Optimization of High-Density Fermentation Conditions for Saccharomycopsis fibuligera Y1402 through Response Surface Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Reagents
2.2. Medium Preparation
2.3. Yeast Activation and Basic Medium Selection
2.4. Optimization of Medium Ingredients
2.4.1. Single-Factor Design of Medium Ingredients
2.4.2. Plackett–Burman Design of Medium Ingredients
2.4.3. Steepest Ascent Test of Medium Ingredients
2.4.4. Response Surface Analysis of Medium Ingredients
2.5. Optimization of Fermentation Conditions
2.5.1. Single-Factor Design of Fermentation Conditions
2.5.2. Plackett–Burman Design of Fermentation Conditions
2.5.3. Steepest Ascent Test of Fermentation Conditions
2.5.4. Response Surface Analysis of Fermentation Conditions
2.6. Scaled-Up Fermentation in a 3 L Fermenter
2.7. Biomass Measurement
2.8. Data Analysis
3. Results and Discussion
3.1. Optimization of Medium Ingredients for S. fibuligera Y1402
3.1.1. Single-Factor Design of Medium Ingredients
3.1.2. Plackett–Burman Design of Medium Ingredients
3.1.3. Steepest Ascent Test of Medium Ingredients
3.1.4. Response Surface Analysis of Medium Ingredients
0.0534X32 − 0.000048X1X2 + 0.000840X1X3 − 0.00755X2X3
3.2. Optimization of Fermentation Conditions for S. fibuligera Y1402
3.2.1. Single-Factor Design of Fermentation Conditions
3.2.2. Plackett–Burman Design of Fermentation Conditions
3.2.3. Steepest Ascent Test of Fermentation Conditions
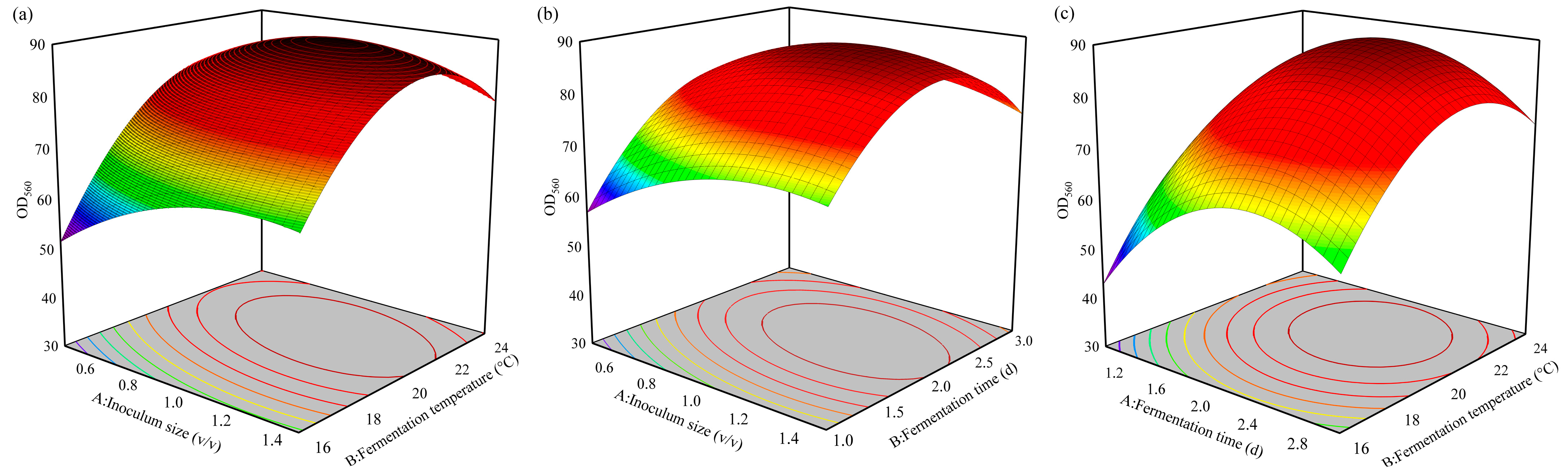
3.2.4. Response Surface Analysis of Fermentation Conditions
1.762X1X2 − 5.70X1X3 − 0.575X2X3
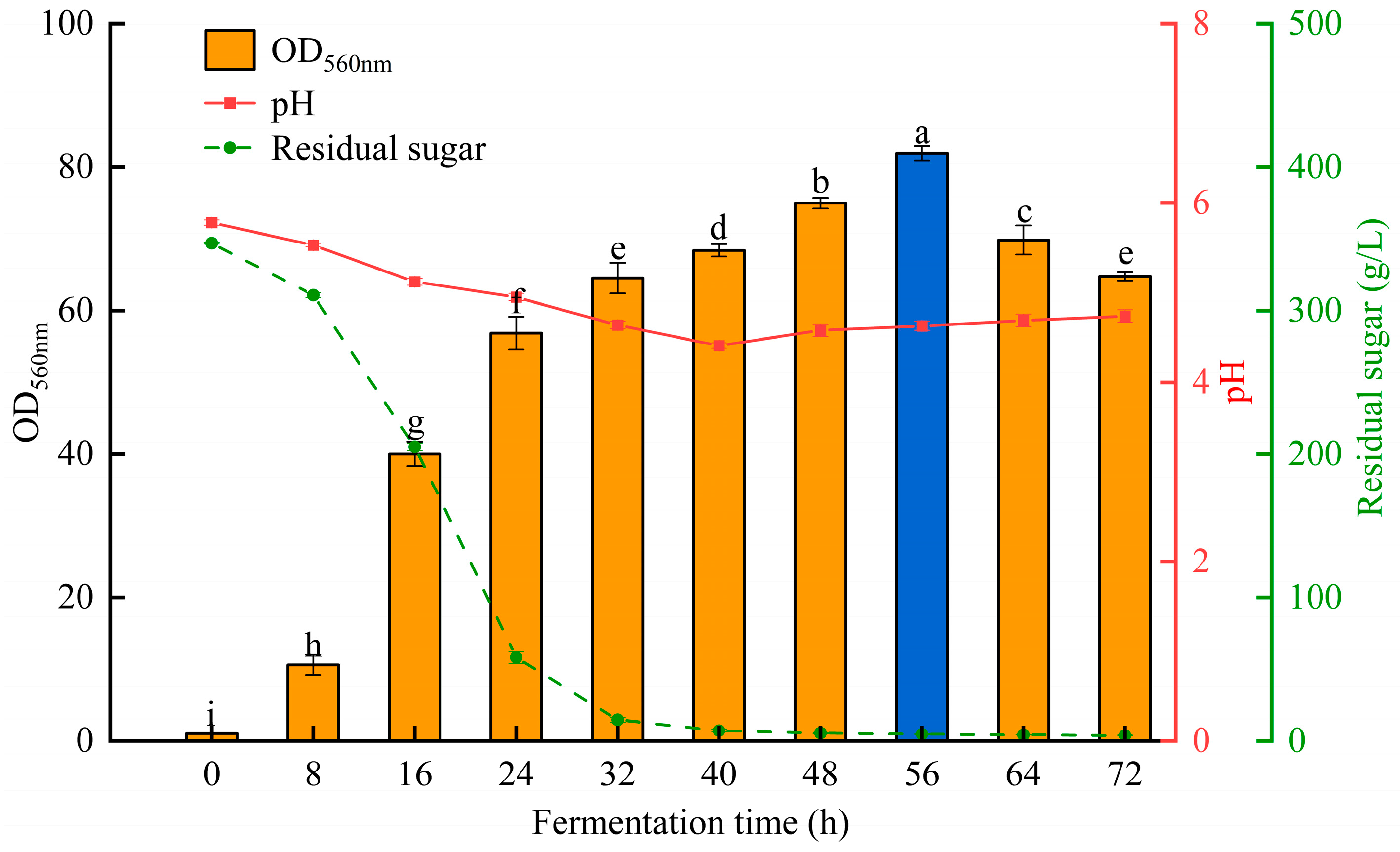
3.3. Scaled-Up Fermentation in a 3 L Fermenter
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chi, Z.M.; Chi, Z.; Liu, G.L.; Wang, F.; Ju, L.; Zhang, T. Saccharomycopsis fibuligera and its applications in biotechnology. Biotechnol. Adv. 2009, 27, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.B.; Zhang, K.Z.; Kang, Z.H.; Yang, J.G. Saccharomycopsis fibuligera in liquor production: A review. Eur. Food Res. Technol. 2021, 247, 1569–1577. [Google Scholar] [CrossRef]
- Hostinová, E. Amylolytic enzymes produced by the yeast Saccharomycopsis fibuligera. Biologia 2002, 57, 247–251. [Google Scholar]
- Natalia, D.; Vidilaseris, K.; Satrimafitrah, P.; Purkan, W.T.I.; Permentier, H.; Fibriansah, G.; Puspasari, F.; Nurachman, Z.; Dijkstra, B.W.; Soemitro, S. Biochemical characterization of a glucoamylase from Saccharomycopsis fibuligera r64. Biologia 2011, 66, 27–32. [Google Scholar] [CrossRef]
- Yu, X.J.; Li, H.J.; Li, J.; Chi, Z.M. Overexpression of acid protease of Saccharomycopsis fibuligera in Yarrowia lipolytica and characterization of the recombinant acid protease for skimmed milk clotting. Biotechnol. Bioproc. Eng. 2010, 15, 467–475. [Google Scholar] [CrossRef]
