Discordant Health Implications and Molecular Mechanisms of Vitamin D in Clinical and Preclinical Studies of Prostate Cancer: A Critical Appraisal of the Literature Data
Abstract
:1. Introduction: The Aim of This Opinion Paper
2. Vitamin D Metabolism, Vitamin D Status, and Biological Actions of Vitamin D
2.1. Vitamin D Metabolism
2.2. Circulating 25(OH)D3 Level as Indicator of the Vitamin D Status
2.3. Calcitriol as Functional Ligand of the Vitamin D Receptor, the Expression of Vitamin D Target Genes, and the Effect on Biological Processes
3. Vitamin D in Preclinical Studies of Prostate Cancer
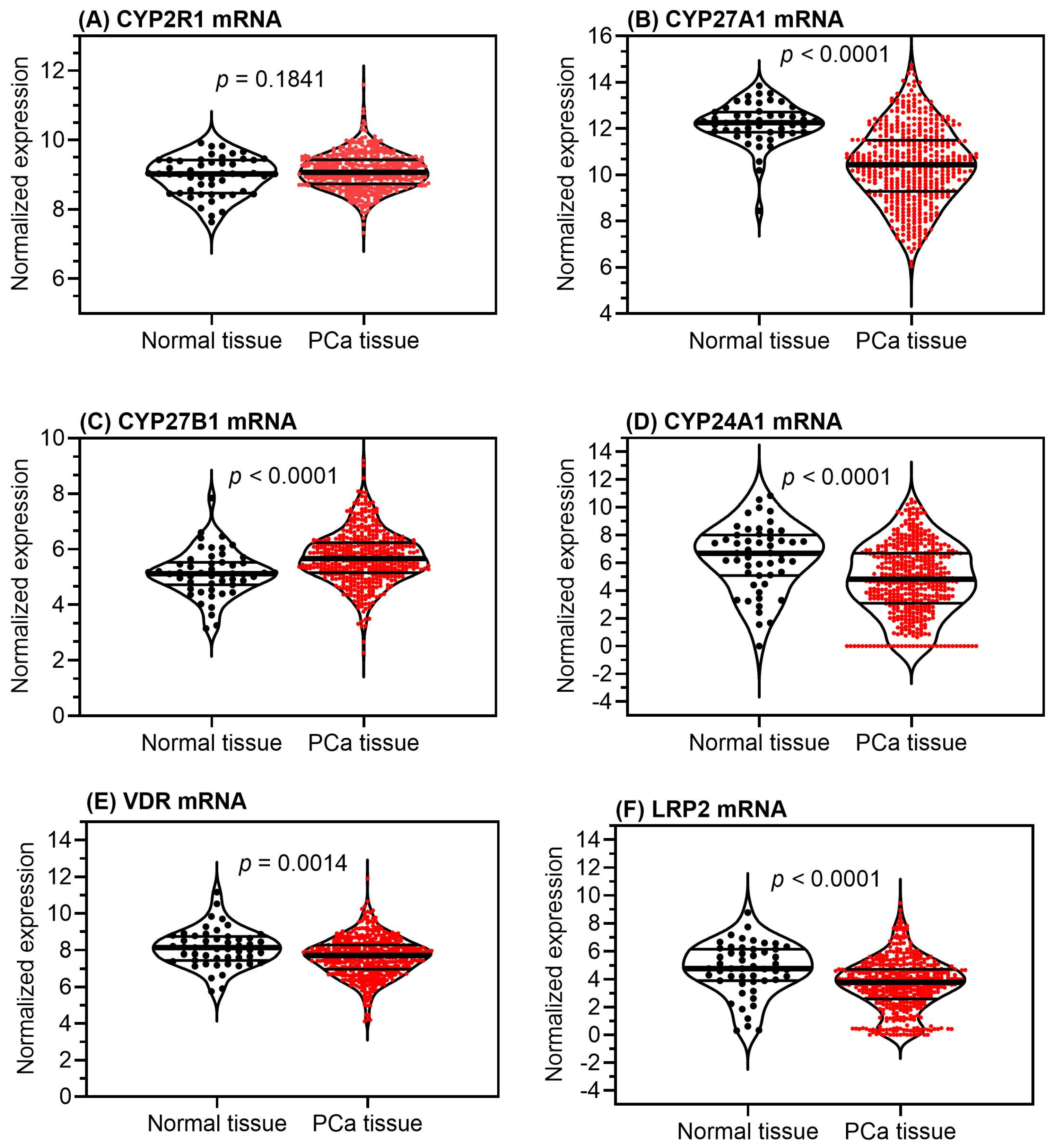
4. Vitamin D in Clinical Studies of Prostate Cancer
5. Discordance between Preclinical and Clinical Vitamin D Related Study Results in Prostate Cancer
6. Conclusions and Outlook for Future Research
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1,25(OH)2D3 | 1,25-dihydroxyvitamin D3, calcitriol |
| 25(OH)D | 25-hydroxyvitamin D as summation of 25(OH)D3 + 25(OH)D2 |
| 25(OH)D2 | 25-hydroxyvitamin D2 |
| 25(OH)D3 | 25-hydroxyvitamin D3 |
| BPH-1 | human benign prostatic hyperplasia cell line |
| C4-2 | androgen-independent cell line derived from LNCaP cell subcutaneous xenograft tumor of castrated mouse |
| CI | confidence interval |
| CWR22R | androgen-sensitive cell line derived from a human PCa xenograft serially propagated in mice that can proliferate without androgens, but show a faster proliferation with androgen as component in the culture medium |
| CYP24A1 | cytochrome P450 family 24 subfamily A member 1 |
| CYP27A1 | cytochrome P450 family 27 subfamily A member 1 |
| CYP27B1 | cytochrome P450 family 27 subfamily B member 1 |
| CYP2R1 | cytochrome P450 family 2 subfamily R member 1 |
| DKK3 | dickkopf WNT signaling pathway inhibitor 3 |
| DU 145 | androgen-independent PCa cell line |
| G6PD | glucose-6-phosphate dehyrogenase |
| HPr1 | cell line derived from normal prostate epithelial cells |
| HPr1AR | cell line derived from normal prostate epithelial cells |
| HR | hazard ratio |
| IGFBP3 | insulin like growth factor binding protein 3 |
| LNCaP | androgen-dependent PCa cell line |
| LRP2 | LDL-receptor-related protein 2 |
| miRNA, miR | microRNA |
| MMP9 | matrix metallopeptidase 9 |
| NFKB1 | nuclear factor kappa B subunit 1 |
| Notch | Notch signalling pathway |
| OR | odds ratio |
| PC-3 | androgen-independent PCa cell line |
| PCa | prostate cancer |
| RCT | randomized controlled clinical trial |
| RR | relative risk |
| RWPE-1 | cell line derived from normal prostate epithelial cells |
| RWPE-2 | androgen-dependent PCa cell line from early stage PCa |
| RXR | retinoid X receptor |
| TCGA | The Cancer Genome Atlas |
| TIMP1 | TIMP metallopeptidase inhibitor 1 |
| VCaP | androgen-dependent PCa cell line |
| VDBP | vitamin D binding protein |
| VDR | vitamin D receptor |
| VDRE | vitamin D reponse elements |
| WNT | WNT signaling pathway |
References
- Mandrola, J.M. Why is vitamin D hype so impervious to evidence? Medscape, 17 February 2022. [Google Scholar]
- Sempos, C.T.; Heijboer, A.C.; Bikle, D.D.; Bollerslev, J.; Bouillon, R.; Brannon, P.M.; DeLuca, H.F.; Jones, G.; Munns, C.F.; Bilezikian, J.P.; et al. Vitamin D assays and the definition of hypovitaminosis D: Results from the First International Conference on Controversies in Vitamin D. Br. J. Clin. Pharmacol. 2018, 84, 2194–2207. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Adler, R.A.; Binkley, N.; Bouillon, R.; Ebeling, P.R.; Lazaretti-Castro, M.; Marcocci, C.; Rizzoli, R.; Sempos, C.T.; Bilezikian, J.P. Controversies in vitamin D: Summary statement from an International Conference. J. Clin. Endocrinol. Metab. 2019, 104, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Bouillon, R.; Binkley, N.; Sempos, C.; Adler, R.A.; Bollerslev, J.; Dawson-Hughes, B.; Ebeling, P.R.; Feldman, D.; Heijboer, A.; et al. Controversies in vitamin D: A statement from the Third International Conference. JBMR Plus 2020, 4, e10417. [Google Scholar] [CrossRef]
- Giustina, A.; Adler, R.A.; Binkley, N.; Bollerslev, J.; Bouillon, R.; Dawson-Hughes, B.; Ebeling, P.R.; Feldman, D.; Formenti, A.M.; Lazaretti-Castro, M.; et al. Consensus statement from 2(nd) International Conference on Controversies in Vitamin D. Rev. Endocr. Metab. Disord. 2020, 21, 89–116. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Bouillon, R.; Dawson-Hughes, B.; Ebeling, P.R.; Lazaretti-Castro, M.; Lips, P.; Marcocci, C.; Bilezikian, J.P. Vitamin D in the older population: A consensus statement. Endocrine 2023, 79, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Wintermeyer, E.; Ihle, C.; Ehnert, S.; Stockle, U.; Ochs, G.; de Zwart, P.; Flesch, I.; Bahrs, C.; Nussler, A.K. Crucial role of vitamin D in the musculoskeletal system. Nutrients 2016, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and extraskeletal actions of vitamin D: Current evidence and outstanding questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef] [PubMed]
