A Combined Experimental/Computational Study of Dicationic Ionic Liquids with Bromide and Tungstate Anions
Abstract
:1. Introduction
2. Results and Discussion
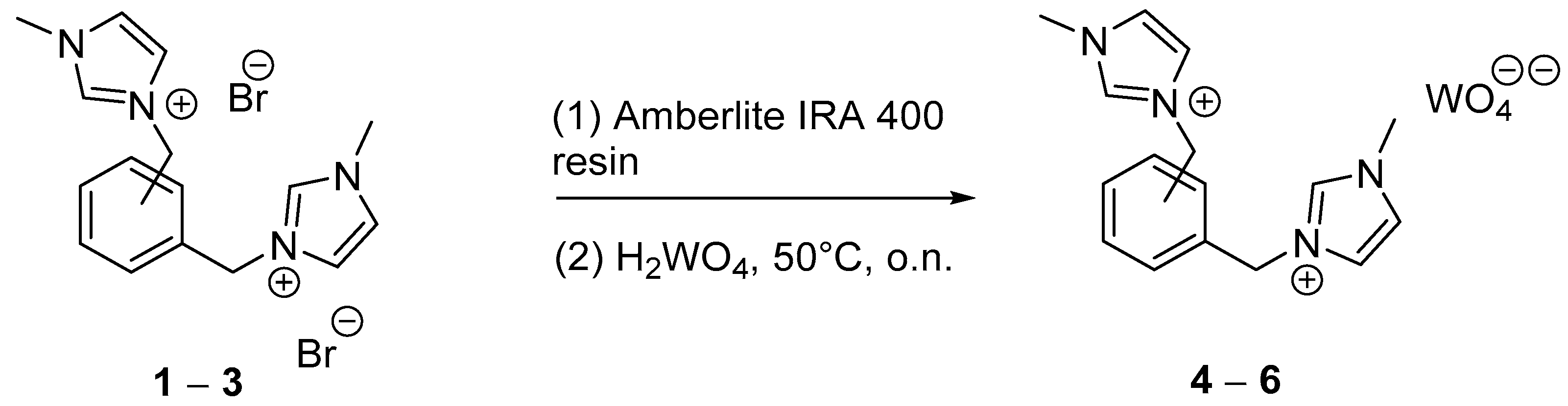
2.1. Synthesis
2.2. Thermal Characterization
2.3. Computational Results—DFT Studies
2.4. Computational Results—Molecular Dynamics Using the GFN2 Force Field
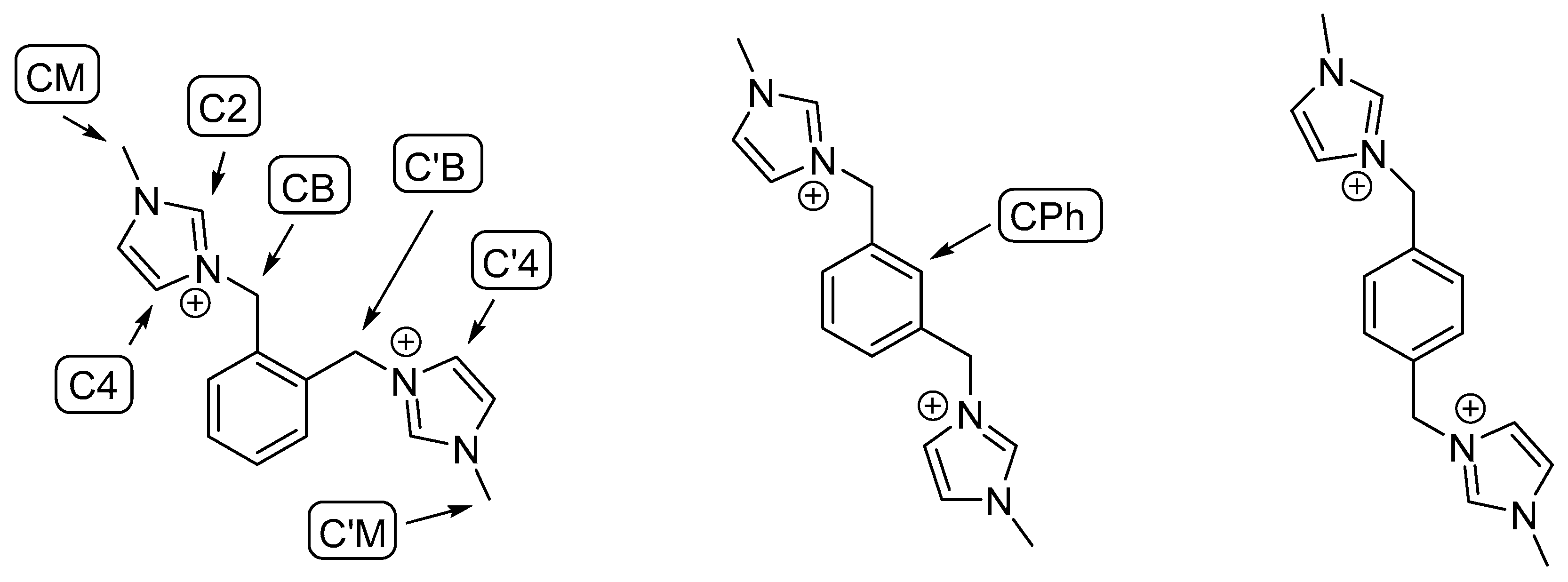
- The electrostatic effect of the double-charged WO42− anion is stronger. This has several effects on the geometry of the dications, the most evident being the stretching of the C2-H and C2′-H bonds (Table 3).
- Differently from the WO42− systems, in the bromide case, there are two different anions that can be displaced independently. The dication/2Br− systems therefore has a larger number of interionic degrees of freedom than the dication/WO42− ones. This was also demonstrated by the existence of the external conformations in the first case only.
- The WO42− ion is bulkier than the bromide one. This lead to a greater sterical hindering and to a more rigid structure.
2.5. Proof-of-Concept Test of Compounds 1–6 as Catalysts for the of Cycloaddition of CO2 to Epoxides
3. Materials and Methods
3.1. General Procedure for the Synthesis of 1,1′-(1,n-Phenylenebis(methylene))bis(3-methylimidazolium) Bromides (1–3)
3.2. 1,1′-(1,2-Phenylenebis(methylene))bis(3-methylimidazolium) Bromide (1)
3.3. 1,1′-(1,3-Phenylenebis(methylene))bis(3-methylimidazolium) Bromide (2)
3.4. 1,1′-(1,4-Phenylenebis(methylene))bis(3-methylimidazolium) Bromide (3)
3.5. General Procedure for the Synthesis of 1,1′-(1,N-phenylenebis(methylene))bis(3-methylimidazolium) Tungstates (4–6)
3.6. 1,1′-(1,2-Phenylenebis(methylene))bis(3-methylimidazolium) Tungstate (4)
3.7. 1,1′-(1,3-Phenylenebis(methylene))bis(3-methylimidazolium) Tungstate (5)
3.8. 1,1′-(1,4-Phenylenebis(methylene))bis(3-methylimidazolium) Tungstate (6)
3.9. General Procedure for the DILs Catalyzed Cycloaddition of CO2 to Epichlorohydrin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Silva, S.S.; Mano, J.F.; Reis, R.L. Ionic liquids in the processing and chemical modification of chitin and chitosan for biomedical applications. Green Chem. 2017, 19, 1208–1220. [Google Scholar] [CrossRef]
- Brandt-Talbot, A.; Gschwend, F.J.V.; Fennell, P.S.; Lammens, T.M.; Tan, B.; Weale, J.; Hallett, J.P. An economically viable ionic liquid for the fractionation of lignocellulosic biomass. Green Chem. 2017, 19, 3078–3102. [Google Scholar] [CrossRef]