- Lee, S.M.; Jung, J.H.; Seo, J.A.; Kim, Y.S. Bioformation of volatile and nonvolatile metabolites by Saccharomycopsis fibuligera kjj81 cultivated under different conditions-carbon sources and cultivation times. Molecules 2018, 23, 2762. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.R.; Zhong, H.Y.; Yang, T.; Lan, C.H.; Zhu, H. Characterization of the key aroma compounds of a sweet rice alcoholic beverage fermented with Saccharomycopsis fibuligera. J. Food Sci. Technol. 2021, 58, 3752–3764. [Google Scholar] [CrossRef]
- Son, E.Y.; Lee, S.M.; Kim, M.; Seo, J.A.; Kim, Y.S. Comparison of volatile and non-volatile metabolites in rice wine fermented by koji inoculated with Saccharomycopsis fibuligera and Aspergillus oryzae. Food Res. Int. 2018, 109, 596–605. [Google Scholar] [CrossRef]
- Zheng, X.W.; Yan, Z.; Nout, M.J.R.; Boekhout, T.; Han, B.Z.; Zwietering, M.H.; Smid, E.J. Characterization of the microbial community in different types of Daqu samples as revealed by 16S rRNA and 26S rRNA gene clone libraries. World J. Microbiol. Biotechnol. 2015, 31, 199–208. [Google Scholar] [CrossRef]
- Nie, Z.Q.; Zheng, Y.; Du, H.U.; Xie, S.K.; Wang, M. Dynamics and diversity of microbial community succession in traditional fermentation of Shanxi aged vinegar. Food Microbiol. 2015, 47, 62–68. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Wu, C.D.; Ding, X.F.; Zheng, J.; Zhou, R.Q. Characterisation of microbial communities in Chinese liquor fermentation starters Daqu using nested PCR-DGGE. World J. Microbiol. Biotechnol. 2014, 30, 3055–3063. [Google Scholar] [CrossRef]
- Li, K.; Zhang, Q.; Zhong, X.T.; Jia, B.H.; Yuan, C.H.; Liu, S.; Che, Z.M.; Xiang, W.L. Microbial diversity and succession in the Chinese Luzhou-flavor liquor fermenting cover lees as evaluated by SSU rRNA profiles. Indian. J. Microbiol. 2013, 53, 425–431. [Google Scholar] [CrossRef]
- Zheng, X.W.; Yan, Z.; Nout, M.J.R.; Smid, E.J.; Zwietering, M.H.; Boekhout, T.; Han, J.S.; Han, B.Z. Microbiota dynamics related to environmental conditions during the fermentative production of Fen-Daqu, a Chinese industrial fermentation starter. Int. J. Food Microbiol. 2014, 182, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.C.; Weng, X.; Zhang, W.; Rao, P.F.; Ni, L. Microbial diversity of traditional fermentation starters for Hong Qu glutinous rice wine as determined by PCR-mediated DGGE. Food Control 2012, 28, 426–434. [Google Scholar] [CrossRef]
- Jin, G.Y.; Zhu, Y.; Xu, Y. Mystery behind Chinese liquor fermentation. Trends Food Sci. Technol. 2017, 63, 18–28. [Google Scholar] [CrossRef]
