Enrichment of Total Flavonoids and Licochalcone A from Glycyrrhiza inflata Bat. Residue Based on a Combined Membrane–Macroporous Resin Process and a Quality-Control Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Purification of TFs from Licorice Residue by a Membrane Method
2.2. MR Screening
2.3. Adsorption Kinetics of TFs from Licorice Residue on the MR HPD-100
2.4. Adsorption Isotherms of TFs from Licorice Residue on the MR HPD-100
2.5. Optimization of Experimental Conditions for Dynamic Adsorption
2.5.1. Determination of Sample Loading Concentration
2.5.2. Determination of the Dynamic Leakage Curve
2.6. Determination of Optimal Application Sequence of Membrane Method and MR Method
2.7. Optimization of the Desorption Conditions of the MR HPD-100
2.8. Validation Experiment
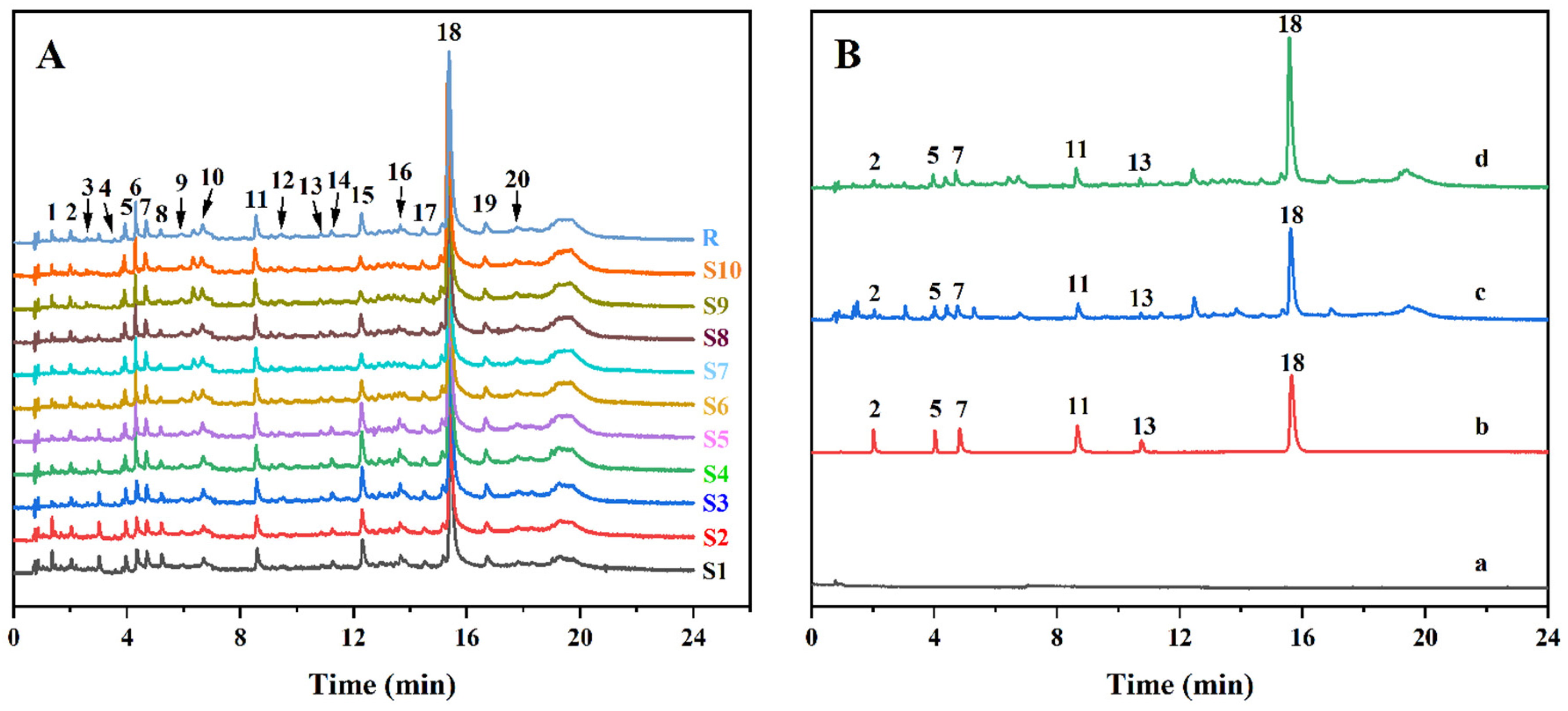
2.9. Establishment of a UPLC Fingerprint
3. Materials and Methods
3.1. Raw Materials and Other Materials
3.2. Reagents and Instruments
3.3. Preparation of an Extract of Licorice Residues
3.4. Determination of TFs and Licochalcone A in Licorice Residue
3.4.1. Determination of TF Content by UV Spectrophotometry
3.4.2. Determination of Licochalcone A by UPLC
3.5. Purification by a Membrane Method
3.5.1. Screening of the Pore Size of A Membrane
3.5.2. Cleaning and Maintenance of the Membrane
3.6. MR Pretreatment
3.7. Tests of Static Adsorption and Desorption
3.7.1. MR Screening
3.7.2. Adsorption Kinetics of TFs on the MR HPD-100
3.7.3. Adsorption Isotherms of TFs on the MR HPD-100
3.8. Optimization of Experimental Conditions for Dynamic Adsorption
3.8.1. Determination of Sample Loading Concentration
3.8.2. Determination of the Dynamic Leakage Curve
3.9. Investigation of Membrane and MR Method Application Order
3.9.1. Effect of MR-Membrane Method on Purification
3.9.2. Effect of the Membrane–MR Method on Purification
3.10. Optimization of Desorption Conditions of the MR HPD-100
3.11. QC of Enriched Substances
3.11.1. Preparation of Mixed Standard and Test Sample
3.11.2. Simultaneous Determination of Six Components by UPLC
3.11.3. Methodology Validation
3.11.4. Establishment of a UPLC Fingerprint
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, M.; Zhao, S.; Yang, S.; Lin, X.; He, X.; Wei, X.; Song, Q.; Li, R.; Fu, C.; Zhang, J.; et al. An “essential herbal medicine”-licorice: A review of phytochemicals and its effects in combination preparations. J. Ethnopharmacol. 2020, 249, 112439. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, L.; Xu, C.; Shi, J.; Chen, S.; Tan, M.; Chen, J.; Zou, L.; Chen, C.; Liu, Z.; et al. A Comprehensive Review for Phytochemical, Pharmacological, and Biosynthesis Studies on Glycyrrhiza spp. Am. J. Chin. Med. 2020, 48, 17–45. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Guo, Z.; Xiao, T.; Liu, H.; Su, G.; Zhao, Y. Enrichment of total flavones and licochalcone A from licorice residues and its hypoglycemic activity. J. Chromatography. B Anal. Technol. Biomed. Life Sci. 2019, 1114–1115, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, Y.; Lin, S. High performance liquid chromatographic analysis of flavonoids in roots of six species of Glycyrrhiza glabra. J. Pharm. 1990, 11, 840–848. [Google Scholar]
- Wang, D.; Liang, J.; Zhang, J.; Wang, Y.; Chai, X. Natural Chalcones in Chinese Materia Medica: Licorice. Evid. Based Complement. Altern. Med. 2020, 2020, 3821248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qing, D.; Sun, Y.; Wang, W.; Ni, H.; Jia, X. Preparation of Glycyrrhiza chalcone A in Glycyrrhiza residue. Xinjiang Tradit. Chin. Med. 2016, 34, 39–40. [Google Scholar]
- Zhang, X. Extraction, Separation, Structure and Hypoglycemic Activity of Alkaline Extracted Polysaccharide from G. inflata Residue. Tianjin Univ. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Zheng, Y.; Wei, J.; Qi, L.; Cheng, J.; Peng, G. A green and efficient protocol for large-scale production of glycyrrhizic acid from licorice roots by combination of polyamide and macroporous resin adsorbent chromatography. J. Sep. Sci. 2013, 36, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhao, M.; Sun, W.; Sun-Waterhouse, D.; Zhuang, M.; Chen, H.; Feng, M.; Lin, L. Absorption and desorption behaviour of the flavonoids from Glycyrrhiza glabra L. leaf on macroporous adsorption resins. Food Chem. 2015, 168, 538–545. [Google Scholar] [CrossRef]
- Yang, M.; Wu, Z.; Wang, F.; Cang, Z.; Strategies, Z.G. Suggestions for the Implementation of Green and Intelligent Manufacturing in Traditional Chinese Medicine. Chin. J. Pharm. 2016, 9, 1205–1210. [Google Scholar]
- Liu, H.; Li, B.; Guo, L.; Pan, L.; Zhu, H.; Tang, Z.; Xing, W.; Cai, Y.; Duan, J.; Wang, M.; et al. Current and Future Use of Membrane Technology in the Traditional Chinese Medicine Industry. Sep. Purif. Rev. 2022, 51, 484–502. [Google Scholar] [CrossRef]
- Su, H.; Yang, R.; Zhao, W.; Hua, X.; Zhang, W. Purification of anthocyanins from purple sweet potato by combination of ultrafiltration and macroporous resin. Food Ind. Sci. Technol. 2016, 37, 268–272. [Google Scholar]
- Zhang, M.; Li, S.; Ren, K.; Yang, Y. Extraction of Total Flavonoids from Chroogomphidius viscidus by Alkali-Soluble Acid Precipitation Method. J. Hainan Norm. Univ. 2019, 32, 170–176. [Google Scholar]