- Toledo Hijo, A.A.C.; Maximo, G.J.; Costa, M.C.; Batista, E.A.C.; Meirelles, A.J.A. Applications of Ionic Liquids in the Food and Bioproducts Industries. ACS Sustain. Chem. Eng. 2016, 4, 5347–5369. [Google Scholar] [CrossRef]
- Berga, L.; Bruce, I.; Nicol, T.W.J.; Holding, A.J.; Isobe, N.; Shimizu, S.; Walker, A.J.; Reid, J.E.S.J. Cellulose dissolution and regeneration using a non-aqueous, non-stoichiometric protic ionic liquid system. Cellulose 2020, 27, 9593–9603. [Google Scholar] [CrossRef]
- Isik, M.; Sardon, H.; Mecerreyes, D. Ionic Liquids and Cellulose: Dissolution, Chemical Modification and Preparation of New Cellulosic Materials. Int. J. Mol. Sci. 2014, 15, 11922–11940. [Google Scholar] [CrossRef]
- Chiappe, C.; Pomelli, C.S. Point-Functionalization of Ionic Liquids: An Overview of Synthesis and Applications. Eur. J. Org. Chem. 2014, 2014, 6120–6139. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.; Headley, A.D. Ionic-Liquid-Supported (ILS) Catalysts for Asymmetric Organic Synthesis. Chem. A Eur. J. 2010, 16, 4426–4436. [Google Scholar] [CrossRef]
- Piatti, E.; Guglielmero, L.; Tofani, G.; Mezzetta, A.; Guazzelli, L.; D’Andrea, F.; Roddaro, S.; Pomelli, C.S. Ionic liquids for electrochemical applications: Correlation between molecular structure and electrochemical stability window. J. Mol. Liq. 2022, 364, 120001. [Google Scholar] [CrossRef]
- Huie, M.M.; DiLeo, R.A.; Marschilok, A.C.; Takeuchi, K.J.; Takeuchi, E.S. Ionic Liquid Hybrid Electrolytes for Lithium-Ion Batteries: A Key Role of the Separator–Electrolyte Interface in Battery Electrochemistry. ACS Appl. Mater. Interfaces 2015, 7, 11724–11731. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Thomas, M.L.; Zhang, S.; Ueno, K.; Yasuda, T.; Dokko, K. Application of Ionic Liquids to Energy Storage and Conversion Materials and Devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar] [CrossRef] [PubMed]
- Jónsson, E. Ionic liquids as electrolytes for energy storage applications—A modelling perspective. Energy Storage Mater. 2020, 25, 827–835. [Google Scholar] [CrossRef]
- Noack, J.; Roznyatovskaya, N.; Herr, T.; Fischer, P. The Chemistry of Redox-Flow Batteries. Angew. Chem. Int. Ed. 2015, 54, 9776–9809. [Google Scholar] [CrossRef] [PubMed]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef] [PubMed]
- Demurtas, M.; Onnis, V.; Zucca, P.; Rescigno, A.; Lachowicz, J.I.; De Villiers Engelbrecht, L.; Nieddu, M.; Ennas, G.; Scano, A.; Mocci, F.; et al. Cholinium-Based Ionic Liquids from Hydroxycinnamic Acids as New Promising Bioactive Agents: A Combined Experimental and Theoretical Investigation. ACS Sustain. Chem. Eng. 2021, 9, 2975–2986. [Google Scholar] [CrossRef]