- Zou, W.; Zhao, C.Q.; Luo, H.B. Diversity and function of microbial community in Chinese strong-flavor Baijiu ecosystem: A review. Front. Microbiol. 2018, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.Y.; Kim, H.J.; Kim, K.S.; Yoo, S.J.; Lee, D.W.; Shin, H.J.; Seo, J.A.; Kang, H.A. Molecular characterization of the Saccharomycopsis fibuligera ATF genes, encoding alcohol acetyltransferase for volatile acetate ester formation. J. Microbiol. 2021, 59, 598–608. [Google Scholar] [CrossRef]
- Farh, M.E.A.; Abdellaoui, N.; Seo, J.A. pH changes have a profound effect on gene expression, hydrolytic enzyme production, and dimorphism in Saccharomycopsis fibuligera. Front. Microbiol. 2021, 12, 672661. [Google Scholar] [CrossRef]
- Cao, T.S.; Chi, Z.; Liu, G.L.; Chi, Z.M. Expression of TPS1 gene from Saccharomycopsis fibuligera A11 in Saccharomyces sp. W0 enhances trehalose accumulation, ethanol tolerance, and ethanol production. Mol. Biotechnol. 2014, 56, 72–78. [Google Scholar] [CrossRef]
- Zhang, X.W.; Peng, S.; Gao, P.P.; Liang, L.H.; Ma, Y.W.; Wang, J. Study on high cell-density culture of Hanseniaspora uvarum BF-345. Food Ferment. Sci. Technol. 2022, 58, 9–18. [Google Scholar] [CrossRef]
- Sun, Y.J.; Huang, H.; Liu, Z.G.; Xiong, T. Optimization of medium composition and culture conditions of Lactobacillus plantarum ncu137. J. Chin. Inst. Food Sci. Technol. 2022, 22, 223–233. [Google Scholar] [CrossRef]
- Li, Y.; Ma, L. Optimization of high density culture condition of lactic acid bacteria. China Brew. 2021, 40, 150–154. [Google Scholar] [CrossRef]
- Zheng, M.; Deng, Z.Y.; Ren, Z.Q.; Li, H.Y. Studies on the high density cultivation of Issatchenkia orientalis. J. Chin. Inst. Food Sci. Technol. 2016, 16, 96–103. [Google Scholar] [CrossRef]
- Ma, M.R.; Liu, X.G.; Li, H.Y.; Zhou, L.Y.; Shan, Q.; Wang, X.W.; Zhang, K. The application of two kinds of Saccharomycopsis fibuligera in mild-flavour Fuqu Baijiu production. Sci. Technol. Food Ind. 2021, 42, 133–139. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Sun, Q.; Liu, X.Y.; Basit, R.A.; Ma, J.H.; Fu, Z.L.; Cheng, L.J.; Fan, G.S.; Teng, C. Screening, identification, and fermentation condition optimization of a high-yield 3-methylthiopropanol yeast and its aroma-producing characteristics. Foods 2024, 13, 418. [Google Scholar] [CrossRef] [PubMed]
- Pepi, M.; Heipieper, H.J.; Fischer, J.; Ruta, M.; Volterrani, M.; Focardi, S.E. Membrane fatty acids adaptive profile in the simultaneous presence of arsenic and toluene in Bacillus sp. ORAs2 and Pseudomonas sp. ORAs5 strains. J. Extremophiles. 2008, 12, 343–349. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhao, Z.B.; Bai, F.W. High-density cultivation of oleaginous yeast Rhodosporidium toruloides Y4 in fed-batch culture. Enzyme Microb. Technol. 2007, 41, 312–317. [Google Scholar] [CrossRef]