- Sobhi, P.; Bahrami, M.; Mahdizadeh, F.; Fazaeli, A.; Babaei, G.; Rezagholizadeh, L. Vitamin D and potential effects on cancers: A review. Mol. Biol. Rep. 2024, 51, 190. [Google Scholar] [CrossRef] [PubMed]
- Emerging Risk Factors Collaboration/EPIC-CVD/Vitamin D Studies Collaboration; Sofianopoulou, E.; Kaptoge, S.K.; Afzal, S.; Jiang, T.; Gill, D.; Gundersen, T.E.; Bolton, T.R.; Allara, E.; Arnold, M.G.; et al. Estimating dose-response relationships for vitamin D with coronary heart disease, stroke, and all-cause mortality: Observational and Mendelian randomisation analyses. Lancet Diabetes Endocrinol. 2024, 12, e2–e11. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Mullooly, M.; Bennett, K.; Crown, J. Vitamin D supplementation: Does it have a preventative or therapeutic role in cancer? Nutr. Cancer 2023, 75, 450–460. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.; Chen, L.J.; Brenner, H.; Schottker, B. Associations of 25-hydroxyvitamin D status and vitamin D supplementation use with mortality due to 18 frequent cancer types in the UK Biobank cohort. Eur. J. Cancer 2023, 191, 113241. [Google Scholar] [CrossRef]
- Seraphin, G.; Rieger, S.; Hewison, M.; Capobianco, E.; Lisse, T.S. The impact of vitamin D on cancer: A mini review. J. Steroid Biochem. Mol. Biol. 2023, 231, 106308. [Google Scholar] [CrossRef] [PubMed]
- Sluyter, J.D.; Manson, J.E.; Scragg, R. Vitamin D and clinical cancer outcomes: A review of meta-analyses. JBMR Plus 2021, 5, e10420. [Google Scholar] [CrossRef]
- Pilz, S.; Trummer, C.; Theiler-Schwetz, V.; Grubler, M.R.; Verheyen, N.D.; Odler, B.; Karras, S.N.; Zittermann, A.; Marz, W. Critical appraisal of large vitamin D randomized controlled trials. Nutrients 2022, 14, 303. [Google Scholar] [CrossRef]
- Song, Z.Y.; Yao, Q.; Zhuo, Z.; Ma, Z.; Chen, G. Circulating vitamin D level and mortality in prostate cancer patients: A dose-response meta-analysis. Endocr. Connect. 2018, 7, R294–R303. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.Y.; Yeh, S.D.; Lee, Y.F. 1alpha,25-dihydroxyvitamin D3 inhibits prostate cancer cell invasion via modulation of selective proteases. Carcinogenesis 2006, 27, 32–42. [Google Scholar] [CrossRef]
- Chung, I.; Han, G.; Seshadri, M.; Gillard, B.M.; Yu, W.D.; Foster, B.A.; Trump, D.L.; Johnson, C.S. Role of vitamin D receptor in the antiproliferative effects of calcitriol in tumor-derived endothelial cells and tumor angiogenesis in vivo. Cancer Res. 2009, 69, 967–975. [Google Scholar] [CrossRef]
- Swami, S.; Krishnan, A.V.; Wang, J.Y.; Jensen, K.; Horst, R.; Albertelli, M.A.; Feldman, D. Dietary vitamin D3 and 1,25-dihydroxyvitamin D3 (calcitriol) exhibit equivalent anticancer activity in mouse xenograft models of breast and prostate cancer. Endocrinology 2012, 153, 2576–2587. [Google Scholar] [CrossRef]
- Giangreco, A.A.; Dambal, S.; Wagner, D.; Van der Kwast, T.; Vieth, R.; Prins, G.S.; Nonn, L. Differential expression and regulation of vitamin D hydroxylases and inflammatory genes in prostate stroma and epithelium by 1,25-dihydroxyvitamin D in men with prostate cancer and an in vitro model. J. Steroid Biochem. Mol. Biol. 2015, 148, 156–165. [Google Scholar] [CrossRef]
- Abu El Maaty, M.A.; Alborzinia, H.; Khan, S.J.; Buttner, M.; Wolfl, S. 1,25(OH)(2)D(3) disrupts glucose metabolism in prostate cancer cells leading to a truncation of the TCA cycle and inhibition of TXNIP expression. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1618–1630. [Google Scholar] [CrossRef] [PubMed]
- Fleet, J.C.; Kovalenko, P.L.; Li, Y.; Smolinski, J.; Spees, C.; Yu, J.G.; Thomas-Ahner, J.M.; Cui, M.; Neme, A.; Carlberg, C.; et al. Vitamin D signaling suppresses early prostate carcinogenesis in TgAPT(121) mice. Cancer Prev. Res. 2019, 12, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Yui, Y.; Watanabe, K.; Kumai, J.; Nishizawa, Y.; Miyaura, C.; Inada, M.; Sasagawa, S. Molecular evidence of IGFBP-3 dependent and independent VD3 action and its nonlinear response on IGFBP-3 induction in prostate cancer cells. BMC Cancer 2020, 20, 802. [Google Scholar] [CrossRef] [PubMed]
