Optimizing Synergistic Silica–Zinc Oxide Coating for Enhanced Flammability Resistance in Cotton Protective Clothing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Coating of Sol–Gel
2.3. Characterization
2.4. Central Composite Design (CCD) for Coating Process
3. Results and Discussion
3.1. Surface Morphology of FR-Finished Cotton Samples
3.2. Thermal Properties
3.3. Comfort Properties
3.4. Analysis of Central Composite Design and RSM Modeling
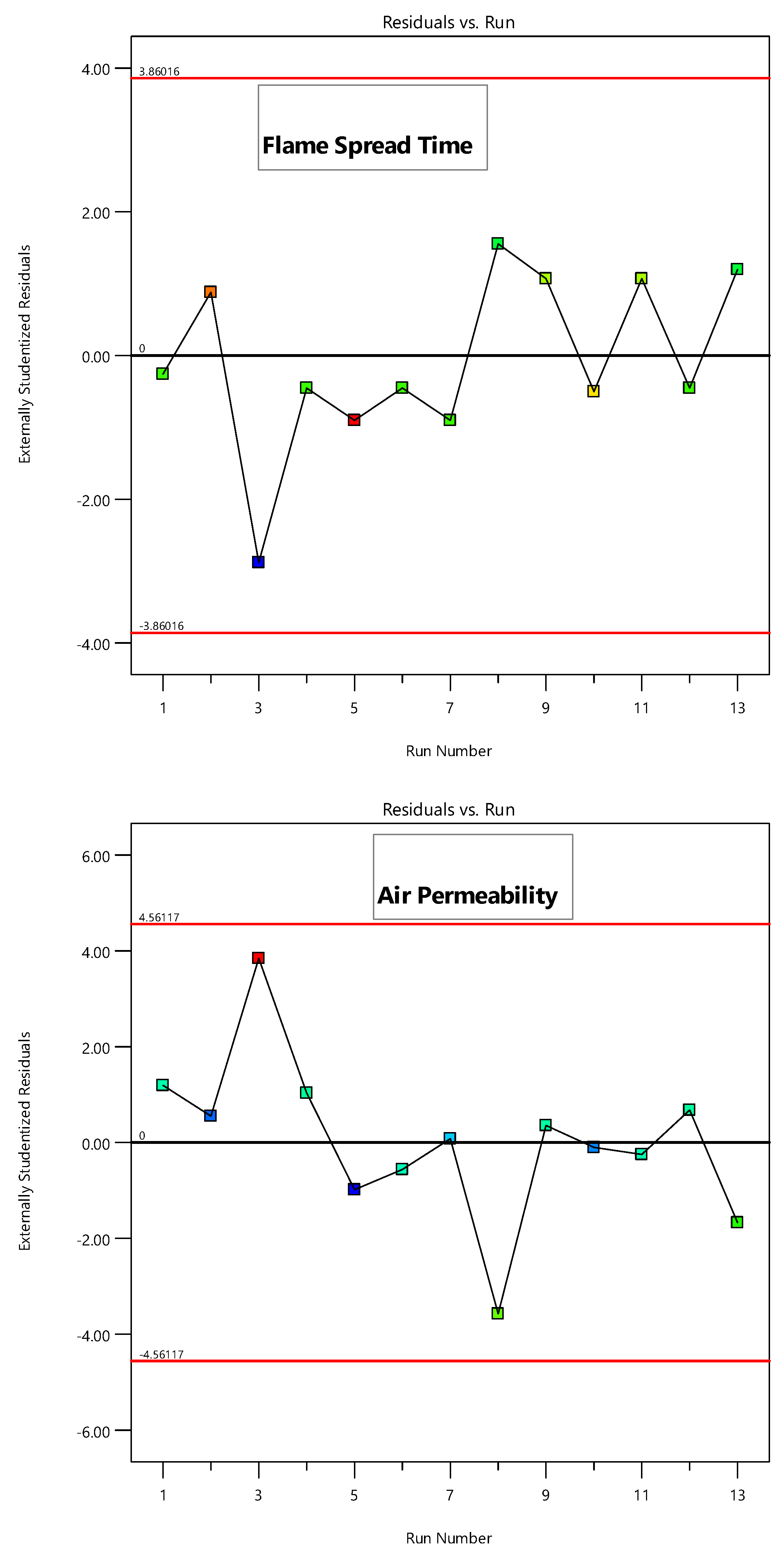
3.4.1. Statistical Analysis and RSM Modeling
3.4.2. Effect of Process Parameters on Process Responses
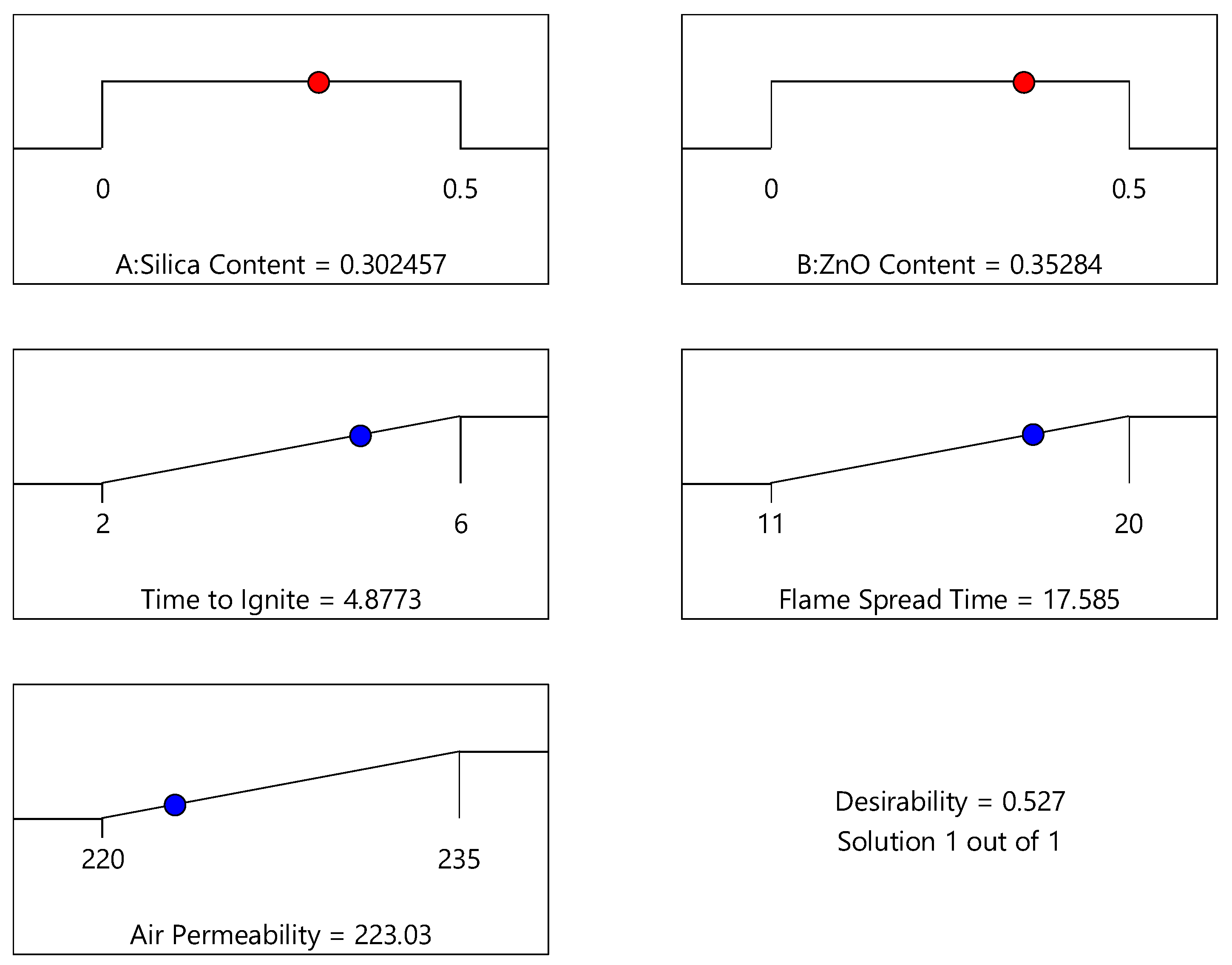
3.4.3. Optimization Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiu, X.; Li, Z.; Li, X.; Zhang, Z. Flame retardant coatings prepared using layer by layer assembly: A review. Chem. Eng. J. 2018, 334, 108–122. [Google Scholar] [CrossRef]
- Ling, C.; Guo, L.; Wang, Z. A review on the state of flame-retardant cotton fabric: Mechanisms and applications. Ind. Crops Prod. 2023, 194, 116264. [Google Scholar] [CrossRef]
- Asmat-Campos, D.; Delfín-Narciso, D.; Juárez-Cortijo, L. Textiles Functionalized with ZnO Nanoparticles Obtained by Chemical and Green Synthesis Protocols: Evaluation of the Type of Textile and Resistance to UV Radiation. Fibers 2021, 9, 10. [Google Scholar] [CrossRef]
- Agnihotri, S.; Sheikh, J.N.; Singh, S.P.; Behera, B.K. Flame-retardant textile structural composites for construction application: A review. J. Mater. Sci. 2024, 59, 1788–1818. [Google Scholar] [CrossRef]
- Nguyen Thi, H.; Vu Thi Hong, K.; Ngo Ha, T.; Phan, D.-N. Application of plasma activation in flame-retardant treatment for cotton fabric. Polymers 2020, 12, 1575. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, M.; Wang, Q.; Li, B. A novel durable flame retardant cotton fabric produced by surface chemical grafting of phosphorus-and nitrogen-containing compounds. Cellulose 2017, 24, 4069–4081. [Google Scholar] [CrossRef]