- Cao, Y.; Mu, T. Comprehensive Investigation on the Thermal Stability of 66 Ionic Liquids by Thermogravimetric Analysis. Ind. Eng. Chem. Res. 2014, 53, 8651–8664. [Google Scholar] [CrossRef]
- Fox, D.M.; Gilman, J.W.; Morgan, A.B.; Shields, J.R.; Maupin, P.H.; Lyon, R.E.; De Long, H.C.; Trulove, P.C. Flammability and Thermal Analysis Characterization of Imidazolium-Based Ionic Liquids. Ind. Eng. Chem. Res. 2008, 47, 6327–6332. [Google Scholar] [CrossRef]
- Kazemiabnavi, S.; Zhang, Z.; Thornton, K.; Banerjee, S. Electrochemical Stability Window of Imidazolium-Based Ionic Liquids as Electrolytes for Lithium Batteries. J. Phys. Chem. B 2016, 120, 5691–5702. [Google Scholar] [CrossRef]
- Cimini, A.; Palumbo, O.; Simonetti, E.; De Francesco, M.; Appetecchi, G.B.; Fantini, S.; Lin, R.; Falgayrat, A.; Paolone, A. Decomposition temperatures and vapour pressures of selected ionic liquids for electrochemical applications. J. Therm. Anal. Calorim. 2020, 142, 1791–1797. [Google Scholar] [CrossRef]
- Pedro, S.N.; Freire, C.S.R.; Silvestre, A.J.D.; Freire, M.G. The Role of Ionic Liquids in the Pharmaceutical Field: An Overview of Relevant Applications. Int. J. Mol. Sci. 2020, 21, 8298. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Cheng, Z. Thermal Stability of Ionic Liquids: Current Status and Prospects for Future Development. Processes 2021, 9, 337. [Google Scholar] [CrossRef]
- Renier, O.; Bousrez, G.; Yang, M.; Hoelter, M.; Mallick, B.; Smetana, V.; Mudring, A.-V. Developing design tools for introducing and tuning structural order in ionic liquids. CrystEngComm 2021, 23, 1785–1795. [Google Scholar] [CrossRef]
- Majhi, D.; Seth, S.; Sarkar, M. Differences in the behavior of dicationic and monocationic ionic liquids as revealed by time resolved-fluorescence, NMR and fluorescence correlation spectroscopy. Phys. Chem. Chem. Phys. 2018, 20, 7844–7856. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Ding, R.; Ellern, A.; Armstrong, D.W. Structure and Properties of High Stability Geminal Dicationic Ionic Liquids. J. Am. Chem. Soc. 2005, 127, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.H. Task-Specific Ionic Liquids. Chem. Lett. 2004, 33, 1072–1077. [Google Scholar] [CrossRef]
- Giernoth, R. Task-Specific Ionic Liquids. Angew. Chem. Int. Ed. 2010, 49, 2834–2839. [Google Scholar] [CrossRef] [PubMed]