- McCray, T.; Pacheco, J.V.; Loitz, C.C.; Garcia, J.; Baumann, B.; Schlicht, M.J.; Valyi-Nagy, K.; Abern, M.R.; Nonn, L. Vitamin D sufficiency enhances differentiation of patient-derived prostate epithelial organoids. iScience 2021, 24, 101974. [Google Scholar] [CrossRef] [PubMed]
- Yetkin, D.; Balli, E.; Ayaz, F. Antiproliferative activity of tamoxifen, vitamin D3 and their concomitant treatment. EXCLI J. 2021, 20, 1394–1406. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Krieger, K.D.; Loitz, C.; Perez, L.M.; Richards, Z.A.; Helou, Y.; Kregel, S.; Celada, S.; Mesaros, C.A.; Bosland, M.; et al. Regulation of prostate androgens by megalin and 25-hydroxyvitamin D status: Mechanism for high prostate androgens in African American Men. Cancer Res. Commun. 2023, 3, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Manousaki, D.; Rosen, C.; Trajanoska, K.; Rivadeneira, F.; Richards, J.B. The health effects of vitamin D supplementation: Evidence from human studies. Nat. Rev. Endocrinol. 2022, 18, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Park, S.; Zuniga, B.; Bera, A.; Song, C.S.; Chatterjee, B. Vitamin D in prostate Cancer. Vitam. Horm. 2016, 100, 321–355. [Google Scholar] [CrossRef]
- Jenkinson, C. The vitamin D metabolome: An update on analysis and function. Cell Biochem. Funct. 2019, 37, 408–423. [Google Scholar] [CrossRef]
- Saponaro, F.; Saba, A.; Zucchi, R. An update on vitamin D metabolism. Int. J. Mol. Sci. 2020, 21, 6573. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Farruggia, M.; Veronese, N.; Barbagallo, M. Vitamin D sources, metabolism, and deficiency: Available compounds and guidelines for its treatment. Metabolites 2021, 11, 255. [Google Scholar] [CrossRef]
- Zhu, J.G.; Ochalek, J.T.; Kaufmann, M.; Jones, G.; Deluca, H.F. CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo. Proc. Natl. Acad. Sci. USA 2013, 110, 15650–15655. [Google Scholar] [CrossRef] [PubMed]
- Quesada-Gomez, J.M.; Bouillon, R. Is calcifediol better than cholecalciferol for vitamin D supplementation? Osteoporos. Int. 2018, 29, 1697–1711. [Google Scholar] [CrossRef]
- Christensen, E.I.; Birn, H.; Storm, T.; Weyer, K.; Nielsen, R. Endocytic receptors in the renal proximal tubule. Physiology 2012, 27, 223–236. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Whitlatch, L.W.; Chen, T.C.; Lokeshwar, B.L.; Holick, M.F. Human prostate cells synthesize 1,25-dihydroxyvitamin D3 from 25-hydroxyvitamin D3. Cancer Epidemiol. Biomark. Prev. 1998, 7, 391–395. [Google Scholar]
- Richards, Z.; Batai, K.; Farhat, R.; Shah, E.; Makowski, A.; Gann, P.H.; Kittles, R.; Nonn, L. Prostatic compensation of the vitamin D axis in African American men. JCI Insight 2017, 2, e91054. [Google Scholar] [CrossRef]
- DeLuca, H.F. Vitamin D: Historical overview. Vitam. Horm. 2016, 100, 1–20. [Google Scholar] [CrossRef]
- Tuckey, R.C.; Cheng, C.Y.S.; Slominski, A.T. The serum vitamin D metabolome: What we know and what is still to discover. J. Steroid Biochem. Mol. Biol. 2019, 186, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Young, M.V.; Schwartz, G.G.; Wang, L.; Jamieson, D.P.; Whitlatch, L.W.; Flanagan, J.N.; Lokeshwar, B.L.; Holick, M.F.; Chen, T.C. The prostate 25-hydroxyvitamin D-1 alpha-hydroxylase is not influenced by parathyroid hormone and calcium: Implications for prostate cancer chemoprevention by vitamin D. Carcinogenesis 2004, 25, 967–971. [Google Scholar] [CrossRef]
- Campolina-Silva, G.H.; Barata, M.C.; Werneck-Gomes, H.; Maria, B.T.; Mahecha, G.A.B.; Belleannee, C.; Oliveira, C.A. Altered expression of the vitamin D metabolizing enzymes CYP27B1 and CYP24A1 under the context of prostate aging and pathologies. J. Steroid Biochem. Mol. Biol. 2021, 209, 105832. [Google Scholar] [CrossRef]
- Vivian, J.; Rao, A.A.; Nothaft, F.A.; Ketchum, C.; Armstrong, J.; Novak, A.; Pfeil, J.; Narkizian, J.; Deran, A.D.; Musselman-Brown, A.; et al. Toil enables reproducible, open source, big biomedical data analyses. Nat. Biotechnol. 2017, 35, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.J.; Craft, B.; Hastie, M.; Repecka, K.; McDade, F.; Kamath, A.; Banerjee, A.; Luo, Y.; Rogers, D.; Brooks, A.N.; et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 2020, 38, 675–678. [Google Scholar] [CrossRef]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Kostenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Nutrition, Novel Foods and Allergens; Turck, D.; Bohn, T.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. Scientific opinion on the tolerable upper intake level for vitamin D, including the derivation of a conversion factor for calcidiol monohydrate. EFSA J. 2023, 21, e08145. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Boucher, B.J.; Bhattoa, H.P.; Lahore, H. Why vitamin D clinical trials should be based on 25-hydroxyvitamin D concentrations. J. Steroid Biochem. Mol. Biol. 2018, 177, 266–269. [Google Scholar] [CrossRef]