- Jahic, M.; Rotticci-Mulder, J.C.; Martinelle, M.; Hul, K.; Enfors, S.O. Modeling of growth and energy metabolism of Pichia pastoris producing a fusion protein. Bioproc. Biosyst. Eng. 2002, 24, 385–393. [Google Scholar] [CrossRef]
- Sohoni, S.V.; Nelapati, D.; Sathe, S.; Javadekar-Subhedar, V.; Gaikaiwari, R.P.; Wangikar, P.P. Optimization of high cell density fermentation process for recombinant nitrilase production in E. coli. Bioresour. Technol. 2015, 188, 202–208. [Google Scholar] [CrossRef]
- Fan, G.S.; Liu, P.X.; Chang, X.; Yin, H.; Cheng, L.J.; Teng, C.; Gong, Y.; Li, X.T. Isolation and identification of a high-yield ethyl caproate-producing yeast from Daqu and optimization of its fermentation. Front. Microbiol. 2021, 12, 663744. [Google Scholar] [CrossRef]
- Park, J.B.; Choi, J.S.; Park, H.W.; Lee, S.B.; Park, H.D. Development of Korean indigenous low-temperature-tolerant yeast for enhancing the flavor of Yakju. Fermentation 2021, 7, 260. [Google Scholar] [CrossRef]
- Gonzalez, C.F.; Farina, J.I.; de Figueroa, L.I.C. Optimized amylolytic enzymes production in Saccharomycopsis fibuligera DSM-70554—An approach to efficient cassava starch utilization. Enzyme Microb. Technol. 2008, 42, 272–277. [Google Scholar] [CrossRef]
- Liang, L.K.; Chi, Z.M.; Wang, X.K.; Zhu, K.L. Properties of trehalose-6-phosphate synthase from Saccharomycopsis fibuligera. Ann. Microbiol. 2006, 56, 241–245. [Google Scholar] [CrossRef]
- Gupta, P.L.; Choi, H.J.; Pawar, R.R.; Jung, S.P.; Lee, S.M. Enhanced biomass production through optimization of carbon source and utilization of wastewater as a nutrient source. J. Environ. Manag. 2016, 184, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.X.; Gao, Y.; Zhou, J.; Wei, L.J.; Chen, J.; Hua, Q. Process optimization with alternative carbon sources and modulation of secondary metabolism for enhanced ansamitocin P-3 production in Actinosynnema pretiosum. J. Biotechnol. 2014, 192, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.B.; Eksriwong, T.; Chauvatcharin, S.; Zhong, J.J. Optimization of carbon source and carbon/nitrogen ratio for cordycepin production by submerged cultivation of medicinal mushroom Cordyceps militaris. Process Biochem. 2005, 40, 1667–1672. [Google Scholar] [CrossRef]
- Fan, G.S.; Cheng, L.J.; Fu, Z.L.; Sun, B.G.; Teng, C.; Jiang, X.Y.; Li, X.T. Screening of yeasts isolated from Baijiu environments for 2-phenylethanol production and optimization of production conditions. 3 Biotech 2020, 10, 275. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Li, X.; Chen, X.; Wang, Z. Optimization of medium components and fermentation conditions of Zygosaccharomyces rouxii. China Condiment. 2015, 40, 61–65. [Google Scholar] [CrossRef]
- Law, B.A.; Sezgin, E.; Sharpe, M.E. Amino acid nutrition of some commercial cheese starters in relation to their growth in peptone-supplemented whey media. J. Dairy Res. 1976, 43, 291–300. [Google Scholar] [CrossRef]
