Titanium Dioxide Nanoparticles Induce Maternal Preeclampsia-like Syndrome and Adverse Birth Outcomes via Disrupting Placental Function in SD Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Culture
2.3. TiO2 NPs Preparation and Exposure Design
2.4. Tissue Collection and Preparation
2.5. Histopathological Analysis and Immunohistochemical Analysis
2.6. Placenta Invasion Ability Assessment
2.7. Immunofluorescence Analysis
2.8. Cell Invasion and Migration Ability Analysis
2.9. Maternal Blood Pressure Monitoring
2.10. Determination of Proteinuria
2.11. Statistical Analysis
3. Results
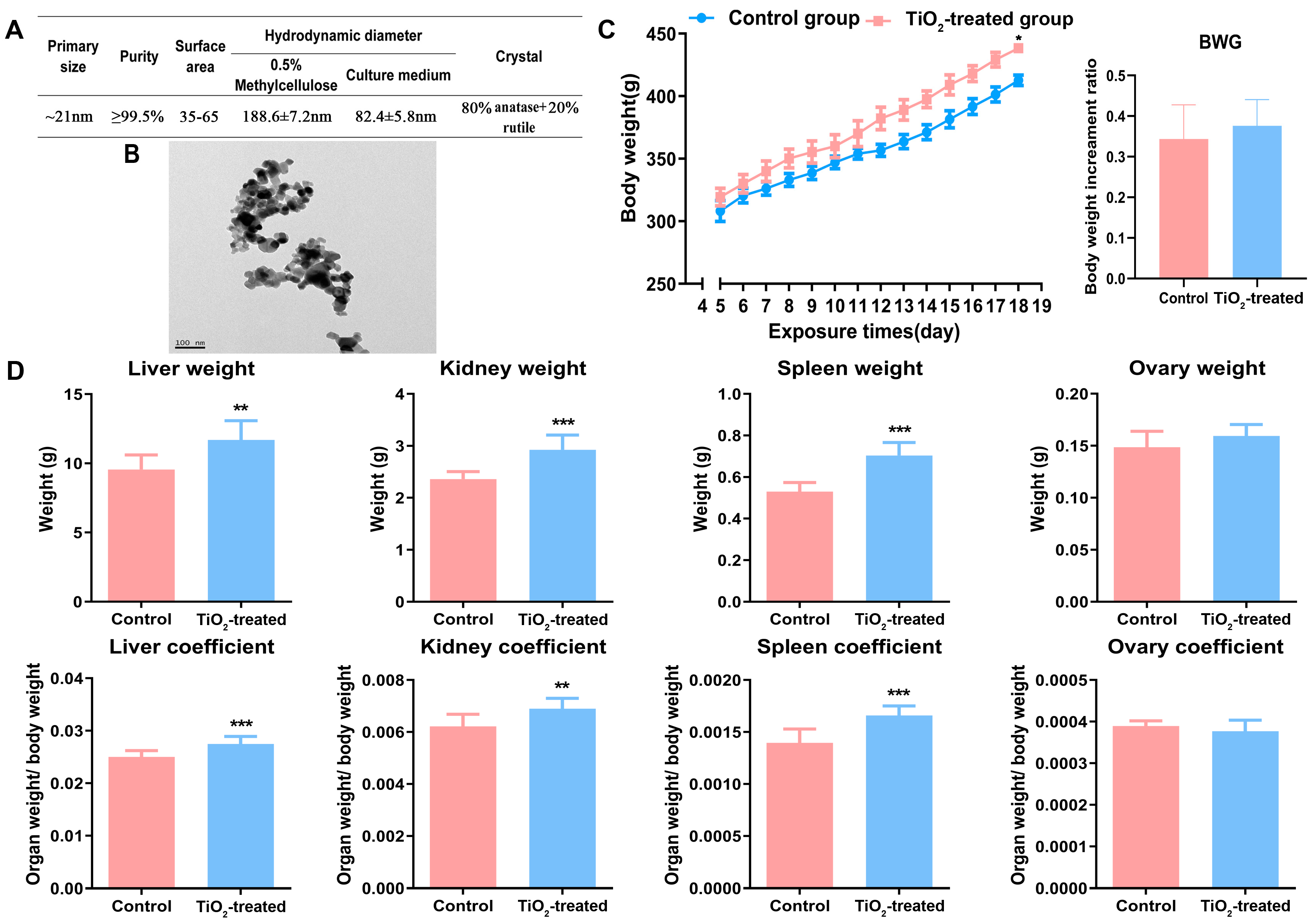
3.1. Main Characteristics of TiO2 NPs
3.2. Effects of TiO2 NPs on Maternal Conditions
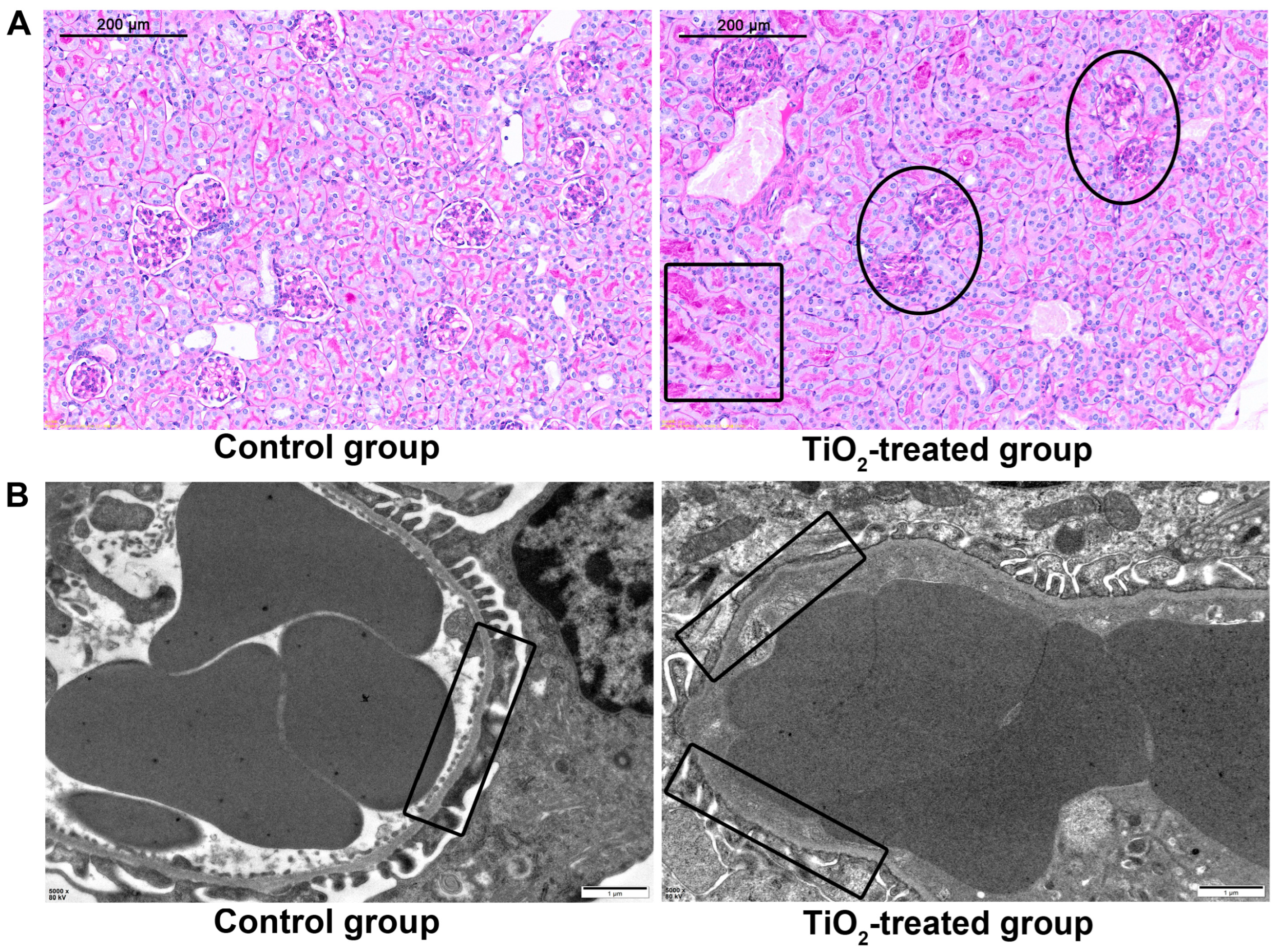
3.3. Pathological Changes of Maternal Organs after TiO2 NPs Exposure
3.4. Effects of TiO2 NPs on Fetal Birth Outcomes
3.5. TiO2 NPs Increased Maternal Mean Arterial Pressure (MAP)
3.6. TiO2 NPs Induced Maternal Proteinuria
3.7. Effects of TiO2 NPs on Placental Infiltration into Uterus
3.8. Effects of TiO2 NPs on Maternal Glomerular Basement Membrane (GBM)
3.9. Effects of TiO2 NPs on the Migration and Invasion Ability of Human Trophoblastic Cells
3.10. Effects of TiO2 NPs on the Autophagy of Human Trophoblastic Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baranowska-Wojcik, E.; Szwajgier, D.; Oleszczuk, P.; Winiarska-Mieczan, A. Effects of Titanium Dioxide Nanoparticles Exposure on Human Health—A Review. Biol. Trace Elem. Res. 2019, 193, 118–129. [Google Scholar] [CrossRef]
- Warheit, D.B.; Donner, E.M. Risk assessment strategies for nanoscale and fine-sized titanium dioxide particles: Recognizing hazard and exposure issues. Food Chem. Toxicol. 2015, 85, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Gulson, B.; McCall, M.J.; Bowman, D.M.; Pinheiro, T. A review of critical factors for assessing the dermal absorption of metal oxide nanoparticles from sunscreens applied to humans, and a research strategy to address current deficiencies. Arch. Toxicol. 2015, 89, 1909–1930. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef]
- Takeda, K.; Shinkai, Y.; Suzuki, K.; Yanagita, S.; Umezawa, M.; Yokota, S.; Tainaka, H.; Oshio, S.; Ihara, T.; Sugamata, M. Health effects of nanomaterials on next generation. Yakugaku Zasshi 2011, 131, 229–236. [Google Scholar] [CrossRef]
