Corrosion Rate and Mechanism of Degradation of Chitosan/TiO2 Coatings Deposited on MgZnCa Alloy in Hank’s Solution
Abstract
:1. Introduction
2. Results and Discussion
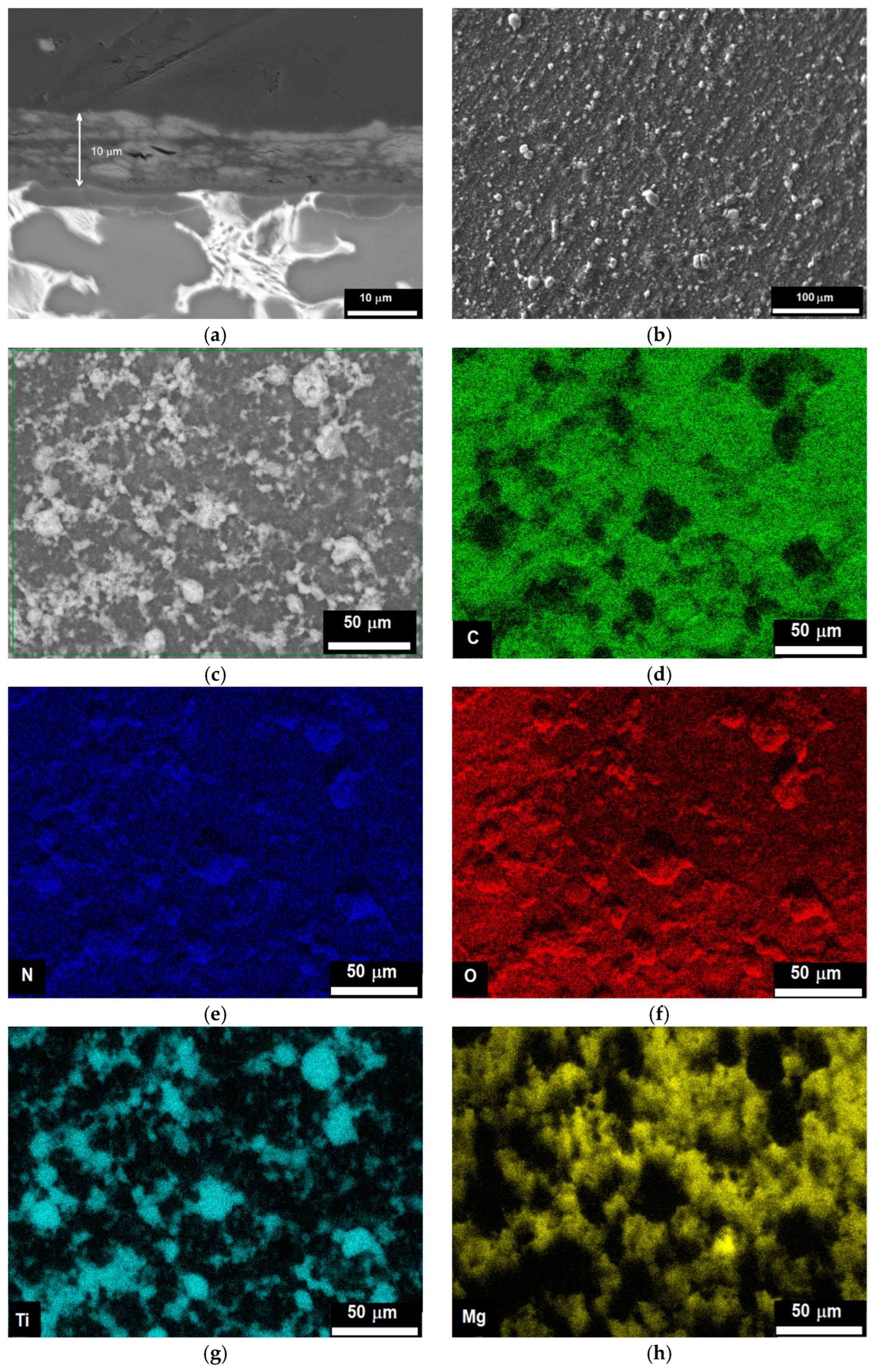
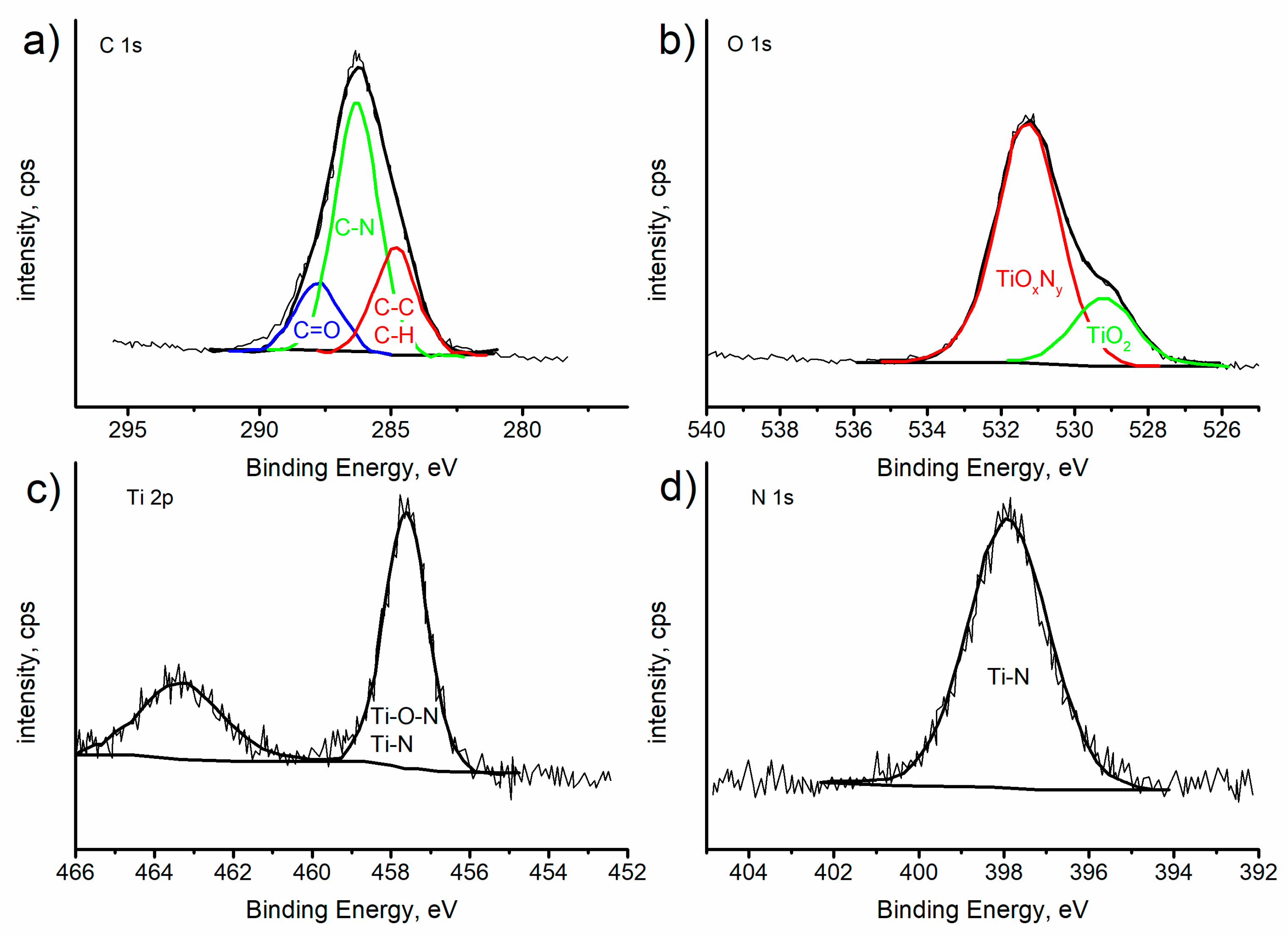
2.1. Structure of the Substrate and the Chitosan/TiO2 Coating
2.2. Corrosion Resistance of Uncoated and Coated Mg19Zn1Ca Alloys
2.3. The Influence of the Chitosan/TiO2 Coating on Corrosion Mechanisms
2.4. Corrosion Rate of Uncoated and Coated Mg19Zn1Ca Alloy
2.5. Corrosion Degradation of Uncoated and Coated Mg19Zn1Ca Alloy
3. Materials and Methods
3.1. Materials
3.2. Coatings Preparation
3.3. Corrosion Measurements
3.4. Characterisation Techniques
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Predko, P.; Rajnovic, D.; Grilli, M.L.; Postolnyi, B.O.; Zemcenkovs, V.; Rijkuris, G.; Pole, E.; Lisnanskis, M. Promising Methods for Corrosion Protection of Magnesium Alloys in the Case of Mg-Al, Mg-Mn-Ce and Mg-Zn-Zr. A Recent Prog. Rev. 2021, 11, 1133. [Google Scholar] [CrossRef]
- Zeng, R.C.; Qi, W.C.; Cui, H.Z.; Zhang, F.; Li, S.Q.; Han, E.H. In vitro corrosion of as-extruded Mg–Ca alloys—The influence of Ca concentration. Corros. Sci. 2015, 96, 23–31. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Ruvalcaba-Gómez, J.M.; Maytorena-Verdugo, C.I.; González-Silva, N.; Romero-Toledo, R.; Aguilera-Aguirre, S.; Pérez-Larios, A.; Montalvo-González, E. Chitosan-TiO2: A Versatile Hybrid Composite. Materials 2020, 13, 81. [Google Scholar] [CrossRef]
- Gonzalez, J.; Hou, R.Q.; Nidadavolu, E.P.S.; Willumeit-Romer, R.; Feyerabend, F. Magnesium degradation under physiological conditions—Best Practice. Bioact. Mater. 2018, 3, 174–185. [Google Scholar] [CrossRef]
- Kozina, I.; Krawiec, H.; Starowicz, M.; Kawalec, M. Corrosion Resistance of MgZn Alloy Covered by Chitosan-Based Coatings. Int. J. Mol. Sci. 2021, 22, 8301. [Google Scholar] [CrossRef]