- Liu, Z.M.; Li, N.; Xiang, X.Y.; Xue, X.L.; Wang, D.P. Screening and identification of a high-yielding β-phenylethanolic yeast and optimization of its proliferation and culture conditions. Food Res. Dev. 2022, 43, 171–179. [Google Scholar] [CrossRef]
- Huang, Z.R.; Wang, Y.; Cui, P.J.; Malina, K.; Bao, Y.H. Optimization of culture conditions for zinc-enriched yeast by response surface methodology. China Brew. 2020, 39, 97–102. [Google Scholar] [CrossRef]
- Yang, S.C.; Lv, X.L.; Miu, Y. Study on optimization of ethanol fermentation medium by using response surface method. J. Anhui Agr. Sci. 2010, 38, 13296–13299. [Google Scholar] [CrossRef]
- Wang, L.; Hu, M.; Wang, J.Y.; Gong, X.F.; Liu, P.L.; Zhan, T.; Li, Y. Optimization of high density fermentation conditions of Kluyveromyces marxianus. Sci. Technol. Food Ind. 2017, 38, 111–118+124. [Google Scholar] [CrossRef]
- Jorgensen, H. Effect of nutrients on fermentation of pretreated wheat straw at very high dry matter content by Saccharomyces cerevisiae. Appl. Biochem. Biotech. 2009, 153, 44–57. [Google Scholar] [CrossRef]
- Xue, C.; Zhao, X.Q.; Yuan, W.J.; Bai, F.W. Improving ethanol tolerance of a self-flocculating yeast by optimization of medium composition. World J. Microbiol. Biotechnol. 2008, 24, 2257–2261. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Xue, C.; Ge, X.M.; Yuan, W.J.; Wang, J.Y.; Bai, F.W. Impact of zinc supplementation on the improvement of ethanol tolerance and yield of self-flocculating yeast in continuous ethanol fermentation. J. Biotechnol. 2009, 139, 55–60. [Google Scholar] [CrossRef]
- Palukurty, M.A.; Telgana, N.K.; Bora, H.S.R.; Mulampaka, S.N. Screening and optimization of metal ions to enhance ethanol production using statistical experimental designs. Afr. J. Microbiol. Res. 2008, 2, 87–94. [Google Scholar] [CrossRef]
- Hu, C.K.; Bai, F.W.; An, L.J. Enhancing ethanol tolerance of a self-flocculating fusant of Schizosaccharomyces pombe and Saccharomyces cerevisiae by Mg2+ via reduction in plasma membrane permeability. Biotechnol. Lett. 2003, 25, 1191–1194. [Google Scholar] [CrossRef]
- Birch, R.M.; Walker, G.M. Influence of magnesium ions on heat shock and ethanol stress responses of Saccharomyces cerevisiae. Enzyme Microb. Technol. 2000, 26, 678–687. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, Y.F.; Lv, J.C.; Liu, C.F. Effect of Cu2+ on CAT, SOD, GSH-Px of Paralichthys olivaceus. J. Huazhong Agric. Univ. 2007, 26, 836–838. [Google Scholar] [CrossRef]
- Wang, T.Y.; Que, F.; Li, F.F.; Li, J.Y. The influence of copper ions on energy metabolism and cell membrane function of Saccharomyce ceremony. Food Sci. Technol. 2017, 42, 12–16. [Google Scholar] [CrossRef]