- Javaherian, M.; Saghanezhad, S.J. Synthesis, Characterization and Applications of Dicationic Ionic Liquids in Organic Synthesis. Mini-Rev. Org. Chem. 2020, 17, 450–464. [Google Scholar] [CrossRef]
- Guglielmero, L.; Mezzetta, A.; Guazzelli, L.; Pomelli, C.S.; D’Andrea, F.; Chiappe, C. Systematic Synthesis and Properties Evaluation of Dicationic Ionic Liquids, and a Glance into a Potential New Field. Front. Chem. 2018, 6, 612. [Google Scholar] [CrossRef] [PubMed]
- Shirota, H.; Mandai, T.; Fukazawa, H.; Kato, T. Comparison between Dicationic and Monocationic Ionic Liquids: Liquid Density, Thermal Properties, Surface Tension, and Shear Viscosity. J. Chem. Eng. Data 2011, 56, 2453–2459. [Google Scholar] [CrossRef]
- Vieira, J.C.; Villetti, M.A.; Frizzo, C.P. Thermal stability and decomposition mechanism of dicationic imidazolium-based ionic liquids with carboxylate anions. J. Mol. Liq. 2021, 330, 115618. [Google Scholar] [CrossRef]
- Bender, C.; Kuhn, B.; Farias, C.; Ziembowicz, F.; Beck, T.; Frizzo, C. Thermal Stability and Kinetic of Decomposition of Mono- and Dicationic Imidazolium-Based Ionic Liquids. J. Braz. Chem. Soc. 2019, 30, 2199–2209. [Google Scholar] [CrossRef]
- Bortolini, O.; Chiappe, C.; Fogagnolo, M.; Massi, A.; Pomelli, C.S. Formation, Oxidation, and Fate of the Breslow Intermediate in the N-Heterocyclic Carbene-Catalyzed Aerobic Oxidation of Aldehydes. J. Org. Chem. 2017, 82, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Bortolini, O.; Chiappe, C.; Fogagnolo, M.; Giovannini, P.P.; Massi, A.; Pomelli, C.S.; Ragno, D. An insight into the mechanism of the aerobic oxidation of aldehydes catalyzed by N-heterocyclic carbenes. Chem. Commun. 2014, 50, 2008–2011. [Google Scholar] [CrossRef] [PubMed]
- Mezzetta, A.; Guglielmero, L.; Mero, A.; Tofani, G.; D’andrea, F.; Pomelli, C.S.; Guazzelli, L. Expanding the Chemical Space of Benzimidazole Dicationic Ionic Liquids. Molecules 2021, 26, 4211. [Google Scholar] [CrossRef]
- Guglielmero, L.; Mezzetta, A.; Pomelli, C.S.; Chiappe, C.; Guazzelli, L. Evaluation of the effect of the dicationic ionic liquid structure on the cycloaddition of CO2 to epoxides. J. CO2 Util. 2019, 34, 437–445. [Google Scholar] [CrossRef]
- Zhang, H.; Li, M.; Yang, B. Design, Synthesis, and Analysis of Thermophysical Properties for Imidazolium-Based Geminal Dicationic Ionic Liquids. J. Phys. Chem. C 2018, 122, 2467–2474. [Google Scholar] [CrossRef]
- Ferdeghini, C.; Mezzetta, A.; D’andrea, F.; Pomelli, C.S.; Guazzelli, L.; Guglielmero, L. The Structure–Property Relationship of Pyrrolidinium and Piperidinium-Based Bromide Organic Materials. Materials 2022, 15, 8483. [Google Scholar] [CrossRef]