- Bentis, A.; Boukhriss, A.; Gmouh, S. Flame-retardant and water-repellent coating on cotton fabric by titania–boron sol–gel method. J. Sol-Gel Sci. Technol. 2020, 94, 719–730. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Sun, J. Layer-by-layer assembly for rapid fabrication of thick polymeric films. Chem. Soc. Rev. 2012, 41, 5998–6009. [Google Scholar] [CrossRef] [PubMed]
- Jelil, R.A. A review of low-temperature plasma treatment of textile materials. J. Mater. Sci. 2015, 50, 5913–5943. [Google Scholar] [CrossRef]
- Coad, B.R.; Lu, Y.; Meagher, L. A substrate-independent method for surface grafting polymer layers by atom transfer radical polymerization: Reduction of protein adsorption. Acta Biomater. 2012, 8, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Bokov, D.; Turki Jalil, A.; Chupradit, S.; Suksatan, W.; Javed Ansari, M.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by sol-gel method: Synthesis and application. Adv. Mater. Sci. Eng. 2021, 2021, 1–21. [Google Scholar] [CrossRef]
- Safdar, F.; Ashraf, M.; Abid, A.; Javid, A.; Iqbal, K. Eco-friendly, efficient and durable flame retardant coating for cotton fabrics using phytic acid/silane hybrid sol. Mater. Chem. Phys. 2024, 311, 128568. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, L.; Liu, J.; Wu, L. Multi-functionalization of cotton fabrics with excellent flame retardant, antibacterial and superhydrophobic properties. Int. J. Biol. Macromol. 2024, 254, 127889. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Figueiras, C.; Pérez-Padilla, Y.; Estrella-Gutiérrez, M.A.; Uc-Cayetano, E.G.; Juárez-Moreno, J.A.; Avila-Ortega, A. Surface Science Engineering through Sol-Gel Process. In Applied Surface Science; Injeti, G., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Najafi, B.; Faizollahzadeh Ardabili, S.; Mosavi, A.; Shamshirband, S.; Rabczuk, T. An intelligent artificial neural network-response surface methodology method for accessing the optimum biodiesel and diesel fuel blending conditions in a diesel engine from the viewpoint of exergy and energy analysis. Energies 2018, 11, 860. [Google Scholar] [CrossRef]