- Zhou, Y.; Shan, H.; Lü, H. Application of preparative high-speed countercurrent chromatography for the separation and purification of three flavonoids from Oroxylum indicum. Biomed. Chromatogr. 2023, 37, e5579. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Meng, X.; Tan, C.; Tong, Y.; Wan, M.; Wang, M.; Zhao, Y.; Deng, H.; Kong, Y.; Ma, Y. Composition and antioxidant activity of anthocyanins from Aronia melanocarpa extracted using an ultrasonic-microwave-assisted natural deep eutectic solvent extraction method. Ultrason. Sonochem. 2022, 89, 106102. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Xu, Y.; Chen, W.; Zhang, Y.; Xu, R. Optimization of Extraction Process and Purification of Flavonoids from Peony Seed Meal by Membrane Method. Sci. Technol. Food Ind. 2023, 44, 258–267. [Google Scholar]
- Wang, X.; Su, J.; Chu, X.; Zhang, X.; Kan, Q.; Liu, R.; Fu, X. Adsorption and Desorption Characteristics of Total Flavonoids from on Macroporous Adsorption Resins. Molecules 2021, 26, 4162. [Google Scholar] [CrossRef]
- Liu, B.; Cao, D.; Meng, Q. Purification of fructo-oligosaccharide by membrane separation process combined with macroporpus resin. Food Mach. 2016, 32, 133–138. [Google Scholar]
- Li, Y.; Zhu, H.; Tang, Z.; Liu, H.; Song, Z.; Ji, S. Isolation and purification of saponins from Cornus saponins by traditional process combined with membrane separation technology. Zhongnan Pharmacol. 2022, 20, 254–261. [Google Scholar]
- Luo, T.; Xiao, X.; Huang, Z.; Miao, Q. Preparation of Compound Double Traditional Chinese Medicine Injection by Ultrafiltration Method and Macroporous Adsorption Resin Method. J. Nanhua Univ. (Nat. Sci. Ed.) 2018, 32, 90–96. [Google Scholar]
- Zhang, C.; Zhao, B.; Xu, H.; Pan, D.; Wu, J.; Zhu, Y. Research and application of carthamus yellow purification technology. China Food Addit. 2022, 33, 87–94. [Google Scholar]
- Li, Q.; Zhang, C.; Chen, L.; Chen, Z. Quality evaluation of Xiao’ er Ganmao granules based on UPLC fingerprint, multi-component determination, and chemometric analysis. J. Pharm. Anal. 2023, 43, 1612–1622. [Google Scholar]
- Zhou, Y.; Lei, R.; Su, J.; Bai, J.; Liu, Y. Quality control research of Sijisanhuang pills based on UPLC fingerprint combined with chemical pattern recognition. J. Pharm. Anal. 2023, 43, 1784–1790. [Google Scholar]
- Hu, Z. Application ofmass spectrometry technique in the study of traditional Chinese medicine fingerprint. Yunnan Chem. Ind. 2020, 47, 28–31. [Google Scholar]
- Fan, X.; Hong, T.; Yang, Q.; Wang, D.; Peng, J.; Xiao, W.; Yang, X.; Hu, X.; Yu, C.; Du, S.; et al. Quality assessment of fried licorice based on fingerprints and chemometrics. Food Chem. 2022, 378, 132121. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiao, Z.; Luo, Y.; Li, X.; Li, J.; Bi, Y.; Liu, J. Study on Enrichment of Total Flavonoids from Licorice Residue by Chemical Conversion Method Based on Fingerprint and Quantitative Analysis of Multi-components with a Single-marker Technique. China J. Tradit. Chin. Med. Inf. 2018, 25, 69–73. [Google Scholar]