- Warheit, D.B. Hazard and risk assessment strategies for nanoparticle exposures: How far have we come in the past 10 years? F1000Research 2018, 7, 376. [Google Scholar] [CrossRef]
- Grande, F.; Tucci, P. Titanium Dioxide Nanoparticles: A Risk for Human Health? Mini Rev. Med. Chem. 2016, 16, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Naserzadeh, P.; Ghanbary, F.; Ashtari, P.; Seydi, E.; Ashtari, K.; Akbari, M. Biocompatibility assessment of titanium dioxide nanoparticles in mice fetoplacental unit. J. Biomed. Mater. Res. A 2018, 106, 580–589. [Google Scholar] [CrossRef]
- Hong, F.; Zhou, Y.; Ji, J.; Zhuang, J.; Sheng, L.; Wang, L. Nano-TiO2 Inhibits Development of the Central Nervous System and Its Mechanism in Offspring Mice. J. Agric. Food Chem. 2018, 66, 11767–11774. [Google Scholar] [CrossRef]
- Burton, G.J.; Fowden, A.L. The placenta: A multifaceted, transient organ. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140066. [Google Scholar] [CrossRef]
- Silva, J.F.; Serakides, R. Intrauterine trophoblast migration: A comparative view of humans and rodents. Cell Adh. Migr. 2016, 10, 88–110. [Google Scholar] [CrossRef]
- Salavati, N.; Smies, M.; Ganzevoort, W.; Charles, A.K.; Erwich, J.J.; Plosch, T.; Gordijn, S.J. The Possible Role of Placental Morphometry in the Detection of Fetal Growth Restriction. Front. Physiol. 2018, 9, 1884. [Google Scholar] [CrossRef]
- Zong, S.; Li, C.; Luo, C.; Zhao, X.; Liu, C.; Wang, K.; Jia, W.; Bai, M.; Yin, M.; Bao, S.; et al. Dysregulated expression of IDO may cause unexplained recurrent spontaneous abortion through suppression of trophoblast cell proliferation and migration. Sci. Rep. 2016, 6, 19916. [Google Scholar] [CrossRef]
- Lyall, F.; Robson, S.C.; Bulmer, J.N. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: Relationship to clinical outcome. Hypertension 2013, 62, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.A.; Gomez, I.; Chiarello, D.I.; Salsoso, R.; Klein, A.D.; Guzman-Gutierrez, E.; Toledo, F.; Sobrevia, L. Role of proteases in dysfunctional placental vascular remodelling in preeclampsia. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1866, 165448. [Google Scholar] [CrossRef]
- Auttachoat, W.; McLoughlin, C.E.; White, K.L., Jr.; Smith, M.J. Route-dependent systemic and local immune effects following exposure to solutions prepared from titanium dioxide nanoparticles. J. Immunotoxicol. 2014, 11, 273–282. [Google Scholar] [CrossRef]