- Georgatsou, E.; Mavrogiannis, L.A.; Fragiadakis, G.S.; Alexandraki, D. The yeast Fre1p/Fre2p cupric reductases facilitate copper uptake and are regulated by the copper-modulated Mac1p activator. J. Biol. Chem. 1997, 272, 13786–13792. [Google Scholar] [CrossRef]
- Hashem, M.; Alamri, S.A.; Asseri, T.A.Y.; Mostafa, Y.S.; Lyberatos, G.; Ntaikou, I. On the optimization of fermentation conditions for enhanced bioethanol yields from starchy biowaste via yeast co-cultures. Sustainability 2021, 13, 1890. [Google Scholar] [CrossRef]
- Liu, P.X.; Chang, X.; Cheng, L.J.; Ding, Z.; Gong, Y.; Li, X.T.; Fan, G.S. Optimization of fermentation conditions for ethyl caproate production from Saccharomyces cerevisiae Y3401. J. Chin. Inst. Food Sci. Technol. 2022, 22, 178–189. [Google Scholar] [CrossRef]
- Matthew, K.P.; Sun, J.C.; Liu, S.Q. Optimization of L-methionine bioconversion to aroma-active methionol by Kluyveromyces lactis using the Taguchi method. J. Food Res. 2013, 2, 90. [Google Scholar] [CrossRef]
- Ma, J.H.; Cheng, L.J.; Zhang, Y.J.; Liu, Y.C.; Sun, Q.; Zhang, J.; Liu, X.Y.; Fan, G.S. Screening of yeasts isolated from Baijiu environments for producing 3-methylthio-1-propanol and optimizing production conditions. Foods 2022, 11, 3616. [Google Scholar] [CrossRef]
- Reddy, L.V.A.; Reddy, O.V.S. Effect of fermentation conditions on yeast growth and volatile composition of wine produced from mango (Mangifera indica L.) fruit juice. Food Bioprod. Process 2011, 89, 487–491. [Google Scholar] [CrossRef]
- Zhang, C.R.; Gao, R.J.; Miao, L.H.; Liu, P.L.; Liao, W.F. Screening and medium optimization of Saccharomycopsis fibuligera with high-yield acid. China Brew. 2021, 40, 153–159. [Google Scholar] [CrossRef]



| Ingredient | Basic Medium | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Glucose | 50 | - | 50 | 70 | 50 | 40 | 40 | - | 100 |
| Sucrose | - | 76 | - | 20 | - | - | - | 67 | - |
| Maltose | - | - | - | 20 | - | - | - | - | - |
| Peptone | - | - | - | - | - | 45 | 40 | - | 10 |
| Tryptone | - | 36 | 20 | 16 | 30 | - | - | - | - |
| Yeast extract | - | - | 10 | 15 | - | - | - | 30 | 10 |
| Urea | 5 | - | - | - | - | - | - | - | - |
| Glycine | 0.5 | - | - | - | - | - | - | - | - |
| (NH4)2SO4 | 0.5 | 1 | 5 | - | - | - | - | - | - |
| KH2PO4 | - | 2 | 1 | 1 | 1.2 | 0.6 | 3.9 | 4.13 | 2 |
| K2HPO4 | - | - | - | 1 | - | - | - | - | - |
| MgSO4 | - | 1.5 | - | 5 | 0.02 | 0.8 | 0.3 | 0.4 | |
| KCl | - | - | 0.4 | - | - | - | - | - | - |
| CaCl2 | - | - | 1.1 | - | - | - | - | - | - |
| FeCl3 | - | - | 0.0025 | - | - | - | - | - | - |
| ZnSO4 | - | - | - | 0.01 | 0.002 | - | - | - | - |
| FeSO4 | - | - | - | 0.006 | 0.002 | - | - | - | - |
| CuSO4 | - | - | - | 0.01 | 0.01 | - | - | - | 0.001 |
| KNO3 | - | - | - | - | - | 14 | - | - | - |