- Luo, L. Preparation and sorghum root effective part Enrichment Process Optimization. Guizhou Univ. 2018. Available online: https://kns.cnki.net/kcms2/article/abstract?v=vRpkk4QO0oj3LXLctQ-yjc0xjxkMAzTP33ptpVwZDCyX5yjssYWgG9xn_awQRZHzZML_XwvcA9WGJn5uYjhqtfgUeNeOyV2EJuED0i2b3LgvgRP07-sApgFzCCkvqnBXOoOeM1AZrsJG5PApXctRIQ==&uniplatform=NZKPT&language=CHS (accessed on 29 April 2024).
- Tsai, W.T.; Chang, C.Y.; Ho, C.Y. Adsorption properties and breakthrough model of 1,1–dichloro–1–fluoroethane on activated carbons. J. Hazard. Mater. 1999, 69, 53–66. [Google Scholar] [CrossRef]
- Singh, A.; Arora, D.; Bala, R.; Khokhar, A.; Kumar, S. Lanthanum nanoparticle (La2O3)-loaded adsorbents for removal of hexavalent chromium: A kinetics, isotherm, and thermodynamic study. J. Environ. Sci. Pollut. Res. Int. 2023, 48, 105415–105428. [Google Scholar] [CrossRef]
- Kurnia, I.; Karnjanakom, S.; Irkham, I.; Haryono, H.; Situmorang, Y.A.; Indarto, A.; Noviyanti, A.R.; Hartati, Y.W.; Guan, G. Enhanced adsorption capacity of activated carbon over thermal oxidation treatment for methylene blue removal: Kinetics, equilibrium, thermodynamic, and reusability studies. J. RSC Adv. 2022, 13, 220–227. [Google Scholar] [CrossRef]
- Fu, Y.; Zu, Y.; Liu, W.; Efferth, T.; Zhang, N.; Liu, X.; Kong, Y. Optimization of luteolin separation from pigeonpea [Cajanus cajan, (L.) Millsp.] leaves by macroporous resins. J. Chromatogr. A 2006, 1137, 145. [Google Scholar] [CrossRef]
- Che Zain, M.S.; Lee, S.Y.; Teo, C.Y.; Shaari, K. Adsorption and Desorption Properties of Total Flavonoids from Oil Palm (Elaeis guineensis Jacq.) Mature Leaf on Macroporous Adsorption Resins. Molecules 2020, 25, 778. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Park, J.J.; Lee, W.Y. Adsorption and desorption characteristics of a phenolic compound from Ecklonia cava on macroporous resin. Food Chem. 2021, 338, 128150. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Du, X. Adsorption of Tea Polyphenols using Microporous Starch: A Study of Kinetics, Equilibrium and Thermodynamics. Molecules 2019, 24, 1449. [Google Scholar] [CrossRef]
- Guo, Z.; Li, J.; Guo, Z.; Guo, Q.; Zhu, B. Phosphorus removal from aqueous solution in parent and aluminum-modified eggshells: Thermodynamics and kinetics, adsorption mechanism, and diffusion process. Environ. Sci. Pollut. Res. Int. 2017, 24, 14525–14536. [Google Scholar] [CrossRef]