- Bouillon, R. Comparative analysis of nutritional guidelines for vitamin D. Nat. Rev. Endocrinol. 2017, 13, 466–479. [Google Scholar] [CrossRef]
- German Nutrition Society. New reference values for vitamin D. Ann. Nutr. Metab. 2012, 60, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J. Clin. Endocrinol. Metab. 2012, 97, 1153–1158. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Bertoldo, F.; Cianferotti, L.; Di Monaco, M.; Falchetti, A.; Fassio, A.; Gatti, D.; Gennari, L.; Giannini, S.; Girasole, G.; Gonnelli, S.; et al. Definition, assessment, and management of vitamin D inadequacy: Suggestions, recommendations, and warnings from the Italian Society for Osteoporosis, Mineral Metabolism and Bone Diseases (SIOMMMS). Nutrients 2022, 14, 4148. [Google Scholar] [CrossRef]
- Pludowski, P.; Kos-Kudla, B.; Walczak, M.; Fal, A.; Zozulinska-Ziolkiewicz, D.; Sieroszewski, P.; Peregud-Pogorzelski, J.; Lauterbach, R.; Targowski, T.; Lewinski, A.; et al. Guidelines for preventing and treating vitamin D deficiency: A 2023 update in Poland. Nutrients 2023, 15, 695. [Google Scholar] [CrossRef]
- Alonso, N.; Zelzer, S.; Eibinger, G.; Herrmann, M. Vitamin D metabolites: Analytical challenges and clinical relevance. Calcif. Tissue Int. 2023, 112, 158–177. [Google Scholar] [CrossRef]
- Binkley, N.; Dawson-Hughes, B.; Durazo-Arvizu, R.; Thamm, M.; Tian, L.; Merkel, J.M.; Jones, J.C.; Carter, G.D.; Sempos, C.T. Vitamin D measurement standardization: The way out of the chaos. J. Steroid Biochem. Mol. Biol. 2017, 173, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Camara, J.E.; Wise, S.A.; Hoofnagle, A.N.; Williams, E.L.; Carter, G.D.; Jones, J.; Burdette, C.Q.; Hahm, G.; Nalin, F.; Kuszak, A.J.; et al. Assessment of serum total 25-hydroxyvitamin D assay commutability of Standard Reference Materials and College of American Pathologists Accuracy-Based Vitamin D (ABVD) Scheme and Vitamin D External Quality Assessment Scheme (DEQAS) materials: Vitamin D Standardization Program (VDSP) Commutability Study 2. Anal. Bioanal. Chem. 2021, 413, 5067–5084. [Google Scholar] [CrossRef]
- Wise, S.A.; Camara, J.E.; Sempos, C.T.; Lukas, P.; Le Goff, C.; Peeters, S.; Burdette, C.Q.; Nalin, F.; Hahm, G.; Durazo-Arvizu, R.A.; et al. Vitamin D Standardization Program (VDSP) intralaboratory study for the assessment of 25-hydroxyvitamin D assay variability and bias. J. Steroid Biochem. Mol. Biol. 2021, 212, 105917. [Google Scholar] [CrossRef]
- Sempos, C.T.; Williams, E.L.; Carter, G.D.; Jones, J.; Camara, J.E.; Burdette, C.Q.; Hahm, G.; Nalin, F.; Duewer, D.L.; Kuszak, A.J.; et al. Assessment of serum total 25-hydroxyvitamin D assays for Vitamin D External Quality Assessment Scheme (DEQAS) materials distributed at ambient and frozen conditions. Anal. Bioanal. Chem. 2022, 414, 1015–1028. [Google Scholar] [CrossRef] [PubMed]
- Wise, S.A.; Camara, J.E.; Burdette, C.Q.; Hahm, G.; Nalin, F.; Kuszak, A.J.; Merkel, J.; Durazo-Arvizu, R.A.; Williams, E.L.; Popp, C.; et al. Interlaboratory comparison of 25-hydroxyvitamin D assays: Vitamin D Standardization Program (VDSP) Intercomparison Study 2—Part 2 ligand binding assays—Impact of 25-hydroxyvitamin D(2) and 24R,25-dihydroxyvitamin D(3) on assay performance. Anal. Bioanal. Chem. 2022, 414, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Dirks, N.F.; Cavalier, E.; Heijboer, A.C. Vitamin D: Marker, measurand & measurement. Endocr. Connect. 2023, 12, e220269. [Google Scholar] [CrossRef]
- Lips, P. Relative value of 25(OH)D and 1,25(OH)2D measurements. J. Bone Miner. Res. 2007, 22, 1668–1671. [Google Scholar] [CrossRef]
- Dirks, N.F.; Martens, F.; Vanderschueren, D.; Billen, J.; Pauwels, S.; Ackermans, M.T.; Endert, E.; Heijer, M.D.; Blankenstein, M.A.; Heijboer, A.C. Determination of human reference values for serum total 1,25-dihydroxyvitamin D using an extensively validated 2D ID-UPLC-MS/MS method. J. Steroid Biochem. Mol. Biol. 2016, 164, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.; Ibrahim, F.; Boudou, P. Evaluation of a new automated assay for the measurement of circulating 1,25-dihydroxyvitamin D levels in daily practice. Clin. Biochem. 2015, 48, 1160–1162. [Google Scholar] [CrossRef] [PubMed]
- Myrtle, J.F.; Haussler, M.R.; Norman, A.W. Evidence for the biologically active form of cholecalciferol in the intestine. J. Biol. Chem. 1970, 245, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Schnoes, H.K.; DeLuca, H.E.; Suda, T.; Cousins, R.J. Isolation and identification of 1,25-dihydroxycholecalciferol. A metabolite of vitamin D active in the intestine. Biochemistry 1971, 10, 2799–2804. [Google Scholar] [PubMed]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef] [PubMed]