- Jovanović, B. Critical review of public health regulations of titanium dioxide, a human food additive. Integr. Environ. Assess. Manag. 2015, 11, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Vercruysse, L.; Caluwaerts, S.; Luyten, C.; Pijnenborg, R. Interstitial trophoblast invasion in the decidua and mesometrial triangle during the last third of pregnancy in the rat. Placenta 2006, 27, 22–33. [Google Scholar] [CrossRef]
- Cotechini, T.; Komisarenko, M.; Sperou, A.; Macdonald-Goodfellow, S.; Adams, M.A.; Graham, C.H. Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. J. Exp. Med. 2014, 211, 165–179. [Google Scholar] [CrossRef]
- Aziz, K.M.A. Association of High Levels of Spot Urine Protein with High Blood Pressure, Mean Arterial Pressure and Pulse Pressure with Development of Diabetic Chronic Kidney Dysfunction or Failure among Diabetic Patients. Statistical Regression Modeling to Predict Diabetic Proteinuria. Curr. Diabetes Rev. 2018, 15, 486–496. [Google Scholar]
- Asare, N.; Duale, N.; Slagsvold, H.H.; Lindeman, B.; Olsen, A.K.; Gromadzka-Ostrowska, J.; Meczynska-Wielgosz, S.; Kruszewski, M.; Brunborg, G.; Instanes, C. Genotoxicity and gene expression modulation of silver and titanium dioxide nanoparticles in mice. Nanotoxicology 2016, 10, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimzadeh Bideskan, A.; Mohammadipour, A.; Fazel, A.; Haghir, H.; Rafatpanah, H.; Hosseini, M.; Rajabzadeh, A. Maternal exposure to titanium dioxide nanoparticles during pregnancy and lactation alters offspring hippocampal mRNA BAX and Bcl-2 levels, induces apoptosis and decreases neurogenesis. Exp. Toxicol. Pathol. 2017, 69, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Yoshioka, Y.; Higashisaka, K.; Mimura, K.; Morishita, Y.; Nozaki, M.; Yoshida, T.; Ogura, T.; Nabeshi, H.; Nagano, K.; et al. Silica and titanium dioxide nanoparticles cause pregnancy complications in mice. Nat. Nanotechnol. 2011, 6, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Grigsby, P.L. Animal Models to Study Placental Development and Function throughout Normal and Dysfunctional Human Pregnancy. Semin. Reprod. Med. 2016, 34, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Bettahar, K.; Pinton, A.; Boisrame, T.; Cavillon, V.; Wylomanski, S.; Nisand, I.; Hassoun, D. Medical induced abortion. J. Gynecol. Obstet. Biol. Reprod. 2016, 45, 1490–1514. [Google Scholar] [CrossRef]