- Ferdeghini, C.; Guazzelli, L.; Pomelli, C.S.; Ciccioli, A.; Brunetti, B.; Mezzetta, A.; Ciprioti, S.V. Synthesis, thermal behavior and kinetic study of N-morpholinium dicationic ionic liquids by thermogravimetry. J. Mol. Liq. 2021, 332, 115662. [Google Scholar] [CrossRef]
- Anthofer, M.H.; Wilhelm, M.E.; Cokoja, M.; Drees, M.; Herrmann, W.A.; Kühn, F.E. Hydroxy-Functionalized Imidazolium Bromides as Catalysts for the Cycloaddition of CO2 and Epoxides to Cyclic Carbonates. ChemCatChem 2015, 7, 94–98. [Google Scholar] [CrossRef]
- Liu, M.; Liang, L.; Liang, T.; Lin, X.; Shi, L.; Wang, F.; Sun, J. Cycloaddition of CO2 and epoxides catalyzed by dicationic ionic liquids mediated metal halide: Influence of the dication on catalytic activity. J. Mol. Catal. A Chem. 2015, 408, 242–249. [Google Scholar] [CrossRef]
- Bahadori, L.; Boyd, R.; Warrington, A.; Shafeeyan, M.; Nokian, P. Evaluation of ionic liquids as electrolytes for vanadium redox flow batteries. J. Mol. Liq. 2020, 317, 114017. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Q.; Shi, X.; Li, Y. Tetrabutylammonium hexafluorophosphate and 1-ethyl-3-methyl imidazolium hexafluorophosphate ionic liquids as supporting electrolytes for non-aqueous vanadium redox flow batteries. J. Power Sources 2012, 203, 201–205. [Google Scholar] [CrossRef]
- Bahadori, L.; Hashim, M.A.; Manan, N.S.A.; Mjalli, F.S.; AlNashef, I.M.; Brandon, N.P.; Chakrabarti, M.H. Investigation of Ammonium- and Phosphonium-Based Deep Eutectic Solvents as Electrolytes for a Non-Aqueous All-Vanadium Redox Cell. J. Electrochem. Soc. 2016, 163, A632–A638. [Google Scholar] [CrossRef]
- Guglielmero, L.; Langroudi, M.M.; Al Khatib, M.; de Oliveira, M.A.C.; Mecheri, B.; De Leo, M.; Mezzetta, A.; Guazzelli, L.; Giglioli, R.; Epifanio, A.D.; et al. Electrochemical and spectroscopic study of vanadyl acetylacetonate–ionic liquids interactions. Electrochim. Acta 2021, 373, 137865. [Google Scholar] [CrossRef]
- Wang, K.; Adidharma, H.; Radosz, M.; Wan, P.; Xu, X.; Russell, C.K.; Tian, H.; Fan, M.; Yu, J. Recovery of rare earth elements with ionic liquids. Green Chem. 2017, 19, 4469–4493. [Google Scholar] [CrossRef]
- Salminen, J.; Blomberg, P.; Mäkinen, J.; Räsänen, L. Environmental aspects of metals removal from waters and gold recovery. AIChE J. 2015, 61, 2739–2748. [Google Scholar] [CrossRef]
- Ahmad, M.G.; Chanda, K. Ionic liquid coordinated metal-catalyzed organic transformations: A comprehensive review. Co-ord. Chem. Rev. 2022, 472, 214769. [Google Scholar] [CrossRef]