- Richards, Z.; McCray, T.; Marsili, J.; Zenner, M.L.; Manlucu, J.T.; Garcia, J.; Kajdacsy-Balla, A.; Murray, M.; Voisine, C.; Murphy, A.B.; et al. Prostate stroma increases the viability and maintains the branching phenotype of human prostate organoids. iScience 2019, 12, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Livingston, S.; Sabir, Z.L.; Haussler, C.A.; Jurutka, P.W. Vitamin D receptor mediates a myriad of biological actions dependent on its 1,25-dihydroxyvitamin D ligand: Distinct regulatory themes revealed by induction of Klotho and Fibroblast Growth Factor-23. JBMR Plus 2021, 5, e10432. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Genomic signaling of vitamin D. Steroids 2023, 198, 109271. [Google Scholar] [CrossRef]
- Molnar, F.; Perakyla, M.; Carlberg, C. Vitamin D receptor agonists specifically modulate the volume of the ligand-binding pocket. J. Biol. Chem. 2006, 281, 10516–10526. [Google Scholar] [CrossRef]
- Carlberg, C. Vitamin D genomics: From in vitro to in vivo. Front. Endocrinol. 2018, 9, 250. [Google Scholar] [CrossRef]
- Carlberg, C. Vitamin D and its target genes. Nutrients 2022, 14, 1354. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Genome-wide (over)view on the actions of vitamin D. Front. Physiol. 2014, 5, 167. [Google Scholar] [CrossRef] [PubMed]
- Trump, D.L.; Aragon-Ching, J.B. Vitamin D in prostate cancer. Asian J. Androl. 2018, 20, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.J.; Trump, D.L. Vitamin D receptor signaling and cancer. Endocrinol. Metab. Clin. N. Am. 2017, 46, 1009–1038. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.J. Vitamin D and the RNA transcriptome: More than mRNA regulation. Front. Physiol. 2014, 5, 181. [Google Scholar] [CrossRef] [PubMed]
- Jones, G. 100 Years of vitamin D: Historical aspects of vitamin D. Endocr. Connect. 2022, 11, e210594. [Google Scholar] [CrossRef] [PubMed]
- Norman, A.W. Minireview: Vitamin D receptor: New assignments for an already busy receptor. Endocrinology 2006, 147, 5542–5548. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Long, M.D.; Battaglia, S.; Hu, Q.; Liu, S.; Sucheston-Campbell, L.E.; Campbell, M.J. VDR regulation of microRNA differs across prostate cell models suggesting extremely flexible control of transcription. Epigenetics 2015, 10, 40–49. [Google Scholar] [CrossRef]
- Carlberg, C.; Velleuer, E. Vitamin D and the risk for cancer: A molecular analysis. Biochem. Pharmacol. 2022, 196, 114735. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M.; Shin, E.A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Gil, A.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and novel actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Munoz, A.; Grant, W.B. Vitamin D and cancer: An historical overview of the epidemiology and mechanisms. Nutrients 2022, 14, 1448. [Google Scholar] [CrossRef] [PubMed]
- Jaroslawska, J.; Carlberg, C. In vivo regulation of signal transduction pathways by vitamin D stabilizes homeostasis of human immune cells and counteracts molecular stress. Int. J. Mol. Sci. 2023, 24, 14632. [Google Scholar] [CrossRef]
- Kovalenko, P.L.; Zhang, Z.; Yu, J.G.; Li, Y.; Clinton, S.K.; Fleet, J.C. Dietary vitamin D and vitamin D receptor level modulate epithelial cell proliferation and apoptosis in the prostate. Cancer Prev. Res. 2011, 4, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Liu, M.D.; Yao, K.; Xu, S.; Yu, D.X.; Xie, D.D.; Xu, D.X. Vitamin D deficiency aggravates growth and metastasis of prostate cancer through promoting EMT in two beta-catenin-related mechanisms. J. Nutr. Biochem. 2023, 111, 109177. [Google Scholar] [CrossRef] [PubMed]
- Muindi, J.R.; Yu, W.D.; Ma, Y.; Engler, K.L.; Kong, R.X.; Trump, D.L.; Johnson, C.S. CYP24A1 inhibition enhances the antitumor activity of calcitriol. Endocrinology 2010, 151, 4301–4312. [Google Scholar] [CrossRef] [PubMed]
- Blutt, S.E.; McDonnell, T.J.; Polek, T.C.; Weigel, N.L. Calcitriol-induced apoptosis in LNCaP cells is blocked by overexpression of Bcl-2. Endocrinology 2000, 141, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.V.; Shinghal, R.; Raghavachari, N.; Brooks, J.D.; Peehl, D.M.; Feldman, D. Analysis of vitamin D-regulated gene expression in LNCaP human prostate cancer cells using cDNA microarrays. Prostate 2004, 59, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.V.; Weigel, N.L. Role of insulin-like growth factor binding proteins in 1alpha,25-dihydroxyvitamin D(3)-induced growth inhibition of human prostate cancer cells. Prostate 2005, 64, 9–19. [Google Scholar] [CrossRef]
