Tackling Losartan Contamination: The Promise of Peroxymonosulfate/Fe(II) Advanced Oxidation Processes
Abstract
:1. Introduction
2. Results and Discussion
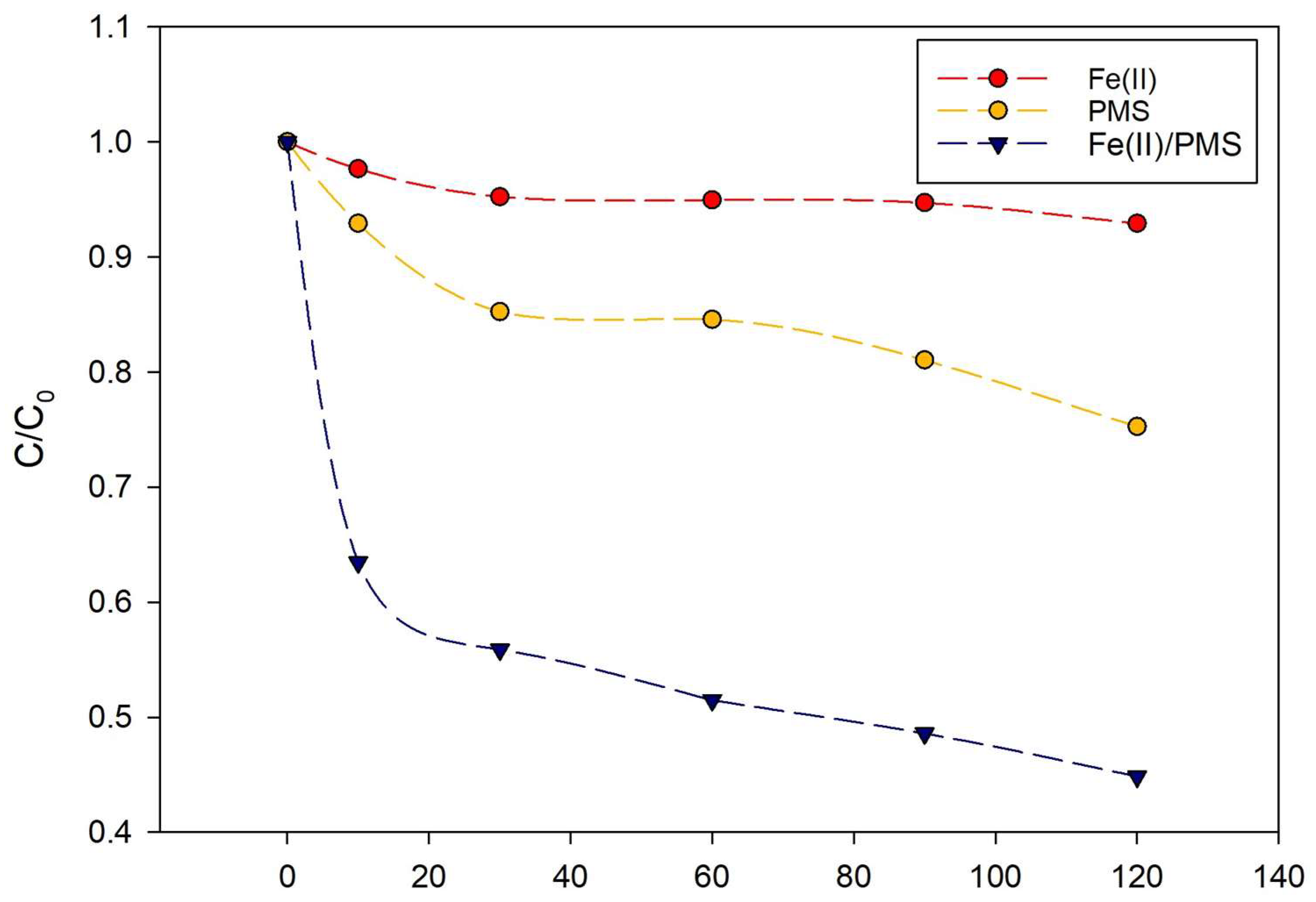
2.1. Degradation Experiments
2.2. Structure Elucidation of Degradation Byproducts DP1–DP9
2.3. Spectral Data
2.4. Kinetics of Losartan Degradation in PMS/Fe(II) System
3. Materials and Methods
3.1. Drug and Reagents
3.2. Peroxymonosulfate Reaction
3.2.1. Apparatus and Equipment
3.2.2. Peroxymonosulfate-Based Advanced Oxidation Reaction
3.2.3. Product Isolation Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osuoha, J.O.; Anyanwu, B.O.; Ejileugha, C. Pharmaceuticals and Personal Care Products as Emerging Contaminants: Need for Combined Treatment Strategy. J. Hazard. Mater. Adv. 2023, 9, 100206. [Google Scholar] [CrossRef]
- Khalid, M.; Abdollahi, M. Environmental Distribution of Personal Care Products and Their Effects on Human Health. Iran J. Pharm. Res. 2021, 20, 216–253. [Google Scholar] [CrossRef]
- Pot, E.J.; Milakovic, M.; Chaumot, A.; Seidensticker, S.; Melling, M.; Supriatin, A.; Sherif, S. Pharmaceutical Pollution of the World’s Rivers. Proc. Natl. Acad. Sci. USA 2022, 119, e2113947119. [Google Scholar] [CrossRef]
- Larsson, D.G.J. Pollution from Drug Manufacturing: Review and Perspectives. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130571. [Google Scholar] [CrossRef]
- Wu, W.M.; Yang, J.; Criddle, C.S. Microplastics Pollution and Reduction Strategies. Front. Environ. Sci. Eng. 2017, 11, 6. [Google Scholar] [CrossRef]
- Rezania, S.; Park, J.; Md Din, M.F.; Mat Taib, S.; Talaiekhozani, A.; Kumar Yadav, K.; Kamyab, H. Microplastics Pollution in Different Aquatic Environments and Biota: A Review of Recent Studies. Mar. Pollut. Bull. 2018, 133, 191–208. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El Nemr, A. Pesticides Pollution: Classifications, Human Health Impact, Extraction and Treatment Techniques. Egypt. Aquat. Res. 2020, 46, 207–220. [Google Scholar] [CrossRef]
- Mali, H.; Shah, C.; Raghunandan, B.H.; Prajapati, A.S.; Patel, D.H.; Trivedi, U.; Subramanian, R.B. Organophosphate Pesticides an Emerging Environmental Contaminant: Pollution, Toxicity, Bioremediation Progress, and Remaining Challenges. J. Environ. Sci. 2023, 127, 234–250. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, L.; Jia, Y.; Zhang, Y.; Dong, Q.; Huang, C. A Study on Environmental Bisphenol A Pollution in Plastics Industry Areas. Water Air Soil Pollut. 2017, 228, 98. [Google Scholar] [CrossRef]