- Guglielmero, L.; Mero, A.; Mezzetta, A.; Tofani, G.; D’Andrea, F.; Pomelli, C.; Guazzelli, L. Novel access to ionic liquids based on trivalent metal–EDTA complexes and their thermal and electrochemical characterization. J. Mol. Liq. 2021, 340, 117210. [Google Scholar] [CrossRef]
- Liu, F.; Yu, J.; Qazi, A.B.; Zhang, L.; Liu, X. Metal-Based Ionic Liquids in Oxidative Desulfurization: A Critical Review. Environ. Sci. Technol. 2021, 55, 1419–1435. [Google Scholar] [CrossRef]
- Bragato, N.; Perosa, A.; Selva, M.; Fiorani, G.; Calmanti, R. Molybdate ionic liquids as halide-free catalysts for CO2 fixation into epoxides. Green Chem. 2023, 25, 4849–4860. [Google Scholar] [CrossRef]
- Calmanti, R.; Selva, M.; Perosa, A. Tungstate ionic liquids as catalysts for CO2 fixation into epoxides. Mol. Catal. 2020, 486, 110854. [Google Scholar] [CrossRef]
- Schmidt, F.; Zehner, B.; Korth, W.; Jess, A.; Cokoja, M. Ionic liquid surfactants as multitasking micellar catalysts for epoxidations in water. Catal. Sci. Technol. 2020, 10, 4448–4457. [Google Scholar] [CrossRef]
- Kimura, T.; Sunaba, H.; Kamata, K.; Mizuno, N. Efficient [WO4]2–-Catalyzed Chemical Fixation of Carbon Dioxide with 2-Aminobenzonitriles to Quinazoline-2,4(1H,3H)-diones. Inorg. Chem. 2012, 51, 13001–13008. [Google Scholar] [CrossRef] [PubMed]
- Kamata, K.; Kimura, T.; Sunaba, H.; Mizuno, N. Scope of chemical fixation of carbon dioxide catalyzed by a bifunctional monomeric tungstate. Catal. Today 2014, 226, 160–166. [Google Scholar] [CrossRef]
- Calmanti, R.; Sargentoni, N.; Selva, M.; Perosa, A. One-Pot Tandem Catalytic Epoxidation—CO2 Insertion of Monounsaturated Methyl Oleate to the Corresponding Cyclic Organic Carbonate. Catalysts 2021, 11, 1477. [Google Scholar] [CrossRef]
- Hu, J.; Ma, J.; Zhu, Q.; Zhang, Z.; Wu, C.; Han, B. Transformation of Atmospheric CO2 Catalyzed by Protic Ionic Liquids: Efficient Synthesis of 2-Oxazolidinones. Angew. Chem. Int. Ed. 2015, 54, 5399–5403. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Wang, Y.; Tao, H.; Yu, Z.; Zhang, Y. Bisimidazolium Tungstate Ionic Liquids: Highly Efficient Catalysts for the Synthesis of Linear Organic Carbonates by the Reaction of Ethylene Carbonate with Alcohols. Catal. Lett. 2023, 153, 62–73. [Google Scholar] [CrossRef]
- Jordão, A.K.; Pinheiro, T.D.N.; Barbosa, E.G. Sodium Tungstate Dihydrate (Na2WO4·2H2O): A Mild Oxidizing and Efficient Reagent in Organic Synthesis. SynOpen 2022, 06, 208–210. [Google Scholar] [CrossRef]