- Bao, B.Y.; Ting, H.J.; Hsu, J.W.; Lee, Y.F. Protective role of 1 alpha, 25-dihydroxyvitamin D3 against oxidative stress in nonmalignant human prostate epithelial cells. Int. J. Cancer 2008, 122, 2699–2706. [Google Scholar] [CrossRef]
- Kovalenko, P.L.; Zhang, Z.; Cui, M.; Clinton, S.K.; Fleet, J.C. 1,25 dihydroxyvitamin D-mediated orchestration of anticancer, transcript-level effects in the immortalized, non-transformed prostate epithelial cell line, RWPE1. BMC Genom. 2010, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A.A.; Montecinos, V.P.; Paredes, R.; Godoy, A.S.; McNerney, E.M.; Tovar, H.; Pantoja, D.; Johnson, C.; Trump, D.; Onate, S.A. Biochemical characterization of nuclear receptors for vitamin D3 and glucocorticoids in prostate stroma cell microenvironment. Biochem. Biophys. Res. Commun. 2011, 412, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Giangreco, A.A.; Vaishnav, A.; Wagner, D.; Finelli, A.; Fleshner, N.; Van der Kwast, T.; Vieth, R.; Nonn, L. Tumor suppressor microRNAs, miR-100 and -125b, are regulated by 1,25-dihydroxyvitamin D in primary prostate cells and in patient tissue. Cancer Prev. Res. 2013, 6, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Erzurumlu, Y.; Aydogdu, E.; Dogan, H.K.; Catakli, D.; Muhammed, M.T.; Buyuksandic, B. 1,25(OH)(2)D(3) induced vitamin D receptor signaling negatively regulates endoplasmic reticulum-associated degradation (ERAD) and androgen receptor signaling in human prostate cancer cells. Cell. Signal. 2023, 103, 110577. [Google Scholar] [CrossRef] [PubMed]
- Germain, L.; Lafront, C.; Paquette, V.; Neveu, B.; Paquette, J.S.; Pouliot, F.; Audet-Walsh, E. Preclinical models of prostate cancer—Modelling androgen dependency and castration resistance in vitro, ex vivo and in vivo. Nat. Rev. Urol. 2023, 20, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.; Trudel, D.; Van der Kwast, T.; Nonn, L.; Giangreco, A.A.; Li, D.; Dias, A.; Cardoza, M.; Laszlo, S.; Hersey, K.; et al. Randomized clinical trial of vitamin D3 doses on prostatic vitamin D metabolite levels and ki67 labeling in prostate cancer patients. J. Clin. Endocrinol. Metab. 2013, 98, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.; Martin, R.M.; Beynon, R.; Harris, R.; Savovic, J.; Zuccolo, L.; Bekkering, G.E.; Fraser, W.D.; Sterne, J.A.; Metcalfe, C. Associations of circulating and dietary vitamin D with prostate cancer risk: A systematic review and dose-response meta-analysis. Cancer Causes Control 2011, 22, 319–340. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shao, X.; Yao, Y.; Xu, L.; Chang, L.; Jiang, Z.; Lin, Z. Positive association between circulating 25-hydroxyvitamin D levels and prostate cancer risk: New findings from an updated meta-analysis. J. Cancer Res. Clin. Oncol. 2014, 140, 1465–1477. [Google Scholar] [CrossRef]
- Gao, J.; Wei, W.; Wang, G.; Zhou, H.; Fu, Y.; Liu, N. Circulating vitamin D concentration and risk of prostate cancer: A dose-response meta-analysis of prospective studies. Ther. Clin. Risk Manag. 2018, 14, 95–104. [Google Scholar] [CrossRef]
- Travis, R.C.; Perez-Cornago, A.; Appleby, P.N.; Albanes, D.; Joshu, C.E.; Lutsey, P.L.; Mondul, A.M.; Platz, E.A.; Weinstein, S.J.; Layne, T.M.; et al. A collaborative analysis of individual participant data from 19 prospective studies assesses circulating vitamin D and prostate cancer risk. Cancer Res. 2019, 79, 274–285. [Google Scholar] [CrossRef]
- Stroomberg, H.V.; Vojdeman, F.J.; Madsen, C.M.; Helgstrand, J.T.; Schwarz, P.; Heegaard, A.M.; Olsen, A.; Tjonneland, A.; Struer Lind, B.; Brasso, K.; et al. Vitamin D levels and the risk of prostate cancer and prostate cancer mortality. Acta Oncol. 2021, 60, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Voutilainen, A.; Virtanen, J.K.; Hantunen, S.; Nurmi, T.; Kokko, P.; Tuomainen, T.P. Multiplicative, additive, and interactive associations of 25-hydroxyvitamin D with lung and prostate cancer. Int. J. Vitam. Nutr. Res. 2024, 94, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Brandstedt, J.; Almquist, M.; Manjer, J.; Malm, J. Vitamin D, PTH, and calcium in relation to survival following prostate cancer. Cancer Causes Control 2016, 27, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Kasperzyk, J.L.; Shui, I.; Hendrickson, W.; Hollis, B.W.; Fall, K.; Ma, J.; Gaziano, J.M.; Stampfer, M.J.; Mucci, L.A.; et al. Prediagnostic plasma vitamin D metabolites and mortality among patients with prostate cancer. PLoS ONE 2011, 6, e18625. [Google Scholar] [CrossRef] [PubMed]