- Mao, Z.; Li, Y.; Dong, T.; Zhang, L.; Zhang, Y.; Li, S.; Hu, H.; Sun, C.; Xia, Y. Exposure to Titanium Dioxide Nanoparticles During Pregnancy Changed Maternal Gut Microbiota and Increased Blood Glucose of Rat. Nanoscale Res. Lett. 2019, 14, 26. [Google Scholar] [CrossRef]
- Hong, F.; Zhou, Y.; Zhao, X.; Sheng, L.; Wang, L. Maternal exposure to nanosized titanium dioxide suppresses embryonic development in mice. Int. J. Nanomed. 2017, 12, 6197–6204. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, M.; Jabeen, F.; Iqbal, R.; Chaudhry, A.S.; Zafar, S.; Ali, M.; Khan, M.S.; Khalid, A.; Shabbir, S.; Asghar, M.S. Assessment of Titanium Dioxide Nanoparticles (TiO2-NPs) Induced Hepatotoxicity and Ameliorative Effects of Cinnamomum cassia in Sprague-Dawley Rats. Biol. Trace Elem. Res. 2018, 182, 57–69. [Google Scholar] [CrossRef]
- Cho, W.S.; Kang, B.C.; Lee, J.K.; Jeong, J.; Che, J.H.; Seok, S.H. Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration. Part. Fibre Toxicol. 2013, 10, 9. [Google Scholar] [CrossRef]
- Alidadi, H.; Khorsandi, L.; Shirani, M. Effects of Quercetin on Tubular Cell Apoptosis and Kidney Damage in Rats Induced by Titanium Dioxide Nanoparticles. Malays. J. Med. Sci. 2018, 25, 72–81. [Google Scholar] [CrossRef]
- Hong, F.; Wu, N.; Ge, Y.; Zhou, Y.; Shen, T.; Qiang, Q.; Zhang, Q.; Chen, M.; Wang, Y.; Wang, L.; et al. Nanosized titanium dioxide resulted in the activation of TGF-beta/Smads/p38MAPK pathway in renal inflammation and fibration of mice. J. Biomed. Mater. Res. A 2016, 104, 1452–1461. [Google Scholar] [CrossRef]
- Mebius, R.E.; Kraal, G. Structure and function of the spleen, Nature reviews. Immunology 2005, 5, 606–616. [Google Scholar]
- Huang, C.; Sun, M.; Yang, Y.; Wang, F.; Ma, X.; Li, J.; Wang, Y.; Ding, Q.; Ying, H.; Song, H.; et al. Titanium dioxide nanoparticles prime a specific activation state of macrophages. Nanotoxicology 2017, 11, 737–750. [Google Scholar] [CrossRef]
- Dhupal, M.; Oh, J.M.; Tripathy, D.R.; Kim, S.K.; Koh, S.B.; Park, K.S. Immunotoxicity of titanium dioxide nanoparticles via simultaneous induction of apoptosis and multiple toll-like receptors signaling through ROS-dependent SAPK/JNK and p38 MAPK activation. Int. J. Nanomed. 2018, 13, 6735–6750. [Google Scholar] [CrossRef]