- Noyori, R.; Aoki, M.; Sato, K. Green oxidation with aqueous hydrogen peroxide. Chem. Commun. 2003, 34, 1977–1986. [Google Scholar] [CrossRef]
- Mero, A.; Guglielmero, L.; Guazzelli, L.; D’andrea, F.; Mezzetta, A.; Pomelli, C.S. A Specific Interaction between Ionic Liquids’ Cations and Reichardt’s Dye. Molecules 2022, 27, 7205. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Alias, Y. Synthesis and Characterization of Novel Dimeric Ionic Liquids by Conventional Approaches. Int. J. Mol. Sci. 2008, 9, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Batsanov, S.S. Van der Waals Radii of Elements. Inorg. Mater. 2001, 37, 871–885. [Google Scholar] [CrossRef]
- Verma, P.L.; Gejji, S.P. Electronic structure and spectral characteristics of alkyl substituted imidazolium based dication-X2 (X = Br, BF4, PF6 and CF3SO3) complexes from theory. J. Mol. Liq. 2019, 293, 111548. [Google Scholar] [CrossRef]
- Bannwarth, C.; Caldeweyher, E.; Ehlert, S.; Hansen, A.; Pracht, P.; Seibert, J.; Spicher, S.; Grimme, S. Extended tight-binding quantum chemistry methods. WIREs Comput. Mol. Sci. 2021, 11, e1493. [Google Scholar] [CrossRef]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB—An Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.L.; Anderson, J.L.; Maginn, E.J.; Brennecke, J.F. Anion Effects on Gas Solubility in Ionic Liquids. J. Phys. Chem. B 2005, 109, 6366–6374. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-Y.; Ma, R.; He, L.-N. Polyoxometalate-based ionic liquids-promoted CO2 conversion. Sci. China Chem. 2016, 59, 507–516. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016.
- Spicher, S.; Grimme, S. Robust Atomistic Modeling of Materials, Organometallic, and Biochemical Systems. Angew. Chem. Int. Ed. 2020, 59, 15665–15673. [Google Scholar] [CrossRef] [PubMed]
- Magill, A.M.; McGuinness, D.S.; Cavell, K.J.; Britovsek, G.J.; Gibson, V.C.; White, A.J.; Williams, D.J.; White, A.H.; Skelton, B.W. Palladium(II) complexes containing mono-, bi- and tridentate carbene ligands. Synthesis, characterisation and application as catalysts in CC coupling reactions. J. Organomet. Chem. 2001, 617–618, 546–560. [Google Scholar] [CrossRef]
- Ibrahim, H.; Koorbanally, N.A.; Ramjugernath, D.; Bala, M.D.; Nyamori, V.O. Synthesis and Characterization of Imidazolium Salts Bearing Fluorinated Anions. Z. Anorg. Allg. Chem. 2012, 638, 2304–2309. [Google Scholar] [CrossRef]











| Compound | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Tpeak (°C) | 334.2 | 361.5 | 355.2 | 214.8 | 251.2 | 260.3 |