- Shui, I.M.; Mucci, L.A.; Kraft, P.; Tamimi, R.M.; Lindstrom, S.; Penney, K.L.; Nimptsch, K.; Hollis, B.W.; Dupre, N.; Platz, E.A.; et al. Vitamin D-related genetic variation, plasma vitamin D, and risk of lethal prostate cancer: A prospective nested case-control study. J. Natl. Cancer Inst. 2012, 104, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Thederan, I.; Chandrasekar, T.; Tennstedt, P.; Knipper, S.; Kuehl, L.; Tilki, D.; Augustin, M.; Heinzer, H.; Zyriax, B.C. Circulating vitamin D and selenium levels and outcome in prostate cancer patients: Lessons from the MARTINI-lifestyle cohort. Eur. Urol. Focus 2021, 7, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Stephan, C.; Lein, M.; Matalon, J.; Kilic, E.; Zhao, Z.; Busch, J.; Jung, K. Serum vitamin D is not helpful for predicting prostate cancer aggressiveness compared with the prostate health index. J. Urol. 2016, 196, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Goulao, B.; Stewart, F.; Ford, J.A.; MacLennan, G.; Avenell, A. Cancer and vitamin D supplementation: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 107, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Lee, D.H.; Greenwood, D.C.; Manson, J.E.; Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality: A meta-analysis of randomized controlled trials. Ann. Oncol. 2019, 30, 733–743. [Google Scholar] [CrossRef]
- Manson, J.E.; Bassuk, S.S.; Buring, J.E.; Group, V.R. Principal results of the VITamin D and OmegA-3 TriaL (VITAL) and updated meta-analyses of relevant vitamin D trials. J. Steroid Biochem. Mol. Biol. 2020, 198, 105522. [Google Scholar] [CrossRef]
- Kuznia, S.; Zhu, A.; Akutsu, T.; Buring, J.E.; Camargo, C.A., Jr.; Cook, N.R.; Chen, L.J.; Cheng, T.D.; Hantunen, S.; Lee, I.M.; et al. Efficacy of vitamin D(3) supplementation on cancer mortality: Systematic review and individual patient data meta-analysis of randomised controlled trials. Ageing Res. Rev. 2023, 87, 101923. [Google Scholar] [CrossRef] [PubMed]
- Boucher, B.J. Why do so many trials of vitamin D supplementation fail? Endocr. Connect. 2020, 9, R195–R206. [Google Scholar] [CrossRef]
- Grant, W.B.; Boucher, B.J.; Al Anouti, F.; Pilz, S. Comparing the evidence from observational studies and randomized controlled trials for nonskeletal health effects of vitamin D. Nutrients 2022, 14, 3811. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Meng, X.; Tian, Q.; Cao, W.; Fan, X.; Wu, L.; Song, M.; Meng, Q.; Wang, W.; Wang, Y. Vitamin D and multiple health outcomes: An umbrella review of observational studies, randomized controlled trials, and Mendelian randomization studies. Adv. Nutr. 2022, 13, 1044–1062. [Google Scholar] [CrossRef] [PubMed]
- Stamp, T.C.; Round, J.M. Seasonal changes in human plasma levels of 25-hydroxyvitamin D. Nature 1974, 247, 563–565. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C.M.; Kazantzidis, A.; Ryan, M.J.; Barber, N.; Sempos, C.T.; Durazo-Arvizu, R.A.; Jorde, R.; Grimnes, G.; Eiriksdottir, G.; Gudnason, V.; et al. Seasonal changes in vitamin -effective UVB availability in Europe and associations with population serum 25-hydroxyvitamin D. Nutrients 2016, 8, 533. [Google Scholar] [CrossRef] [PubMed]
- Bai, K.; Dong, H.; Liu, L.; She, X.; Liu, C.; Yu, M.; Liang, Z.; Lin, H.; Ke, P.; Huang, X.; et al. Serum 25-hydroxyvitamin D status of a large Chinese population from 30 provinces by LC-MS/MS measurement for consecutive 3 years: Differences by age, sex, season and province. Eur. J. Nutr. 2023, 62, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.L.; Santana, K.V.; Ribeiro, H. The effect of sunlight exposure on vitamin D status in countries of low and high latitudes: A systematic literature review. Curr. Nutr. Rep. 2023, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr. Rev. 2014, 72, 48–54. [Google Scholar] [CrossRef]
- Marcinkowska, E.; Wallace, G.R.; Brown, G. The use of 1alpha,25-dihydroxyvitamin D(3) as an anticancer agent. Int. J. Mol. Sci. 2016, 17, 729. [Google Scholar] [CrossRef]
- Morris, M.J.; Smaletz, O.; Solit, D.; Kelly, W.K.; Slovin, S.; Flombaum, C.; Curley, T.; Delacruz, A.; Schwartz, L.; Fleisher, M.; et al. High-dose calcitriol, zoledronate, and dexamethasone for the treatment of progressive prostate carcinoma. Cancer 2004, 100, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Jia, X.; Chi, K.; de Wit, R.; Berry, W.R.; Albers, P.; Henick, B.; Waterhouse, D.; Ruether, D.J.; Rosen, P.J.; et al. Randomized, open-label phase III trial of docetaxel plus high-dose calcitriol versus docetaxel plus prednisone for patients with castration-resistant prostate cancer. J. Clin. Oncol. 2011, 29, 2191–2198. [Google Scholar] [CrossRef] [PubMed]