- Czyrski, A.; Jarzębski, H. Response surface methodology as a useful tool for evaluation of the recovery of the fluoroquinolones from plasma—The study on applicability of box-behnken design, central composite design and doehlert design. Processes 2020, 8, 473. [Google Scholar] [CrossRef]
- Qadir, D.; Nasir, R.; Mannan, H.A.; Mukhtar, H. Optimization and performance studies of NFDK membrane for ionic separation from aqueous solutions. Chem. Pap. 2022, 76, 1815–1831. [Google Scholar] [CrossRef]
- Karim-nejad, M.M.; Nazari, A.; Davodi-roknabadi, A. Efficient flame retardant of mercerized cotton through cross-linking with citric acid and ZnO nanoparticles optimized by RSM models. J. Text. Inst. 2015, 106, 1115–1126. [Google Scholar] [CrossRef]
- Forouharshad, M.; Montazer, M.; Moghadam, M.B.; Saligheh, O. Flame retardant wool using zirconium oxychloride in various acidic media optimized by RSM. Thermochim. Acta 2011, 516, 29–34. [Google Scholar] [CrossRef]
- Saleemi, S.; Naveed, T.; Riaz, T.; Memon, H.; Awan, J.A.; Siyal, M.I.; Xu, F.; Bae, J. Surface functionalization of cotton and PC fabrics using SiO2 and ZnO nanoparticles for durable flame retardant properties. Coatings 2020, 10, 124. [Google Scholar] [CrossRef]
- ASTM D1230–1294; Standard Test Method for Flammability of Apparel Textiles. ASTM International: West Conshohocken, PA, USA, 2001.
- AATCC TM197; Vertical Wicking of Textiles. American Association of Textile Chemists and Colorists: The Triangle Science Park, NC, USA, 2011.
- ISO 139:2005; Textiles—Standard Atmosphere for Conditioning and Testing. ISO: Geneva, Switzerland, 2005.
- ASTM D5035-11(2019); Standard Test Method for Breaking Force and Elongation of Textile Fabrics (Strip Method). ASTM International: West Conshohocken, PA, USA, 2019.