| Corn plasm | - | - | - | - | - | 15 | - | 16 | - |
| Yeast nutrient | - | - | - | - | - | 3 | - | - | - |
| Factor | Level |
|---|---|
| Glucose concentration (g/L) | 100, 200, 300, 400 and 500 |
| Yeast extract concentration (g/L) | 10, 20, 30, 40, 50 and 60 |
| Peptone concentration (g/L) | 10, 20, 30, 40, 50 and 60 |
| KH2PO4 concentration (g/L) | 1, 2, 4, 8 and 10 |
| MgSO4 concentration (g/L) | 0.4, 1, 2, 4 and 6 |
| CuSO4 concentration (g/L) | 0.01, 0.05, 0.1, 0.5 and 1 |
| Initial pH | 4.5, 5.0, 5.5, 6.0, 6.5 and 7.0 |
| Inoculum size (v/v) | 0.5%, 1%, 2%, 4%, 6%, 8% and 10% |
| Liquid volume (mL/250 mL) | 12.5, 25, 37.5, 50 and 62.5 |
| Rotation speed (r/min) | 90, 120, 150, 180 and 200 |
| Fermentation temperature (°C) | 15, 20, 25, 30, 35 and 40 |
| Fermentation time (d) | 1, 2, 3, 4, 5, 6 and 7 |
| Test Number | X1 | X2 | X3 | X4 | X5 | X6 | OD560nm |
|---|---|---|---|---|---|---|---|
| 1 | 1 | −1 | 1 | −1 | −1 | −1 | 40 |
| 2 | 1 | 1 | −1 | 1 | 1 | −1 | 36.5 |
| 3 | −1 | −1 | −1 | −1 | −1 | −1 | 41.95 |
| 4 | 1 | −1 | 1 | 1 | −1 | 1 | 25 |
| 5 | −1 | −1 | 1 | 1 | 1 | −1 | 30 |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | 43.5 |
| 7 | 0 | 0 | 0 | 0 | 0 | 0 | 44 |
| 8 | 1 | 1 | −1 | 1 | −1 | −1 | 40.83 |
| 9 | −1 | 1 | −1 | −1 | −1 | 1 | 25 |
| 10 | 1 | 1 | 1 | −1 | 1 | 1 | 41 |
| 11 | −1 | 1 | 1 | −1 | 1 | −1 | 35 |
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | 41.5 |
| 13 | −1 | 1 | 1 | 1 | −1 | 1 | 35 |
| 14 | 1 | −1 | −1 | −1 | 1 | 1 | 41.63 |
| 15 | −1 | −1 | −1 | 1 | 1 | 1 | 36 |
| Test Number | Variable | OD560nm | ||
|---|---|---|---|---|
| Glucose Concentration (g/L) | Yeast Extract Concentration (g/L) | KH2PO4 Concentration (g/L) | ||
| 1 | 200 | 30 | 2 | 33.37 |
| 2 | 250 | 25 | 3 | 38.12 |
| 3 | 300 | 20 | 4 | 40.10 |
| 4 | 350 | 15 | 5 | 46.68 |
| 5 | 400 | 10 | 6 | 39.90 |
| Test Number | Glucose Concentration (g/L) | Yeast Extract Concentration (g/L) | KH2PO4 Concentration (g/L) | OD560nm | |||
|---|---|---|---|---|---|---|---|
| X1 | Code X1 | X2 | Code X2 | X3 | Code X3 | Y | |
| 1 | 300 | −1 | 15 | 0 | 4 | −1 | 39.61 |
| 2 | 400 | 1 | 15 | 0 | 4 | −1 | 40.53 |
| 3 | 350 | 0 | 20 | 1 | 4 | −1 | 48.52 |
| 4 | 350 | 0 | 20 | 1 | 6 | 1 | 47.45 |
| 5 | 300 | −1 | 20 | 1 | 5 | 0 | 40.75 |
| 6 | 350 | 0 | 15 | 0 | 5 | 0 | 53.24 |
| 7 | 400 | 1 | 10 | −1 | 5 | 0 | 44.15 |
| 8 | 350 | 0 | 10 | −1 | 4 | −1 | 42.17 |
| 9 | 400 | 1 | 20 | 1 | 5 | 0 | 42.25 |
| 10 | 300 | −1 | 10 | −1 | 5 | 0 | 40.26 |
| 11 | 350 | 0 | 15 | 0 | 5 | 0 | 51.45 |
| 12 | 350 | 0 | 15 | 0 | 5 | 0 | 53.25 |
| 13 | 350 | 0 | 10 | −1 | 6 | 1 | 48.60 |
| 14 | 350 | 0 | 15 | 0 | 5 | 0 | 53.14 |
| 15 | 350 | 0 | 15 | 0 | 5 | 0 | 53.40 |
| 16 | 300 | −1 | 15 | 0 | 6 | 1 | 41.09 |
| 17 | 400 | 1 | 15 | 0 | 6 | 1 | 50.41 |