- Li, C.Q.; Xu, D.K.; Chen, X.B.; Wang, B.J.; Wu, R.Z.; Han, E.H.; Birbilis, N. Composition and microstructure dependent corrosion behaviour of Mg-Li alloys. Electrochim. Acta 2018, 260, 55–64. [Google Scholar] [CrossRef]
- Du, Y.Z.; Qiao, X.G.; Zheng, M.Y.; Wang, D.B.; Wu, K.; Golovin, I.S. Effect of microalloying with Ca on the microstructure and mechanical properties of Mg-6 mass % Zn alloys. Mater. Desn. 2016, 98, 285–293. [Google Scholar] [CrossRef]
- Jiang, P.; Blawert, C.; Zheludkevich, M.L. The Corrosion Performance and Mechanical Properties of Mg-Zn Based Alloys—A Review. Corros. Mater. Degrad. 2020, 1, 92–158. [Google Scholar] [CrossRef]
- Li, C.Y.; Fan, X.L.; Zeng, R.C.; Cui, L.Y.; Li, S.Q.; Zhang, F.; He, Q.K.; Kannan, M.B.; Jiang, H.W.; Chen, D.C.; et al. Corrosion resistance of in-situ growth of nano-sized Mg(OH)2 on micro-arc oxidized magnesium alloy AZ31—Influence of EDTA. J. Mater. Sci. Technol. 2019, 35, 1088–1098. [Google Scholar] [CrossRef]
- Zhu, J.; Jia, H.; Liao, K.; Li, X. Improvement on corrosion resistance of micro-arc oxidized AZ91D magnesium alloy by a pore-sealing coating. J. Alloys Compd. 2021, 889, 161460. [Google Scholar] [CrossRef]
- Han, L.; Li, X.; Xue, F.; Chu, C.; Bai, J. Biocorrosion behavior of micro-arc-oxidized AZ31 magnesium alloy in different simulated dynamic physiological environments. Surf. Coat. Technol. 2019, 361, 240–248. [Google Scholar] [CrossRef]
- Gu, C.D.; Yan, W.; Zhang, J.L.; Tu, J.P. Corrosion resistance of AZ31B magnesium alloy with a conversion coating produced from a choline chloride—Urea based deep eutectic solvent. Corros. Sci. 2016, 106, 108–116. [Google Scholar] [CrossRef]
- Francis, A.; Yang, Y.; Boccaccini, A.R. A new strategy for developing chitosan conversion coating on magnesium substrates for orthopedic implants. Appl. Surf. Sci. 2019, 466, 854–862. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, X.; Yin, X.; Wang, J.; Zhu, G.; Yu, S.; Liu, E.; Wang, B.; Yang, X. Preparation of robust, self-cleaning and anti-corrosion superhydrophobic Ca-P/chitosan (CS) composite coating on AZ31 magnesium alloy. Surf. Coat. Technol. 2022, 432, 128074. [Google Scholar] [CrossRef]