- Chen, Y.; Ding, H.; Sun, S. Preparation and Characterization of ZnO Nanoparticles Supported on Amorphous SiO2. Nanomaterials 2017, 7, 217. [Google Scholar] [CrossRef]
- Teli, M.D.; Annaldewar, B.N. Superhydrophobic and ultraviolet protective nylon fabrics by modified nano silica coating. J. Text. Inst. 2017, 108, 460–466. [Google Scholar] [CrossRef]
- Bentis, A.; Boukhriss, A.; Grancaric, A.M.; El Bouchti, M.; El Achaby, M.; Gmouh, S. Flammability and combustion behavior of cotton fabrics treated by the sol gel method using ionic liquids combined with different anions. Cellulose 2019, 26, 2139–2153. [Google Scholar] [CrossRef]
- Mondal, S.; Adak, B.; Mukhopadhyay, S. 11 Functional and smart textiles for military and defence applications. In Smart and Functional Textiles; De Gruyter: Berlin, Germany, 2023; p. 397. [Google Scholar]
- Saleemi, S.; Malik, S.A.; Syed, U.; Tanwari, A. Investigation of wash durability of silica nanoparticle coated 100% cotton reactive dyed fabric treated by sol-gel technique. J. Eng. Fibers Fabr. 2014, 9, 155892501400900402. [Google Scholar] [CrossRef]
- Lomov, S.V.; Truevtzev, A.; Cassidy, C. A predictive model for the fabric-to-yarn bending stiffness ratio of a plain-woven set fabric. Text. Res. J. 2000, 70, 1088–1096. [Google Scholar] [CrossRef]
- Lammens, N.; Kersemans, M.; Luyckx, G.; Van Paepegem, W.; Degrieck, J. Improved accuracy in the determination of flexural rigidity of textile fabrics by the Peirce cantilever test (ASTM D1388). Text. Res. J. 2014, 84, 1307–1314. [Google Scholar] [CrossRef]
- Islam, S.R.; Wang, W.; Junjie, P.; Jiang, J.; Shao, H.; Chen, N. Study on thermal, mechanical, and wettability properties of three-dimensional weft-knitted spacer fabrics with various silica aerogels coating. Text. Res. J. 2023, 93, 3611–3629. [Google Scholar] [CrossRef]