| Test Number | X1 | X2 | X3 | X4 | X5 | OD560nm |
|---|---|---|---|---|---|---|
| 1 | 5 | 1.5 | 150 | 15 | 3 | 65.28 |
| 2 | 7 | 0.5 | 90 | 25 | 3 | 65.50 |
| 3 | 7 | 1.5 | 90 | 25 | 3 | 71.77 |
| 4 | 6 | 1 | 120 | 20 | 2 | 89.30 |
| 5 | 7 | 0.5 | 150 | 15 | 1 | 2.37 |
| 6 | 5 | 0.5 | 150 | 25 | 3 | 60.17 |
| 7 | 7 | 1.5 | 90 | 15 | 1 | 6.46 |
| 8 | 6 | 1 | 120 | 20 | 2 | 87.63 |
| 9 | 7 | 1.5 | 150 | 15 | 3 | 89.96 |
| 10 | 5 | 1.5 | 90 | 25 | 1 | 56.33 |
| 11 | 5 | 0.5 | 90 | 15 | 1 | 3.38 |
| 12 | 7 | 0.5 | 150 | 25 | 1 | 75.20 |
| 13 | 6 | 1 | 120 | 20 | 2 | 87.73 |
| 14 | 5 | 0.5 | 90 | 15 | 3 | 63.26 |
| 15 | 5 | 1.5 | 150 | 25 | 1 | 70.24 |
| Test Number | Variable | OD560nm | ||
|---|---|---|---|---|
| Inoculum Size (v/v) | Fermentation Temperature (°C) | Fermentation Time (h) | ||
| 1 | 0.50 | 15.0 | 24 | 23.78 |
| 2 | 0.75 | 17.5 | 36 | 37.82 |
| 3 | 1.00 | 20.0 | 48 | 86.40 |
| 4 | 1.25 | 22.5 | 60 | 83.77 |
| 5 | 1.50 | 25.0 | 72 | 66.10 |
| Test Number | Inoculum Size (v/v) | Fermentation Temperature (°C) | Fermentation Time (h) | OD560nm | |||
|---|---|---|---|---|---|---|---|
| X1 | Code X1 | X2 | Code X2 | X3 | Code X3 | Y | |
| 1 | 1.0 | 0 | 20 | 0 | 2 | 0 | 87.3 |
| 2 | 1.0 | 0 | 20 | 0 | 2 | 0 | 87.8 |
| 3 | 0.5 | −1 | 20 | 0 | 3 | 1 | 74.6 |
| 4 | 1.0 | 0 | 20 | 0 | 2 | 0 | 87.7 |
| 5 | 1.5 | 1 | 16 | −1 | 2 | 0 | 67.3 |
| 6 | 0.5 | −1 | 24 | 1 | 2 | 0 | 76.4 |
| 7 | 1.0 | 0 | 20 | 0 | 2 | 0 | 88.0 |
| 8 | 0.5 | −1 | 16 | −1 | 2 | 0 | 53.8 |
| 9 | 1.0 | 0 | 20 | 0 | 2 | 0 | 87.9 |
| 10 | 1.5 | 1 | 24 | 1 | 2 | 0 | 75.8 |
| 11 | 1.5 | 1 | 20 | 0 | 1 | −1 | 68.7 |
| 12 | 1.0 | 0 | 16 | −1 | 1 | −1 | 42.3 |
| 13 | 1.0 | 0 | 16 | −1 | 3 | 1 | 55.4 |
| 14 | 1.0 | 0 | 24 | 1 | 1 | −1 | 70.2 |
| 15 | 1.5 | 1 | 20 | 0 | 3 | 1 | 76.5 |
| 16 | 1.0 | 0 | 24 | 1 | 3 | 1 | 74.2 |
| 17 | 0.5 | −1 | 20 | 0 | 1 | −1 | 55.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, H.; Sun, Q.; Wang, L.; Fu, Z.; Zhou, T.; Ma, J.; Liu, X.; Fan, G.; Teng, C. Optimization of High-Density Fermentation Conditions for Saccharomycopsis fibuligera Y1402 through Response Surface Analysis. Foods 2024, 13, 1546. https://doi.org/10.3390/foods13101546
Yuan H, Sun Q, Wang L, Fu Z, Zhou T, Ma J, Liu X, Fan G, Teng C. Optimization of High-Density Fermentation Conditions for Saccharomycopsis fibuligera Y1402 through Response Surface Analysis. Foods. 2024; 13(10):1546. https://doi.org/10.3390/foods13101546
Chicago/Turabian StyleYuan, Hongyang, Qi Sun, Lanshuang Wang, Zhilei Fu, Tianze Zhou, Jinghao Ma, Xiaoyan Liu, Guangsen Fan, and Chao Teng. 2024. "Optimization of High-Density Fermentation Conditions for Saccharomycopsis fibuligera Y1402 through Response Surface Analysis" Foods 13, no. 10: 1546. https://doi.org/10.3390/foods13101546





