; Nagueh, S.F.; Sengupta, P.P.; et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur. J. Echocardiogr. 2011, 12, 167–205. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Mondillo, S.; Galderisi, M.; Mandoli, G.E.; Ballo, P.; Nistri, S.; Capo, V.; D’Ascenzi, F.; D’Andrea, A.; Esposito, R.; et al. L’ecocardiografia speckle tracking: Roadmap per la misurazione e l’utilizzo clinico [Speckle tracking echocardiography: A practical guide]. G. Ital. Cardiol. 2017, 18, 253–269. (In Italian) [Google Scholar] [CrossRef] [PubMed]
- Kuppahally, S.S.; Akoum, N.; Burgon, N.S.; Badger, T.J.; Kholmovski, E.G.; Vijayakumar, S.; Rao, S.N.; Blauer, J.; Fish, E.N.; Dibella, E.V.; et al. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: Relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ. Cardiovasc. Imaging 2010, 3, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Caputo, M.; Mondillo, S.; Ballo, P.; Palmerini, E.; Lisi, M.; Marino, E.; Galderisi, M. Feasibility and reference values of left atrial longitudinal strain imaging by two-dimensional speckle tracking. Cardiovasc. Ultrasound 2009, 7, 6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cameli, M.; Lisi, M.; Righini, F.M.; Mondillo, S. Novel echocardiographic techniques to assess left atrial size, anatomy and function. Cardiovasc. Ultrasound 2012, 10, 4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2014, 64, e1–e76, Erratum in J. Am. Coll. Cardiol. 2014, 64, 2305–2307. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, X.; Wei, B.; Ren, T.; Huang, N.; Escobar, C.; Pang, P.Y.K.; Liu, X.; Zhou, H. Associations between waist circumference, central obesity, and the presence of non-valvular atrial fibrillation patients with heart failure. J. Thorac. Dis. 2024, 16, 2049–2059. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Conen, D.; Glynn, R.J.; Sandhu, R.K.; Tedrow, U.B.; Albert, C.M. Risk factors for incident atrial fibrillation with and without left atrial enlargement in women. Int. J. Cardiol. 2013, 168, 1894–1899. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cameli, M.; Ciccone, M.M.; Maiello, M.; Modesti, P.A.; Muiesan, M.L.; Scicchitano, P.; Novo, S.; Palmiero, P.; Saba, P.S.; Pedrinelli, R.; et al. Speckle tracking analysis: A new tool for left atrial function analysis in systemic hypertension: An overview. J. Cardiovasc. Med. 2016, 17, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Pedersson, P.R.; Skaarup, K.G.; Lassen, M.C.H.; Olsen, F.J.; Iversen, A.Z.; Jørgensen, P.G.; Biering-Sørensen, T. Left atrial strain is associated with long-term mortality in acute coronary syndrome patients. Int. J. Cardiovasc. Imaging 2024, 40, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Norton, J.; Foy, A.; Ba, D.M.; Liu, G.; Leslie, D.; Zhang, Y.; Naccarelli, G.V. Obese patients with new onset atrial fibrillation/flutter have higher risk of hospitalization, cardioversions, and ablations. Am. Heart J. Plus 2024, 40, 100375. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hatem, S.N.; Redheuil, A.; Gandjbakhch, E. Cardiac adipose tissue and atrial fibrillation: The perils of adiposity. Cardiovasc. Res. 2016, 109, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, A.S.; Gelpi, G.; Mangini, A.; Carugo, S.; Ruscica, M.; Macchi, C. Exploring the role of epicardial adipose-tissue-derived extracellular vesicles in cardiovascular diseases. iScience 2024, 27, 109359. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Venteclef, N.; Guglielmi, V.; Balse, E.; Gaborit, B.; Cotillard, A.; Atassi, F.; Amour, J.; Leprince, P.; Dutour, A.; Clément, K.; et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur. Heart J. 2015, 36, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Carbone, F.; Mach, F.; Montecucco, F. The role of adipocytokines in atherogenesis and atheroprogression. Curr. Drug Targets 2015, 16, 295–320. [Google Scholar] [CrossRef] [PubMed]
- Liberale, L.; Bonaventura, A.; Vecchiè, A.; Casula, M.; Dallegri, F.; Montecucco, F.; Carbone, F. The Role of Adipocytokines in Coronary Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 10, Erratum in Curr. Atheroscler. Rep. 2017, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Gaborit, B.; Sengenes, C.; Ancel, P.; Jacquier, A.; Dutour, A. Role of Epicardial Adipose Tissue in Health and Disease: A Matter of Fat? Compr. Physiol. 2017, 7, 1051–1082. [Google Scholar] [CrossRef] [PubMed]


| Variables | I Tertile (BMI < 25.3) N = 127 | II Tertile (25.3 ≤ BMI ≤ 29.3) N = 137 | III Tertile (BMI > 29.3) N = 131 | Pa | Pb | Pc |
|---|---|---|---|---|---|---|
| Age (years) | 71.9 ± 11.4 | 69.8 ± 11.5 | 70.1 ± 10.0 | NS | NS | NS |
| SBP (mmHg) | 127 ± 18 | 130 ± 17 | 129 ± 21 | NS | NS | NS |
| DBP (mmHg) | 74 ± 10 | 78 ± 10 | 77 ± 11 | <0.01 | NS | <0.001 |
| BMI (kg/m2) | 22.6 ± 2.1 | 27.1 ± 1.2 | 33.0 ± 3.6 | <0.0001 | <0.0001 | <0.0001 |
| CHA2DS2-VASc | 3.2 ± 1.6 | 3.3 ± 1.7 | 3.4 ± 1.7 | NS | NS | NS |
| LV Mass Index | 42.0 ± 13.9 | 45.7 ± 15.5 | 49.0 ± 13.7 | NS | NS | 0.001 |
| LV EF (%) | 55.1 ± 10.3 | 57.2 ± 8.9 | 55.7 ± 8.7 | NS | NS | NS |
| LV GLS (%) | 18.7 ± 5.7 | 18.9 ± 4.7 | 18.5 ± 5.1 | NS | NS | NS |
| E/e’ | 11.6 ± 5.5 | 11.9 ± 7.1 | 18.5 ± 5.1 | NS | NS | NS |
| LA volume index | 45.0 ± 16.1 | 43.9 ± 14.3 | 47.0 ± 14.3 | NS | NS | NS |
| PALS (%) | 19.0 ± 11.5 | 17.8 ± 10.6 | 14.2 ± 8.3 | NS | 0.03 | 0.02 |
| Dependent Variable | Determinants | β Coefficient | p-Value |
|---|---|---|---|
| PALS | CHA2DS2-VASc | −0.180 | <0.001 |
| LV mass index | −0.112 | 0.04 | |
| LV EF | 0.228 | <0.0001 | |
| E/e’ | −0.141 | <0.01 | |
| PAPs | −0.153 | 0.006 | |
| BMI | −0.140 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pucci, M.; Gammaldi, V.; Capece, L.M.; Paoletta, D.; Iervolino, A.; Pontoriero, M.; Iacono, M.; Megaro, P.; Esposito, R. Association between Obesity and Atrial Function in Patients with Non-Valvular Atrial Fibrillation: An Echocardiographic Study. J. Clin. Med. 2024, 13, 2895. https://doi.org/10.3390/jcm13102895
Pucci M, Gammaldi V, Capece LM, Paoletta D, Iervolino A, Pontoriero M, Iacono M, Megaro P, Esposito R. Association between Obesity and Atrial Function in Patients with Non-Valvular Atrial Fibrillation: An Echocardiographic Study. Journal of Clinical Medicine. 2024; 13(10):2895. https://doi.org/10.3390/jcm13102895
Chicago/Turabian StylePucci, Martina, Vittoria Gammaldi, Luca Maria Capece, Daniele Paoletta, Adelaide Iervolino, Mariateresa Pontoriero, Marina Iacono, Pasquale Megaro, and Roberta Esposito. 2024. "Association between Obesity and Atrial Function in Patients with Non-Valvular Atrial Fibrillation: An Echocardiographic Study" Journal of Clinical Medicine 13, no. 10: 2895. https://doi.org/10.3390/jcm13102895