- Mahmud, S.; Sultana, M.Z.; Pervez, M.N.; Habib, M.A.; Liu, H.-H. Surface Functionalization of “Rajshahi Silk” Using Green Silver Nanoparticles. Fibers 2017, 5, 35. [Google Scholar] [CrossRef]
- Alongi, J.; Han, Z.; Bourbigot, S. Intumescence: Tradition versus novelty. A comprehensive review. Prog. Polym. Sci. 2015, 51, 28–73. [Google Scholar] [CrossRef]
- Naidua, T.; Qadir, D.; Nasir, R.; Mannan, H.A.; Mukhtar, H.; Maqsood, K.; Ali, A.; Abdulrahman, A. Utilization of moringa oleifera and nanofiltration membrane to treat palm oil mill effluent (POME). Mater. Und Werkst. 2021, 52, 346–356. [Google Scholar] [CrossRef]
- Masor, A.; Nasir, R.; Mannan, H.; Qadir, D.; Sharif, R.; Mohshim, D.; Mukhtar, H.; Muhammad, A. Performance optimization of polyetherimide-zeolite 4A mixed matrix membranes for carbon dioxide/methane separation process using response surface methodology. Mater. Und Werkst. 2023, 54, 571–585. [Google Scholar] [CrossRef]
- Liu, H.; Li, P.; Xu, Y.-J.; Zhu, P.; Liu, Y. Eco-friendly flame-retardant coatings based on γ-ureidopropyltriethoxysilane for cotton fabrics with improved flame retardancy and mechanical properties. Sustain. Mater. Technol. 2024, 39, e00821. [Google Scholar] [CrossRef]
- Bakar, N.A.; Yusop, H.M.; Ismail, W.W.; Zulkifli, N.F. Sol-Gel Finishing for Protective Fabrics. Biointerface Res. Appl. Chem 2023, 13, 283. [Google Scholar]
- Adalarasan, R.; Santhanakumar, M.; Shanmuga Sundaram, A. Optimization of weld characteristics of friction welded AA 6061-AA 6351 joints using grey-principal component analysis (G-PCA). J. Mech. Sci. Technol. 2014, 28, 301–307. [Google Scholar] [CrossRef]
- Shen, R.; Fan, T.; Quan, Y.; Ma, R.; Zhang, Z.; Li, Y.; Wang, Q. Thermal stability and flammability of cotton fabric with TiO2 coatings based on biomineralization. Mater. Chem. Phys. 2022, 282, 125986. [Google Scholar] [CrossRef]