| Tonset (°C) | 310.7 | 296.0 | 328.7 | 205.3 | 233.2 | 248.1 |
| Tstart 5% (°C) | 281.2 | 284.5 | 299.7 | 217.2 | 240.2 | 253.1 |
| Isomer | Ortho | Meta | Para | |||
|---|---|---|---|---|---|---|
| Conformer | Internal | External | Internal | External | Internal | External |
| Energies | ||||||
| Abs. Energies (Ha) | −5988.938627 | −5988.939888 | −5988.945324 | −5988.927851 | −5988.936105 | −5988.930137 |
| ∆E + ∆ZPE (KJ/mol) | +17.58 | +14.27 | 0.00 | +45.87 | +24.20 | +39.87 |
| ∆∆E + ∆∆ZPE (KJ/mol) | −3.31 | +45.87 | +15.67 | |||
| distances/Å | ||||||
| H(C2)-Br1 | 2.49 | - | 2.66 | - | 2.48 | 2.97 |
| H(C2)-Br2 | - | 2.50 | 3.38 | 2.25 | - | 3.01 |
| H′(C2)-Br1 | - | 2.41 | 2.42 | 2.89 | 2.48 | 2.36 |
| H′(C2)-Br2 | 2.43 | - | 3.95 | - | - | - |
| H(C4)-Br1 | 2.80 | - | - | 2.53 | - | - |
| H′(C4)-Br2 | 2.63 | - | - | - | - | - |
| H1(CB)-Br2 | 2.65 | 2.64 | 2.79 | - | - | - |
| H1′(CB)-Br1 | 2.89 | 2.48 | - | - | - | - |
| H2′(CB)-Br2 | - | 2.70 | - | - | - | - |
| H1(CM)-Br1 | - | - | - | - | 2.98 | 2.63 |
| H2(CM)-Br2 | - | - | - | - | 2.84 | - |
| H1′(CM)-Br1 | - | - | - | - | 2.98 | 2.86 |
| H2′(CM)-Br2 | - | - | - | - | 2.85 | - |
| Isomer | Ortho | Meta | Para |
|---|---|---|---|
| Abs. energies (Ha) | −1209.542873 | −1209.586818 | −1209.573244 |
| ∆E + ∆ZPE (KJ/mol) | +115.38 | 0.00 | +35.64 |
| C2-H | 1.08 | 1.16 | 1.09 |
| C2′-H | 1.10 | 1.08 | 1.13 |
| H(C2)-W | 2.78 | 2.82 | |
| H′(C2)-W | 2.67 | 2.80 | 2.80 |
| H(C4)-W | 3.13 | ||
| H(C5)-W | 3.21 | ||
| H(CPh)-W | 2.91 | ||
| W-O (average) | 1.78 | 1.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso Gomes, G.; Ferdeghini, C.; Guglielmero, L.; D’Andrea, F.; Guazzelli, L.; Mezzetta, A.; Pomelli, C.S. A Combined Experimental/Computational Study of Dicationic Ionic Liquids with Bromide and Tungstate Anions. Molecules 2024, 29, 2131. https://doi.org/10.3390/molecules29092131
Cardoso Gomes G, Ferdeghini C, Guglielmero L, D’Andrea F, Guazzelli L, Mezzetta A, Pomelli CS. A Combined Experimental/Computational Study of Dicationic Ionic Liquids with Bromide and Tungstate Anions. Molecules. 2024; 29(9):2131. https://doi.org/10.3390/molecules29092131
Chicago/Turabian StyleCardoso Gomes, Guelber, Claudio Ferdeghini, Luca Guglielmero, Felicia D’Andrea, Lorenzo Guazzelli, Andrea Mezzetta, and Christian Silvio Pomelli. 2024. "A Combined Experimental/Computational Study of Dicationic Ionic Liquids with Bromide and Tungstate Anions" Molecules 29, no. 9: 2131. https://doi.org/10.3390/molecules29092131