- Liu, J.; Li, Q.; Liu, R.; Yin, Y.; Chen, X.; Bi, K. Enrichment and purification of six Aconitum alkaloids from Aconiti kusnezoffii radix by macroporous resins and quantification by HPLCMS. J. Chromatogr. B 2014, 960, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wu, G.; Dong, Z.; Yang, D.; Bo, Y.; An, M.; Zhao, L. Natural deep eutectic solvents as tailored and sustainable media for the extraction of five compounds from compound liquorice tablets and their comparison with conventional organic solvents. RSC Adv. 2021, 11, 37649–37660. [Google Scholar] [CrossRef]
- Yu, P.; Li, Q.; Feng, Y.; Ma, S.; Chen, Y.; Li, G. Extraction and Analysis of Six Effective Components in Glycyrrhiza uralensis Fisch by Deep Eutectic Solvents (DES) Combined with Quantitative Analysis of Multi-Components by Single Marker (QAMS) Method. Molecules 2021, 26, 1310. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, X.; Xiao, Z.; Li, X.; Li, J.; Luo, Y. Study on the quality standard of flavonoid extract in licorice residue. China J. Tradit. Chin. Med. Inf. 2017, 24, 67–70. [Google Scholar]
- Paun, G.; Neagu, E.; Parvulescu, V.; Anastasescu, M.; Petrescu, S.; Albu, C.; Nechifor, G.; Radu, G.L. New Hybrid Nanofiltration Membranes with Enhanced Flux and Separation Performances Based on Polyphenylene Ether-Ether-Sulfone/Polyacrylonitrile/SBA-15. Membranes 2022, 12, 689. [Google Scholar] [CrossRef]
- Conidi, C.; Fucà, L.; Drioli, E.; Cassano, A. A Membrane-Based Process for the Recovery of Glycyrrhizin and Phenolic Compounds from Licorice Wastewaters. Molecules 2019, 24, 2279. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yan, S.; Zhou, H.; Wu, H.; Wang, S.; Yong, X.; Jun, Z. Separation and purification of glabridin from a deep eutectic solvent extract of Glycyrrhiza glabra residue by macroporous resin and its mechanism. Sep. Purif. Technol. 2023, 315, 123731. [Google Scholar] [CrossRef]
- Fan, R. Separation of Glycyrrhizic Acid and Its Derivants from Hydrolyzation in Subcritical Water by Macroporous Resin. Molecules 2020, 25, 4305. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.P.; Yu, Z.F.; Yue, T.L.; Quek, S.Y. Adsorption isotherm, thermodynamics and kinetics studies of polyphenols separation from kiwifruit juice using adsorbent resin. J. Food Eng. 2013, 116, 195–201. [Google Scholar] [CrossRef]
- Andrew Ofudje, E.; Sodiya, E.F.; Olanrele, O.S.; Akinwunmi, F. Adsorption of Cd2+ onto apatite surface: Equilibrium, kinetics and thermodynamic studies. J. Heliyon 2023, 9, e12971. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Kong, X.Z.; Zhang, C.M.; Chen, Y.M.; Hua, Y.F. Adsorption of soy isoflavones by activated carbon: Kinetics, thermodynamics and influence of soy oligosaccharides. J. Chem. Eng. 2013, 215–216, 113–121. [Google Scholar] [CrossRef]
- Phojaroen, J.; Raita, M.; Champreda, V.; Laosiripojana, N.; Assabumrungrat, S.; Chuetor, S. Thermodynamic and Kinetic Equilibrium for Adsorption of Cellulosic Xylose of Commercial Cation-Exchange Resins. J. ACS Omega 2024, 9, 3006–3016. [Google Scholar] [CrossRef]