- Hong, F.; Wang, L. Nanosized titanium dioxide-induced premature ovarian failure is associated with abnormalities in serum parameters in female mice. Int. J. Nanomed. 2018, 13, 2543–2549. [Google Scholar] [CrossRef]
- Zhao, X.; Ze, Y.; Gao, G.; Sang, X.; Li, B.; Gui, S.; Sheng, L.; Sun, Q.; Cheng, J.; Cheng, Z.; et al. Nanosized TiO2-induced reproductive system dysfunction and its mechanism in female mice. PLoS ONE 2013, 8, e59378. [Google Scholar] [CrossRef]
- Gao, G.; Ze, Y.; Li, B.; Zhao, X.; Zhang, T.; Sheng, L.; Hu, R.; Gui, S.; Sang, X.; Sun, Q.; et al. Ovarian dysfunction and gene-expressed characteristics of female mice caused by long-term exposure to titanium dioxide nanoparticles. J. Hazard. Mater. 2012, 243, 19–27. [Google Scholar] [CrossRef]
- Philbrook, N.A.; Winn, L.M.; Afrooz, A.R.; Saleh, N.B.; Walker, V.K. The effect of TiO(2) and Ag nanoparticles on reproduction and development of Drosophila melanogaster and CD-1 mice. Toxicol. Appl. Pharmacol. 2011, 257, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Pijnenborg, R.; Vercruysse, L.; Hanssens, M. The uterine spiral arteries in human pregnancy: Facts and controversies. Placenta 2006, 27, 939–958. [Google Scholar] [CrossRef]
- Yin, F.; Zhu, Y.; Zhang, M.; Yu, H.; Chen, W.; Qin, J. A 3D human placenta-on-a-chip model to probe nanoparticle exposure at the placental barrier. Toxicol. In Vitro 2019, 54, 105–113. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, X.; Zhou, Y.; Yu, D.; Deng, Y.; Ouyang, J.; Yang, B.; Luo, D.; Zhang, D.; Kuang, H. Gestational exposure to titanium dioxide nanoparticles impairs the placentation through dysregulation of vascularization, proliferation and apoptosis in mice. Int. J. Nanomed. 2018, 13, 777–789. [Google Scholar] [CrossRef]
- Huang, X.; Han, X.; Huang, Z.; Yu, M.; Zhang, Y.; Fan, Y.; Xu, B.; Zhou, K.; Song, L.; Wang, X.; et al. Maternal pentachlorophenol exposure induces developmental toxicity mediated by autophagy on pregnancy mice. Ecotoxicol. Environ. Saf. 2019, 169, 829–836. [Google Scholar] [CrossRef]
- Dai, X.; Liu, R.; Li, N.; Yi, J. Titanium dioxide nanoparticles induce in vitro autophagy. Hum. Exp. Toxicol. 2019, 38, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Valentini, X.; Rugira, P.; Frau, A.; Tagliatti, V.; Conotte, R.; Laurent, S.; Colet, J.M.; Nonclercq, D. Hepatic and Renal Toxicity Induced by TiO2 Nanoparticles in Rats: A Morphological and Metabonomic Study. J. Toxicol. 2019, 2019, 5767012. [Google Scholar] [CrossRef]