- Alongi, J.; Carosio, F.; Frache, A.; Malucelli, G. Layer by Layer coatings assembled through dipping, vertical or horizontal spray for cotton flame retardancy. Carbohydr. Polym. 2013, 92, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Wang, H.; Yao, Y.; Wang, R. Mg(OH)2 and PDMS-coated cotton fabrics for excellent oil/water separation and flame retardancy. Cellulose 2019, 26, 6879–6890. [Google Scholar] [CrossRef]
- Rajendran, V.; Dhineshbabu, N.; Kanna, R.R.; Kaler, K.V. Enhancement of thermal stability, flame retardancy, and antimicrobial properties of cotton fabrics functionalized by inorganic nanocomposites. Ind. Eng. Chem. Res. 2014, 53, 19512–19524. [Google Scholar] [CrossRef]
- Castellano, A.; Colleoni, C.; Iacono, G.; Mezzi, A.; Plutino, M.R.; Malucelli, G.; Rosace, G. Synthesis and characterization of a phosphorous/nitrogen based sol-gel coating as a novel halogen-and formaldehyde-free flame retardant finishing for cotton fabric. Polym. Degrad. Stab. 2019, 162, 148–159. [Google Scholar] [CrossRef]
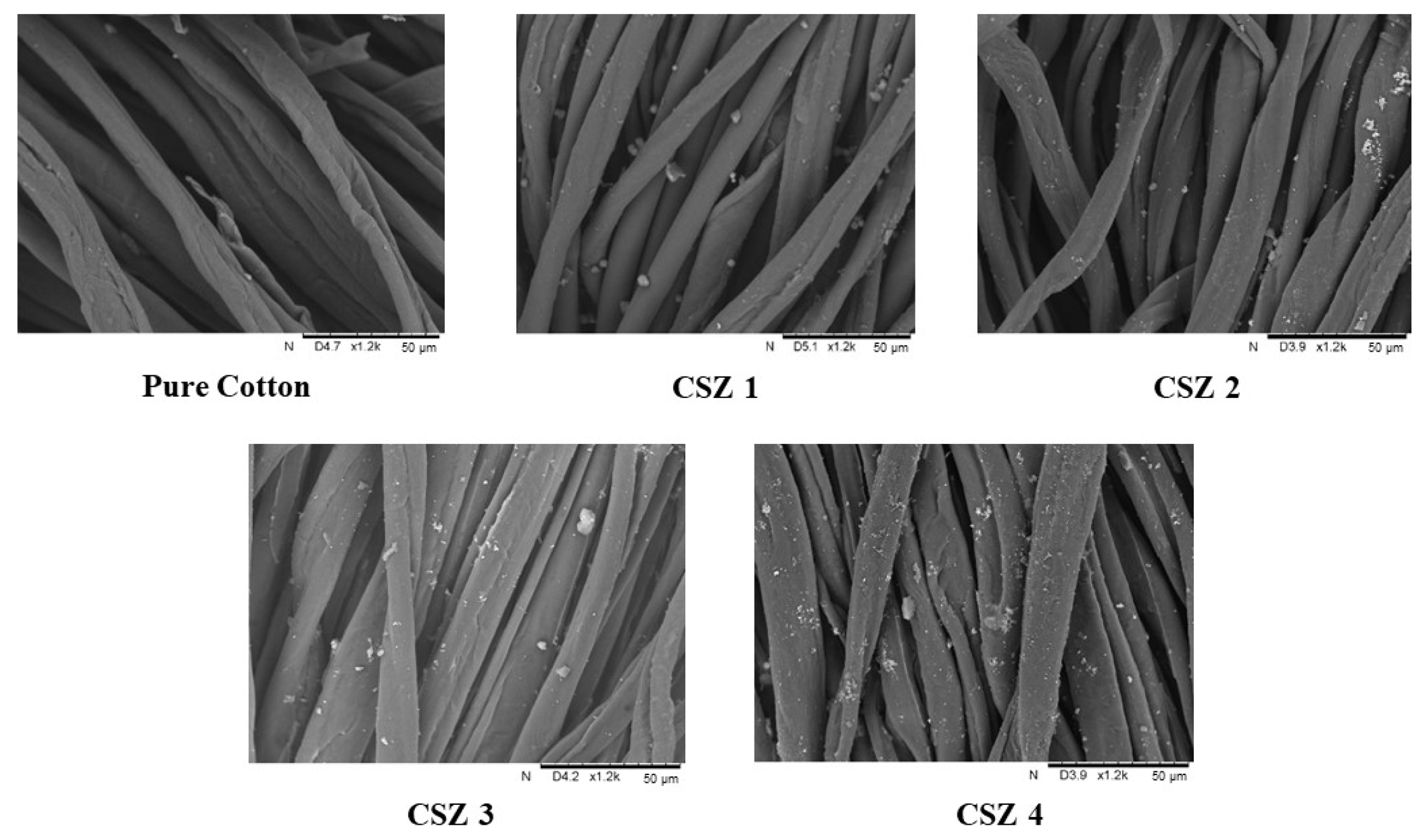







| No. | Samples | Silica (%) | Zinc Oxide (%) | Thickness (mm) | Fabric Structure | Add on (%) |
|---|---|---|---|---|---|---|
| 1 | Pure Cotton | 0 | 0 | 0.20 ± 0.03 | Plain weave | - |
| 2 | CSZ 1 | 0.25 | 0 | 0.24 ± 0.02 | 1.51 | |
| 3 | CSZ 2 | 0 | 0.25 | 0.25 ± 0.05 | 1.54 | |
| 4 | CSZ 3 | 0.25 | 0.25 | 0.27 ± 0.02 | 1.66 | |
| 5 | CSZ 4 | 0.5 | 0.5 | 0.30 ± 0.04 | 1.98 |
| Design | Design Model | Factors | Code-Level | Responses | No of Runs | |||
|---|---|---|---|---|---|---|---|---|
| Actual | Coded | −α | 0 | +α | ||||
| Central composite design (CCD) | Quadratic | Silica Content (%) | A | 0 | 0.25 | 0.50 | Response 1: Time to Ignite (s) Response 2: Flame Spread Timing (s) Response 3: Air Permeability (mL/s) | 13 |
| ZnO Content (%) | B | 0 | 0.25 | 0.50 | ||||
| Run | Factor 1 A: Silica Content (%) | Factor 2 B: ZnO Content (%) | Response 1 Time to Ignite (s) | Response 2 Flame Spread Timing (s) | Response 3: Air Permeability (mL/s) |