- Fu, P.; Kawamura, K. Ubiquity of Bisphenol A in the Atmosphere. Environ. Pollut. 2010, 158, 3138–3143. [Google Scholar] [CrossRef]
- Trifuoggi, M.; Donadio, C.; Ferrara, L.; Stanislao, C.; Toscanesi, M.; Arienzo, M. Levels of Pollution of Rare Earth Elements in the Surface Sediments from the Gulf of Pozzuoli (Campania, Italy). Mar. Pollut. Bull. 2018, 136, 374–384. [Google Scholar] [CrossRef]
- Ramos, S.J.; Dinali, G.S.; Oliveira, C.; Martins, G.C.; Moreira, C.G.; Siqueira, J.O.; Guilherme, L.R.G. Rare Earth Elements in the Soil Environment. Curr. Pollut. Rep. 2016, 2, 28–50. [Google Scholar] [CrossRef]
- Timmermans, P.B.; Wong, P.C.; Chiu, A.T.; Herblin, W.F.; Benfield, P.; Carini, D.J.; Lee, R.J.; Wexler, R.R.; Anne Saye, J.M.; Smith, R.D. Angiotensin II Receptors and Angiotensin II Receptor Antagonists. Pharmacol. Rev. 1993, 45, 205–251. [Google Scholar]
- Eworuke, E.; Shinde, M.; Hou, L.; Paterson, M.J.; Jensen, P.B.; Maro, J.C.; Rai, A.; Scarnecchia, D.; Pennap, D.; Woronow, D.; et al. Valsartan, Losartan and Irbesartan Use in the USA, UK, Canada and Denmark after the Nitrosamine Recalls: A Descriptive Cohort Study. BMJ Open 2023, 13, e070985. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; de Pedro, C.; Paxeus, N. Effluent from Drug Manufactures Contains Extremely High Levels of Pharmaceuticals. J. Hazard. Mater. 2007, 148, 751–755. [Google Scholar] [CrossRef]
- Huerta-Fontela, M.; Galceran, M.T.; Ventura, F. Occurrence and Removal of Pharmaceuticals and Hormones through Drinking Water Treatment. Water Res. 2011, 45, 1432–1442. [Google Scholar] [CrossRef]
- Cortez, F.S.; da Silva Souza, L.; Guimarães, L.L.; Almeida, J.E.; Pusceddu, F.H.; Maranho, L.A.; Mota, L.G.; Nobre, C.R.; Moreno, B.B.; Abessa, D.M.d.S.; et al. Ecotoxicological Effects of Losartan on the Brown Mussel Perna Perna and Its Occurrence in Seawater from Santos Bay (Brazil). Sci. Total Environ. 2018, 637–638, 1363–1371. [Google Scholar] [CrossRef]