| MR | Particle Size (mm) | Specific Surface Area (m2·g−1) | Average Pore Diameter (Å) | Polarity |
|---|---|---|---|---|
| HPD-100 | 0.3–1.25 | 500–550 | 85–90 | Non |
| AB-8 | 0.3–1.25 | 480–520 | 130–140 | Weak |
| HPD-300 | 0.3–1.25 | 800–870 | 50–55 | Non |
| HPD-500 | 0.3–1.25 | 500–550 | 100–120 | Non |
| XDA-1 | 0.3–1.25 | 1000–1100 | 85–95 | Non |
| S-8 | 0.3–1.25 | 100–120 | 280–300 | Polarity |
| Dynamics Model | Kinetic Equation | Parameters |
|---|---|---|
| Pseudo-first order model | ln(qe − qt) = lnqe − K1t | K1 = 0.0081 min |
| qe = 0.419 mg·g−1 | ||
| R2 = 0.88173 | ||
| Pseudo-second order model | t/qt = 1/K2qe2 + t/qe | K2 = 0.1304 |
| qe = 6.674 | ||
| R2 = 0.999997 | ||
| Intra-particle diffusion model | qt = Kdt1/2 + C | Kd1 = 0.1004 |
| C1 = 5.823 | ||
| R2 = 0.9087 | ||
| Kd2 = 0.00929 | ||
| C1 = 6.456 | ||
| R2 = 0.9173 | ||
| Kd3 = 5.176 × 10−4 | ||
| C1 = 6.65 | ||
| R2 = 0.5425 |
| Model | T/(°C) | Equations | Parameters | ||
|---|---|---|---|---|---|
| KF [(mg·g−1) (mL·mg−1) 1/n] | n | R2 | |||
| Freundlich | 25 | lnqe = 0.651 lnCe + 1.977 | 7.220 | 1.535 | 0.974 |
| 35 | lnqe = 0.732 lnCe + 1.850 | 6.359 | 1.367 | 0.986 | |
| 45 | lnqe = 0.735 lnCe + 1.564 | 4.778 | 1.361 | 0.988 | |
| KL (mL·mg−1) | qm (mg·g−1) | R2 | |||
| Langmuir | 25 | = 0.0530Ce + 0.0775 | 0.684 | 18.875 | 0.994 |
| 35 | = 0.0460Ce + 0.103 | 0.446 | 21.715 | 0.996 | |
| 45 | = 0.0597Ce + 0.139 | 0.429 | 16.742 | 0.993 | |
| KT (L·mg−1) | BT (J·mol−1) | R2 | |||
| Temkin | 25 | qe = 4.041 lnCe + 7.942 | 7.137 | 4.041 | 0.988 |
| 35 | qe = 4.048 lnCe + 7.157 | 5.859 | 4.048 | 0.973 | |
| 45 | qe = 3.077 lnCe + 5.386 | 5.756 | 3.077 | 0.967 | |
| Serial Number | Application Sequence | Purity of Total Flavonoid (%) | Change in Purity (Multiplication Factor) | Content of Licochalcone A (mg·g−1) | Change in Content (Multiplication Factor) |
|---|---|---|---|---|---|
| ① | MR–membrane method | 47.6 ± 1.26 | 1.45 | 15.23 ± 0.53 | 0.31 |
| ② | Membrane–MR method | 68.7 ± 1.23 | 2.09 | 19.94 ± 1.13 | 1.71 |
| Independent Factor | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Elution volume | 4 | 5 | 6 |
| Ethanol concentration | 70 | 80 | 90 |
| Elution flow rate | 1.5 | 3 | 4.5 |
| run | A (BV) | B (%) | C (mL·min−1) | Recovery Rate (%) | Purity (%) |
|---|---|---|---|---|---|
| 1 | 5 | 70 | 1.5 | 72.3 | 66 |
| 2 | 6 | 80 | 4.5 | 87.3 | 68.7 |
| 3 | 6 | 70 | 3 | 74 | 65.2 |
| 4 | 5 | 90 | 1.5 | 83.6 | 70.7 |
| 5 | 5 | 80 | 3 | 93.3 | 78.6 |
| 6 | 5 | 70 | 4.5 | 75.6 | 65.9 |
| 7 | 5 | 80 | 3 | 92.8 | 78.3 |
| 8 | 5 | 80 | 3 | 88.4 | 77 |
| 9 | 4 | 80 | 1.5 | 87 | 72.9 |
| 10 | 4 | 80 | 4.5 | 86.5 | 73.5 |