- Qi, Z.; Zhao, Y.; Ji, M.; Wang, G.; Ying, L.; Wang, Z.; Krit, B. Preparation of chitosan/phosphate composite coating on Mg alloy (AZ31B) via one-step chemical conversion method. Resour. Chem. Mater. 2023, 2, 39–48. [Google Scholar] [CrossRef]
- Cordoba, L.C.; Marques, A.; Taryba, M.; Coradin, T.; Montemor, F. Hybrid coatings with collagen and chitosan for improved bioactivity of Mg alloys. Surf. Coat. Technol. 2018, 341, 103–113. [Google Scholar] [CrossRef]
- Witecka, A.; Valet, S.; Basista, M.; Boccaccini, A.R. Electrophoretically deposited high molecular weight chitosan/bioactive glass composite coatings on WE43 magnesium alloy. Surf. Coat. Technol. 2021, 418, 127232. [Google Scholar] [CrossRef]
- Gao, F.; Hu, Y.; Gong, Z.; Liu, T.; Gong, T.; Liu, S.; Zhang, C.; Quan, L.; Kaveendran, B.; Pan, C. Fabrication of chitosan/heparinized graphene oxide multilayer coating to improve corrosion resistance and biocompatibility of magnesium alloys. Mater. Sci. Eng. C 2019, 104, 109947. [Google Scholar] [CrossRef]
- Hahn, B.D.; Park, D.S.; Choi, J.J.; Ryu, J.; Yoon, W.H.; Choi, J.H.; Kim, H.E.; Kim, S.G. Aerosol deposition of hydroxyapatite–chitosan composite coatings on biodegradable magnesium alloy. Surf. Coat. Technol. 2011, 205, 3112–3118. [Google Scholar] [CrossRef]
- Müller, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; Sanz, Y.E.; Lagaron, J.M.; Miesbauer, O.; Bianchin, A.; Hankin, S.; Bölz, U.; et al. Review on the processing and properties of polymer nanocomposites and nanocoatings and their applications in the packaging, automotive and solar energy fields. Nanomaterials 2017, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Chen, B.Y.; Den, W. Chitosan as a natural polymer for heterogeneous catalysts support: A short review on its applications. Appl. Sci. 2015, 5, 1272–1283. [Google Scholar] [CrossRef]