- Ladhari, A.; La Mura, G.; Di Marino, C.; Di Fabio, G.; Zarrelli, A. Sartans: What They Are for, How They Degrade, Where They Are Found and How They Transform. Sustain. Chem. Pharm. 2021, 20, 100409. [Google Scholar] [CrossRef]
- Siciliano, A.; Medici, A.; Guida, M.; Libralato, G.; Saviano, L.; Previtera, L.; Di Fabio, G.; Zarrelli, A. Newly Discovered Irbesartan Disinfection Byproducts via Chlorination: Investigating Potential Environmental Toxicity. Appl. Sci. 2023, 13, 8170. [Google Scholar] [CrossRef]
- Nödler, K.; Hillebrand, O.; Idzik, K.; Strathmann, M.; Schiperski, F.; Zirlewagen, J.; Licha, T. Occurrence and Fate of the Angiotensin II Receptor Antagonist Transformation Product Valsartan Acid in the Water Cycle—A Comparative Study with Selected β-Blockers and the Persistent Anthropogenic Wastewater Indicators Carbamazepine and Acesulfame. Water Res. 2013, 47, 6650–6659. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced Oxidation Process-Mediated Removal of Pharmaceuticals from Water: A Review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Zrinyi, N.; Pham, A.L.T. Oxidation of Benzoic Acid by Heat-Activated Persulfate: Effect of Temperature on Transformation Pathway and Product Distribution. Water Res. 2017, 120, 43–51. [Google Scholar] [CrossRef]
- Ma, J.; Li, H.; Chi, L.; Chen, H.; Chen, C. Changes in Activation Energy and Kinetics of Heat-Activated Persulfate Oxidation of Phenol in Response to Changes in PH and Temperature. Chemosphere 2017, 189, 86–93. [Google Scholar] [CrossRef]
- Yang, S.; Wang, P.; Yang, X.; Wei, G.; Zhang, W.; Shan, L. A Novel Advanced Oxidation Process to Degrade Organic Pollutants in Wastewater: Microwave-Activated Persulfate Oxidation. J. Environ. Sci. 2009, 21, 1175–1180. [Google Scholar] [CrossRef]
- Gabet, A.; Métivier, H.; de Brauer, C.; Mailhot, G.; Brigante, M. Hydrogen Peroxide and Persulfate Activation Using UVA-UVB Radiation: Degradation of Estrogenic Compounds and Application in Sewage Treatment Plant Waters. J. Hazard. Mater. 2021, 405, 124693. [Google Scholar] [CrossRef]
- Wacławek, S.; Lutze, H.V.; Sharma, V.K.; Xiao, R.; Dionysiou, D.D. Revisit the Alkaline Activation of Peroxydisulfate and Peroxymonosulfate. Curr. Opin. Chem. Eng. 2022, 37, 100854. [Google Scholar] [CrossRef]