| 11 | 4 | 70 | 3 | 70.1 | 64.3 |
| 12 | 5 | 80 | 3 | 89 | 77.3 |
| 13 | 5 | 80 | 3 | 90.7 | 77.3 |
| 14 | 6 | 80 | 1.5 | 88 | 67.6 |
| 15 | 5 | 90 | 4.5 | 86.3 | 71 |
| 16 | 6 | 90 | 3 | 85 | 65.8 |
| 17 | 4 | 90 | 3 | 82.6 | 72.8 |
| RECOVERY RATE | Purity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | R2 = 0.9645 | RA2 = 0.9188 | RP2 = 0.7774 | R2 = 0.9895 | RA2 = 0.9760 | RP2 = 0.9013 | ||||||
| Adequate precision | 12.907 | 21.715 | ||||||||||
| Source | SS | df | MS | F-Value | p-value | SS | df | MS | F-Value | p-value | ||
| Model | 774.65 | 9 | 86.07 | 21.11 | 0.0003 | 406.68 | 9 | 45.19 | 73.18 | <0.0001 | ||
| A (elution volume) | 8.20 | 1 | 8.20 | 2.01 | 0.1991 | 32.80 | 1 | 32.80 | 53.13 | 0.0002 | ||
| B (ethanol concentration) | 258.78 | 1 | 258.78 | 63.46 | <0.0001 | 44.65 | 1 | 44.65 | 72.31 | <0.0001 | ||
| C (elution flow rate) | 2.88 | 1 | 2.88 | 0.71 | 0.4285 | 0.45 | 1 | 0.45 | 0.73 | 0.4209 | ||
| AB | 0.56 | 1 | 0.56 | 0.14 | 0.7213 | 15.60 | 1 | 15.60 | 25.27 | 0.0015 | ||
| AC | 1 × 10−2 | 1 | 1 × 10−2 | 2.452 × 10−3 | 0.9619 | 0.063 | 1 | 0.063 | 0.10 | 0.7597 | ||
| BC | 0.090 | 1 | 0.090 | 0.022 | 0.8861 | 0.040 | 1 | 0.040 | 0.065 | 0.8064 | ||
| A2 | 28.08 | 1 | 28.08 | 6.89 | 0.0342 | 74.27 | 1 | 74.27 | 120.28 | <0.0001 | ||
| B2 | 449.52 | 1 | 449.52 | 110.24 | <0.0001 | 176.53 | 1 | 176.53 | 285.88 | <0.0001 | ||
| C2 | 4.71 | 1 | 4.71 | 1.15 | 0.3182 | 33.60 | 1 | 33.60 | 54.42 | 0.0002 | ||
| Residual | 28.54 | 7 | 4.08 | 4.32 | 7 | 0.63 | ||||||
| Lack of fit | 9.29 | 3 | 3.10 | 0.64 | 0.6263 | 2.34 | 3 | 0.78 | 1.58 | 0.3269 | ||
| Pure error | 19.25 | 4 | 4.81 | 1.98 | 4 | 0.50 | ||||||
| Correlation total | 803.20 | 16 | 411.00 | 16 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, Z.; Wang, Y.; Wu, Y.; Miao, L.; Ma, Y.; Wei, L.; Chen, W.; Li, H. Enrichment of Total Flavonoids and Licochalcone A from Glycyrrhiza inflata Bat. Residue Based on a Combined Membrane–Macroporous Resin Process and a Quality-Control Study. Molecules 2024, 29, 2282. https://doi.org/10.3390/molecules29102282
Wang X, Zhang Z, Wang Y, Wu Y, Miao L, Ma Y, Wei L, Chen W, Li H. Enrichment of Total Flavonoids and Licochalcone A from Glycyrrhiza inflata Bat. Residue Based on a Combined Membrane–Macroporous Resin Process and a Quality-Control Study. Molecules. 2024; 29(10):2282. https://doi.org/10.3390/molecules29102282
Chicago/Turabian StyleWang, Xiaoxia, Zhou Zhang, Yun Wang, Yayi Wu, Li Miao, Yue Ma, Lihua Wei, Wen Chen, and Hong Li. 2024. "Enrichment of Total Flavonoids and Licochalcone A from Glycyrrhiza inflata Bat. Residue Based on a Combined Membrane–Macroporous Resin Process and a Quality-Control Study" Molecules 29, no. 10: 2282. https://doi.org/10.3390/molecules29102282