|---|---|---|---|---|---|
| 1 | 0 | 0.50 | 3 | 16 | 225 |
| 2 | 0.50 | 0.25 | 5 | 19 | 221.5 |
| 3 | 0 | 0 | 2 | 11 | 235 |
| 4 | 0.25 | 0.25 | 4 | 16 | 225.3 |
| 5 | 0.50 | 0.50 | 6 | 20 | 220 |
| 6 | 0.25 | 0.25 | 4.6 | 16 | 224.8 |
| 7 | 0.50 | 0 | 3 | 16 | 223 |
| 8 | 0 | 0.25 | 3 | 15 | 229 |
| 9 | 0.25 | 0.25 | 4.2 | 17 | 225.1 |
| 10 | 0.25 | 0.50 | 5 | 18 | 222 |
| 11 | 0.25 | 0.25 | 4 | 17 | 224.9 |
| 12 | 0.25 | 0.25 | 4.5 | 16 | 225.2 |
| 13 | 0.25 | 0 | 3 | 15 | 228 |
| No. | Samples | Water Content (%) | Wicking Length (mm) | Bending Length (mm) | Air Permeability (mL/s) | Tensile Strength (N) |
|---|---|---|---|---|---|---|
| 1 | Pure Cotton | 72 | 60 | 11 | 235 | 432.9 |
| 2 | CSZ 1 | 60.6 | 35 | 11.6 | 230 | 522.7 |
| 3 | CSZ 2 | 58.8 | 45 | 11.2 | 229 | 555.4 |
| 4 | CSZ 3 | 54.2 | 38 | 11.8 | 225 | 555.5 |
| 5 | CSZ 4 | 53.7 | 32 | 12.6 | 220 | 580.2 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 14.26 | 5 | 2.85 | 56.76 | <0.0001 | Significant |
| A-Silica Content | 6.00 | 1 | 6.00 | 119.41 | <0.0001 | |
| B-ZnO Content | 6.00 | 1 | 6.00 | 119.41 | <0.0001 | |
| AB | 1.00 | 1 | 1.00 | 19.90 | 0.0029 | |
| A2 | 0.3902 | 1 | 0.3902 | 7.77 | 0.0270 | |
| B2 | 0.3902 | 1 | 0.3902 | 7.77 | 0.0270 | |
| Lack of Fit | 0.0397 | 3 | 0.0132 | 0.1698 | 0.9115 | not significant |
| R2 | 0.9759 | |||||
| Adjusted R2 | 0.9587 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 52.17 | 2 | 26.08 | 56.67 | <0.0001 | significant |
| A-Silica Content | 28.17 | 1 | 28.17 | 61.20 | <0.0001 | |
| B-ZnO Content | 24.00 | 1 | 24.00 | 52.14 | <0.0001 | |
| Lack of Fit | 3.40 | 6 | 0.5671 | 1.89 | 0.2799 | not significant |
| R2 | 0.9189 | |||||
| Adjusted R2 | 0.9027 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 173.53 | 5 | 34.70 | 301.80 | <0.0001 | significant |
| A-Silica Content | 100.04 | 1 | 100.04 | 870 | <0.0001 | |
| B-ZnO Content | 60.17 | 1 | 60.17 | 523.23 | <0.0001 | |