- Ben-Eltriki, M.; Deb, S.; Guns, E.S. Calcitriol in combination therapy for prostate cancer: Pharmacokinetic and pharmacodynamic interactions. J. Cancer 2016, 7, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Morgentaler, A.; Traish, A.M. Shifting the paradigm of testosterone and prostate cancer: The saturation model and the limits of androgen-dependent growth. Eur. Urol. 2009, 55, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.; Dias, A.G.; Schnabl, K.; Van der Kwast, T.; Vieth, R. Determination of 1,25-dihydroxyvitamin D concentrations in human colon tissues and matched serum samples. Anticancer Res. 2012, 32, 259–263. [Google Scholar] [PubMed]
- Milani, C.; Katayama, M.L.; de Lyra, E.C.; Welsh, J.; Campos, L.T.; Brentani, M.M.; Maciel Mdo, S.; Roela, R.A.; del Valle, P.R.; Goes, J.C.; et al. Transcriptional effects of 1,25 dihydroxyvitamin D(3) physiological and supra-physiological concentrations in breast cancer organotypic culture. BMC Cancer 2013, 13, 119. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Munoz, A. An update on vitamin D signaling and cancer. Semin. Cancer Biol. 2022, 79, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Martens, P.J.; Gysemans, C.; Verstuyf, A.; Mathieu, A.C. Vitamin D’s effect on immune function. Nutrients 2020, 12, 1248. [Google Scholar] [CrossRef]
- Carlberg, C.; Haq, A. The concept of the personal vitamin D response index. J. Steroid Biochem. Mol. Biol. 2018, 175, 12–17. [Google Scholar] [CrossRef]
- Zheng, S.; Zhu, Z.; Ding, C. How can we design a proper trial for vitamin D treatment of diseases? Facts and numbers. J. Cachexia Sarcopenia Muscle 2023, 14, 1146–1149. [Google Scholar] [CrossRef]

| Reference, Year | Study Object a | Calcitriol (nM) Treatment | Effects of Calcitriol |
|---|---|---|---|
| Blutt et al., [88] 2000 | LNCaP cells | 10–100 | Cell growth inhibition Enhanced apoptosis |
| Krishnan et al., [89] 2004 | LNCaP cells | 50 | Numerous up-and down-regulated genes |
| Stewart et al., [90] 2005 | LNCaP, DU 145, PC-3, C4-2, LAPC-4 cells | 100 | Cell growth inhibition independent on IGFBP3 |
| Bao et al., [18] 2006 | LNCaP, PC-3, DU 145 cells | 100 | Decreased PCa cell invasion by TIMP1 modulated MMP9 |
| Bao et al., [91] 2008 | DU 145, BPH-1, RWPE-1, CWR22R cells | 100 | Antioxidative effects in non-malignant prostate cells through G6PD activation |
| Kovalenko et al., [92] 2010 | RWPE-1 cells | 100 | Multiple calcitriol-regulated tran-scripts of anticancerogenic path-ways (WNT, Notch, NFKB1) |
| Hidalgo et al., [93] 2011 | Benign- and cancer-associated prostate fibroblasts | 100 | Cell-dependent VDR-mediated transcriptional activities |
| Giangreco et al., [94] 2013 | RWPE-1, RWPE-2, LNCaP, primary cells isolated from human prostate | 10–100 | Upregulation of tumor suppressive miRNAs (miR-100, miR-125b) |
| Singh et al., [79] 2015 | RWPE-1, RWPE-2, HPr1, HPr1AR, LNCaP, C4-2, PC-3 cells | 100 | Identification of VDR-regulated miRNA patterns and patterns of miRNA and mRNA co-regulation |
| Abu El Maaty et al., [22] 2017 | LNCaP, VCaP, DU 145, PC-3 cells | 100 | Disruption of glucose metabolism and the tricarboxylic acid cycle |
| McCray et al., [25] 2021 | Patient-derived benign prostate epithelial organoids | 10–50 | Inhibition of WNT activity and suppression of DKK3 |
| Erzurumlu et al. [95] 2023 | LNCaP, 22Rv1 cells | 2.5–100 | Inhibition of the androgen receptor signaling and tumor formation of LNCaP cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fendler, A.; Stephan, C.; Ralla, B.; Jung, K. Discordant Health Implications and Molecular Mechanisms of Vitamin D in Clinical and Preclinical Studies of Prostate Cancer: A Critical Appraisal of the Literature Data. Int. J. Mol. Sci. 2024, 25, 5286. https://doi.org/10.3390/ijms25105286
Fendler A, Stephan C, Ralla B, Jung K. Discordant Health Implications and Molecular Mechanisms of Vitamin D in Clinical and Preclinical Studies of Prostate Cancer: A Critical Appraisal of the Literature Data. International Journal of Molecular Sciences. 2024; 25(10):5286. https://doi.org/10.3390/ijms25105286
Chicago/Turabian StyleFendler, Annika, Carsten Stephan, Bernhard Ralla, and Klaus Jung. 2024. "Discordant Health Implications and Molecular Mechanisms of Vitamin D in Clinical and Preclinical Studies of Prostate Cancer: A Critical Appraisal of the Literature Data" International Journal of Molecular Sciences 25, no. 10: 5286. https://doi.org/10.3390/ijms25105286