- Balaji, J.; Sethuraman, M.G. Chitosan-doped-hybrid/TiO2 nanocomposite-based sol-gel coating for the corrosion resistance of aluminum metal in 3.5% NaCl medium. Int. J. Biol. Macromol. 2017, 104, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Ledwing, P.; Kot, M.; Moskalewicz, T.; Dubiel, B. Electrophoretic deposition of nc-TiO2/chitosan composite coatings on X2CrNiMo17-12-2 stainless steel. Arch. Metall. Mater. 2012, 62, 405–410. [Google Scholar] [CrossRef]
- Cai, S.; Lei, T.; Li, N.; Feng, F. Effects of Zn on microstructure, mechanical properties and corrosion behavior of Mg–Zn alloys. Mater. Sci. Eng. C 2012, 32, 2570–2577. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Zhao, C.; Li, J.; Song, Y.; Xie, C.; Tao, H.; Zhang, Y.; He, Y.; Jiang, Y.; et al. Research on an Mg–Zn alloy as a degradable biomateriał. Acta Biomater. 2010, 6, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Deepak, J.R.; Arunkumar, T.; Ravipati, S.V.S.D.; Varma, S.S. XRD investigation of biodegradable magnesium rare earth alloy. Mater. Today Proc. 2021, 47, 4676–4681. [Google Scholar] [CrossRef]
- Gil-Santos, A.; Szakacs, G.; Moelans, N.; Hort, N.; van der Biest, O. Microstructure and mechanical characterization of cast Mg-Ca-Si alloys. J. Alloys Compd. 2017, 694, 767–776. [Google Scholar] [CrossRef]
- Burda, C.; Lou, Y.; Chen, X.; Samia, A.C.S.; Stout, J.; Gole, J.L. Enhanced Nitrogen Doping in TiO2 Nanoparticles. Nano Lett. 2003, 3, 1049–1051. [Google Scholar] [CrossRef]
- Avcu, E.; Bastan, F.E.; Abdullahb, H.Z.; Rehman, M.A.U.; Avcu, Y.Y.; Boccaccini, A.R. Electrophoretic deposition of chitosan-based composite coatings for biomedical applications: A review. Prog. Mater. Sci. 2019, 103, 69–108. [Google Scholar] [CrossRef]
- Pawlak, A.; Mucha, M. Thermogravimetric and FTIR studies of chitosan blends. Thermochim. Acta 2003, 396, 153–166. [Google Scholar] [CrossRef]
- Rahimi, M.; Aghdam, R.M.; Sohi, M.H.; Rezayan, A.H.; Ettelaei, M. Improving biocompatibility and corrosion resistance of anodized AZ31 Mg alloy by electrospun chitosan/mineralized bone allograft (MBA) nanocoatings. Surf. Coat. Technol. 2021, 405, 126627. [Google Scholar] [CrossRef]