| AB | 12.25 | 1 | 12.25 | 106.53 | <0.0001 | |
| A2 | 0.58 | 1 | 0.58 | 5.10 | 0.0587 | |
| B2 | 0.12 | 1 | 0.12 | 1.06 | 0.3369 | |
| Lack of Fit | 0.69 | 3 | 0.21 | 4.91 | 0.0792 | not significant |
| R2 | 0.9954 | |||||
| Adjusted R2 | 0.9921 |
| No. | Silica Content | ZnO Content | Time to Ignite (s) | Flame Spread Time (s) | Air Permeability (mL/s) | Desirability |
|---|---|---|---|---|---|---|
| 1 | 0.302 | 0.353 | 4.876 | 17.582 | 223.035 | 0.527 |
| No. | Time to Ignite (s) | Flame Spread Time (s) | Air Permeability (mL/s) |
|---|---|---|---|
| 1 | 4.84 | 17.49 | 223.53 |
| 2 | 4.77 | 17.33 | 221.87 |
| 3 | 4.81 | 17.41 | 224.63 |
| Analysis | Actual Mean | Actual Median | Std Dev | N | SE Pred | 95% PI low | Data Mean | 95% PI High |
|---|---|---|---|---|---|---|---|---|
| Time to Ignite (s) | 4.78402 | 4.78402 | 0.224157 | 3 | 0.159072 | 4.40787 | 4.80667 | 5.16016 |
| Flame Spread Time (s) | 17.3617 | 17.3617 | 0.678422 | 3 | 0.445898 | 16.3682 | 17.41 | 18.3552 |
| Air Permeability (mL/s) | 223.317 | 223.317 | 0.339104 | 3 | 0.240645 | 222.748 | 223.343 | 223.886 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleemi, S.; Mannan, H.A.; Riaz, T.; Hai, A.M.; Zeb, H.; Khan, A.K. Optimizing Synergistic Silica–Zinc Oxide Coating for Enhanced Flammability Resistance in Cotton Protective Clothing. Fibers 2024, 12, 44. https://doi.org/10.3390/fib12050044
Saleemi S, Mannan HA, Riaz T, Hai AM, Zeb H, Khan AK. Optimizing Synergistic Silica–Zinc Oxide Coating for Enhanced Flammability Resistance in Cotton Protective Clothing. Fibers. 2024; 12(5):44. https://doi.org/10.3390/fib12050044
Chicago/Turabian StyleSaleemi, Sidra, Hafiz Abdul Mannan, Tabinda Riaz, Abdul Moqeet Hai, Hassan Zeb, and Amber Khalil Khan. 2024. "Optimizing Synergistic Silica–Zinc Oxide Coating for Enhanced Flammability Resistance in Cotton Protective Clothing" Fibers 12, no. 5: 44. https://doi.org/10.3390/fib12050044