- Fontana, L.; Leso, V.; Marinaccio, A.; Cenacchi, G.; Papa, V.; Leopold, K.; Schindl, R.; Bocca, B.; Alimonti, A.; Iavicoli, I. The effects of palladium nanoparticles on the renal function of female Wistar rats. Nanotoxicology 2015, 9, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Luthe, S.K.; Nasu, M. Blood pressure and acute kidney injury. Crit. Care 2017, 21, 28. [Google Scholar] [CrossRef]
- Niu, L.; Shao, M.; Liu, Y.; Hu, J.; Li, R.; Xie, H.; Zhou, L.; Shi, L.; Zhang, R.; Niu, Y. Reduction of oxidative damages induced by titanium dioxide nanoparticles correlates with induction of the Nrf2 pathway by GSPE supplementation in mice. Chem.-Biol. Interact. 2017, 275, 133–144. [Google Scholar] [CrossRef]
- Hong, F.; Hong, J.; Wang, L.; Zhou, Y.; Liu, D.; Xu, B.; Yu, X.; Sheng, L. Chronic exposure to nanoparticulate TiO2 causes renal fibrosis involving activation of the Wnt pathway in mouse kidney. J. Agric. Food Chem. 2015, 63, 1639–1647. [Google Scholar] [CrossRef]
- Mao, Z.; Yao, M.; Li, Y.; Fu, Z.; Li, S.; Zhang, L.; Zhou, Z.; Tang, Q.; Han, X.; Xia, Y. miR-96-5p and miR-101-3p as potential intervention targets to rescue TiO2 NP-induced autophagy and migration impairment of human trophoblastic cells. Biomater. Sci. 2018, 6, 3273–3283. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Miao, D.; Hu, H.; Xue, P.; Zhou, K.; Mao, Z. Titanium Dioxide Nanoparticles Induce Maternal Preeclampsia-like Syndrome and Adverse Birth Outcomes via Disrupting Placental Function in SD Rats. Toxics 2024, 12, 367. https://doi.org/10.3390/toxics12050367
Li H, Miao D, Hu H, Xue P, Zhou K, Mao Z. Titanium Dioxide Nanoparticles Induce Maternal Preeclampsia-like Syndrome and Adverse Birth Outcomes via Disrupting Placental Function in SD Rats. Toxics. 2024; 12(5):367. https://doi.org/10.3390/toxics12050367
Chicago/Turabian StyleLi, Haixin, Dandan Miao, Haiting Hu, Pingping Xue, Kun Zhou, and Zhilei Mao. 2024. "Titanium Dioxide Nanoparticles Induce Maternal Preeclampsia-like Syndrome and Adverse Birth Outcomes via Disrupting Placental Function in SD Rats" Toxics 12, no. 5: 367. https://doi.org/10.3390/toxics12050367