- Leon, A.; Reuquen, P.; Garín, C.; Segura, R.; Vargas, P.; Zapata, P.; Orihuela, P.A. FTIR and Raman Characterization of TiO2 Nanoparticles Coated with Polyethylene Glycol as Carrier for 2-Methoxyestradiol. Appl. Sci. 2017, 7, 49. [Google Scholar] [CrossRef]
- Gharagozlou, M.; Naghibi, S. Preparation of vitamin B12–TiO2 nanohybrid studied by TEM, FTIR and optical analysis techniques. Mater. Sci. Semicond. Proc. 2015, 35, 166–173. [Google Scholar] [CrossRef]
- Xing, Y.; Li, X.; Guo, X.; Li, W.; Chen, J.; Liu, Q.; Xu, Q.; Wang, Q.; Yang, H.; Shui, Y.; et al. Effects of different TiO2 nanoparticles concentrations on the physical and antibacterial activities of chitosan-based coating film. Nanomaterials 2020, 10, 1365. [Google Scholar] [CrossRef] [PubMed]
- Hussein, E.M.; Desoky, W.M.; Hanafy, M.F.; Guirguis, O.W. Effect of TiO2 nanoparticles on the structural configurations and thermal, mechanical, and optical properties of chitosan/TiO2 nanoparticle composites. J. Phys. Chem. Solids 2021, 152, 109983. [Google Scholar] [CrossRef]
- Vieira, R.S.; Oliveira, M.L.M.; Guibal, E.; Rodríguez-Castellón, E.; Beppu, M.M. Copper, mercury and chromium adsorption on natural and crosslinked chitosan films: An XPS investigation of mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2011, 374, 108–114. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Wang, J.; Wu, J.; Li, D.; Liu, M.; Su, P. Nitrogen-doped TiO2 nanoparticles better TiO2 nanotube array photo-anodes for dye sensitized solar cells. Electrochim. Acta 2014, 137, 744–750. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Kong, D.; Yang, Y.; Wang, Y.; Guo, Y.; Lu, Y.; Kim, J.-K.; Luo, Y. In situ growth of Sn nanoparticles confined carbon-based TiO2 /TiN composite with long-term cycling stability for sodium-ion batteries. Electrochim. Acta 2021, 367, 137450. [Google Scholar] [CrossRef]