- Medici, A.; Lavorgna, M.; Isidori, M.; Russo, C.; Orlo, E.; Luongo, G.; Di Fabio, G.; Zarrelli, A. Advanced Oxidation Process of Valsartan by Activated Peroxymonosulfate: Chemical Characterization and Ecotoxicological Effects of Its Byproducts. Sci. Total Environ. 2024, 908, 168337. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Aravind, U.K.; Aravindakumar, C.T. Ultrasound Based AOP for Emerging Pollutants: From Degradation to Mechanism. Environ. Sci. Pollut. Res. 2017, 24, 6261–6269. [Google Scholar] [CrossRef]
- Joseph, C.G.; Li Puma, G.; Bono, A.; Krishnaiah, D. Sonophotocatalysis in Advanced Oxidation Process: A Short Review. Ultrason. Sonochem. 2009, 16, 583–589. [Google Scholar] [CrossRef]
- Verdini, F.; Calcio Gaudino, E.; Canova, E.; Colia, M.C.; Cravotto, G. Highly Efficient Tetracycline Degradation under Simultaneous Hydrodynamic Cavitation and Electrical Discharge Plasma in Flow. Ind. Eng. Chem. Res. 2023, 62, 19311–19322. [Google Scholar] [CrossRef]
- Xiao, S.; Cheng, M.; Zhong, H.; Liu, Z.; Liu, Y.; Yang, X.; Liang, Q. Iron-mediated activation of persulfate and peroxymonosulfate in both homogeneous and heterogeneous ways: A review. Chem. Eng. J. 2020, 384, 123265. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, L.; Jiang, M.; Yang, Y.; Yang, P.; Lu, J.; Ferronato, C.C.; Chovelon, J.M. Ferrous-activated peroxymonosulfate oxidation of antimicrobial agent sulfaquinoxaline and structurally related compounds in aqueous solution: Kinetics, products, and transformation pathways. Environ. Sci. Pollut. Res. 2017, 24, 19535–19545. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medici, A.; Luongo, G.; Pedatella, S.; Previtera, L.; Di Fabio, G.; Zarrelli, A. Tackling Losartan Contamination: The Promise of Peroxymonosulfate/Fe(II) Advanced Oxidation Processes. Molecules 2024, 29, 2237. https://doi.org/10.3390/molecules29102237
Medici A, Luongo G, Pedatella S, Previtera L, Di Fabio G, Zarrelli A. Tackling Losartan Contamination: The Promise of Peroxymonosulfate/Fe(II) Advanced Oxidation Processes. Molecules. 2024; 29(10):2237. https://doi.org/10.3390/molecules29102237
Chicago/Turabian StyleMedici, Antonio, Giovanni Luongo, Silvana Pedatella, Lucio Previtera, Giovanni Di Fabio, and Armando Zarrelli. 2024. "Tackling Losartan Contamination: The Promise of Peroxymonosulfate/Fe(II) Advanced Oxidation Processes" Molecules 29, no. 10: 2237. https://doi.org/10.3390/molecules29102237