- Kuznetsov, M.V.; Zhuravlev, J.F.; Zhilyaev, V.A.; Gubanov, V.A. XPS study of the nitrides, oxides and oxynitrides of titanium. J. Electron. Spectros. Relat. Phenom. 1992, 58, 1–9. [Google Scholar] [CrossRef]
- Kravanja, K.A.; Suhadolnik, L.; Bele, M.; Maver, U.; Rozanc, J.; Knez, Z.; Marevci, M.K.; Finsgar, M. The synthesis, surface analysis, and cellular response of titania and titanium oxynitride nanotube arrays prepared on TiAl6V4 for potential biomedical applications. J. Mater. Res. Technol. 2023, 24, 4074–4090. [Google Scholar] [CrossRef]
- Winston Revie, R.; Uhlig, H.H. Corrosion and Corrosion Control; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Fajardo, S.; Miguélez, L.; Arenas, M.A.; de Damborenea, J.; Llorente, I.; Feliu, S. Corrosion resistance of pulsed laser modified AZ31 Mg alloy surfaces. J. Magnes. Alloy. 2022, 10, 756–768. [Google Scholar] [CrossRef]
- Zou, Q.; Le, Q.; Chen, X.; Jia, Y.; Ban, C.; Wang, T.; Wang, H.; Guo, R.; Ren, L.; Atrens, A. The influence of Ga alloying on Mg-Al-Zn alloys as anode material for Mg-air primary batteries. Electrochim. Acta 2022, 401, 139372. [Google Scholar] [CrossRef]
- Li, Z.; Guo, X.; Zhang, Y.; Liu, G.; Zhang, S. Abnormal Blood Glucose Concentration on Degradation Behavior of AZ31 Magnesium Alloy. Chem. Res. Chin. Univ. 2020, 36, 1217–1226. [Google Scholar] [CrossRef]
- Shetty, S.; Nayak, J.; Shetty, A.N. Influence of sulfate ion concentration and pH on the corrosion of Mg-Al-Zn-Mn (GA9) magnesium alloy. J. Magnes. Alloy. 2015, 3, 258–270. [Google Scholar] [CrossRef]
- Wang, N.; Wang, R.; Peng, C.; Peng, B.; Feng, Y.; Hu, C. Discharge behaviour of Mg-Al-Pb and Mg-Al-Pb-In alloys as anodes for Mg-air battery. Electrochim. Acta 2014, 149, 193–205. [Google Scholar] [CrossRef]
- Li, S.; Li, H.; Zhao, C.; Wang, Z.; Liu, K.; Du, W. Effects of Ca addition on microstructure, electrochemical behavior and magnesium-air battery performance of Mg-2Zn-xCa alloys. J. Electroanal. Chem. 2022, 904, 115944. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A.; John, D.S. An hydrogen evolution method for the estimation of the corrosion rate of magnesium alloys. In Mgnesium Technologies; Hryn, J.N., Ed.; Wiley: New Orleans, LA, USA, 2001; pp. 254–262. [Google Scholar] [CrossRef]
- Ghali, E.; Dietzel, W.; Kainer, K.U. Testing of general and localized corrosion of magnesium alloys: A critical review. J. Mater. Eng. Perform. 2004, 13, 517–529. [Google Scholar] [CrossRef]
- Zhou, H.; Jingyao, L.; Jian, L.; Ruan, O.; Jina, W.; Yu, Z.; Li, W.; Chu, P.K. Calcium phosphate coating on biomedical WE43 magnesium alloy pretreated with a magnesium phosphate layer for corrosion protection. Surf. Coat. Technol. 2020, 401, 126248. [Google Scholar] [CrossRef]
- Ha, S.W.; Kirch, M.; Birchler, F.; Eckert, K.L.; Mayer, J.; Wintermantel, E.; Sittig, C.; Pfund-Klingenfuss, I.; Textor, M.; Spencer, N.D.; et al. Surface activation of polyetheretherketone (PEEK) and formation of calcium phosphate coatings by precipitation. J. Mater. Sci. Mater. Med. 1997, 8, 683–690. [Google Scholar] [CrossRef]
- Ong, J.L.; Hoppe, C.A.; Cardenas, H.L.; Cavin, R.; Carnes, D.L.; Sogal, A.; Raikar, G.N. Osteoblast precursor cell activity on HA surfaces of different treatments. J. Biomed. Mater. Res. 1998, 39, 176–183. [Google Scholar] [CrossRef]
- Ong, J.L.; Lucas, L.C.; Raikar, G.N.; Weimer, J.J.; Gregory, J.C. Surface characterization of ion-beam sputter-deposited Ca-P coatings after in vitro immersion. Colloids Surf. A Physicochem. Eng. Asp. 1994, 87, 151–162. [Google Scholar] [CrossRef]
- Acheson, J.G.; Robinson, L.; McKillop, S.; Wilson, S.; McIvor, J.M.; Meenan, B.J.; Boyd, A.R. TOFSIMS and XPS characterisation of strontium in amorphous calcium phosphate sputter deposited coatings. Mater. Charact. 2021, 171, 110739. [Google Scholar] [CrossRef]
- Omar, S.A.; Ballarre, J.; Schreiner, W.H.; Cere, S.M. Micro Raman and XPS surface analysis to understand the electrochemical behaviour of AZ31 and AZ91 magnesium alloys as temporary implant materials. Mater. Today Commun. 2022, 31, 103557. [Google Scholar] [CrossRef]





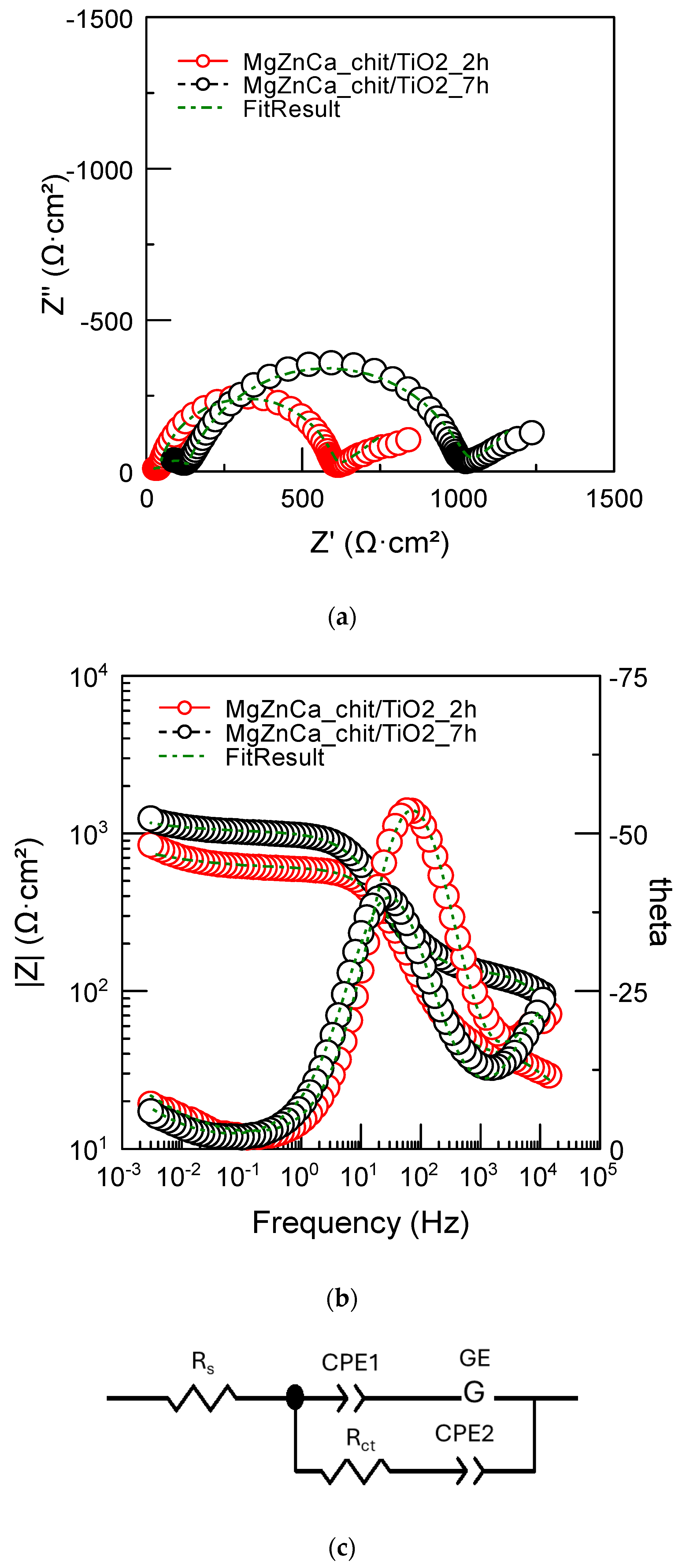




| Rs (Ω·cm2) | Rct (Ω·cm2) | Y (S·cm−2·sn) | n | RL (Ω·cm2) | L (H·cm2) | |
|---|---|---|---|---|---|---|
| 2 h | 66.0 ± 0.4 | 191.9 ± 1.8 | 2.4 × 10−4 ± 4 × 10−6 | 0.84 ± 0.009 | 436.5 ± 21 | 1117.0 ± 87 |
| 7 h | 68.0 ± 0.67 | 239.6 ± 2.5 | 2.9 × 10−4 ± 1.3 × 10−5 | 0.84 ± 0.003 | 489.5 ± 27.0 | 2229.0 ± 127.0 |
| Rs (Ω·cm2) | Rct (Ω·cm2) | Y1 (S·cm−2·sn) | n1 | Y2 (S·cm−2·sn) | n2 | GE-T (S·cm−2·s0.5) | GE-P | |
|---|---|---|---|---|---|---|---|---|
| 2 h | 13.2 ± 2.0 | 585.9 ± 4.9 | 2.6 × 10−5 ± 2.2 × 10−7 | 0.9 ± 0.005 | 0.029 ± 4.4 × 10−4 | 0.42 ± 0.017 | 2.1 × 10−4 ± 1.2 × 10−5 | 2938 ± 946 |
| 7 h | 33 ± 0.8 | 991.2 ± 7.3 | 3.4 × 10−5 ± 3.8 × 10−7 | 0.82 ± 0.006 | 5.8 × 10−5 ± 2.2 × 10−7 | 0.49 ± 0.02 | 5.8 × 10−5 ± 3.0 × 10−6 | 27,652 ± 449 |
| Step | Time, s | Speed, rps |
|---|---|---|
| S1 | 5 | 250 |
| S3 | 30 | 1000 |
| S3 | 60 | 1500 |
| drying | 60 | 250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krawiec, H.; Kozina, I.; Starowicz, M.; Lekka, M.; Zanella, C.; Fedrizzi, L.; Fedel, M.; Deflorian, F. Corrosion Rate and Mechanism of Degradation of Chitosan/TiO2 Coatings Deposited on MgZnCa Alloy in Hank’s Solution. Int. J. Mol. Sci. 2024, 25, 5313. https://doi.org/10.3390/ijms25105313
Krawiec H, Kozina I, Starowicz M, Lekka M, Zanella C, Fedrizzi L, Fedel M, Deflorian F. Corrosion Rate and Mechanism of Degradation of Chitosan/TiO2 Coatings Deposited on MgZnCa Alloy in Hank’s Solution. International Journal of Molecular Sciences. 2024; 25(10):5313. https://doi.org/10.3390/ijms25105313
Chicago/Turabian StyleKrawiec, Halina, Iryna Kozina, Maria Starowicz, Maria Lekka, Caterina Zanella, Lorenzo Fedrizzi, Michele Fedel, and Flavio Deflorian. 2024. "Corrosion Rate and Mechanism of Degradation of Chitosan/TiO2 Coatings Deposited on MgZnCa Alloy in Hank’s Solution" International Journal of Molecular Sciences 25, no. 10: 5313. https://doi.org/10.3390/ijms25105313







