Functional Analysis of the Major Pilin Proteins of Type IV Pili in Streptococcus sanguinis CGMH010
Abstract
:1. Introduction
2. Results
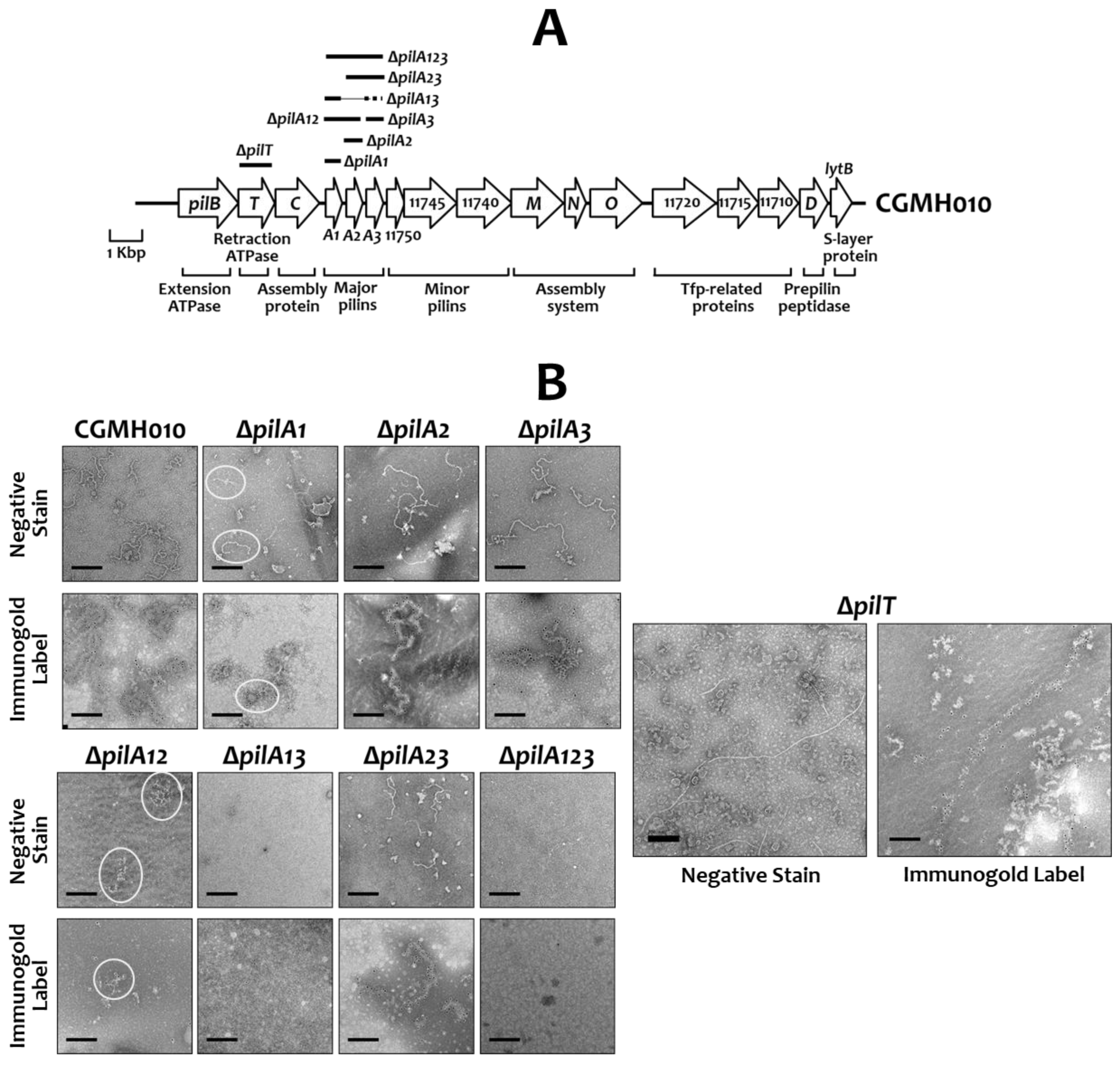
2.1. Sequence Analysis of the PilA Proteins and Generation of Anti-PilA Antisera
2.2. The Effect of PilA Proteins in the Assembly of Tfp in S. sanguinis CGMH010
2.3. The Twitching Activity of Tfp Composed of Different PilA Proteins
2.4. Wild-Type Tfp Were Essential for Maintaining Biofilm Structure
2.5. Motile Tfp Are Essential for the Invasion of Host Cells by S. sanguinis
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Generation of pilA Knockout Derivatives of S. sanguinis CGMH010
4.3. Expression of the Pilin Proteins and Preparation of Pilin Protein-Specific Antibody
4.4. Protein Structure Prediction
4.5. Purification of Extracellular Tfp
4.6. Immunogold Labeling, Negative Staining, and TEM Observation
4.7. Gel Electrophoresis and Western Blot Analysis
4.8. Examination of Twitching Motility
4.9. Preparation of Flow-Cell Biofilm and Examination by CLSM
4.10. Invasion Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denise, R.; Abby, S.S.; Rocha, E.P.C. Diversification of the type IV filament superfamily into machines for adhesion, protein secretion, DNA uptake, and motility. PLoS Biol. 2019, 17, e3000390. [Google Scholar] [CrossRef] [PubMed]
- Daum, B.; Gold, V. Twitch or swim: Towards the understanding of prokaryotic motion based on the type IV pilus blueprint. Biol. Chem. 2018, 399, 799–808. [Google Scholar] [CrossRef]
- Giltner, C.L.; Nguyen, Y.; Burrows, L.L. Type IV pilin proteins: Versatile molecular modules. Microbiol. Mol. Biol. Rev. MMBR 2012, 76, 740–772. [Google Scholar] [CrossRef] [PubMed]
- Craig, L.; Forest, K.T.; Maier, B. Type IV pili: Dynamics, biophysics and functional consequences. Nat. Rev. Microbiol. 2019, 17, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Pelicic, V. Type IV pili: E pluribus unum? Mol. Microbiol. 2008, 68, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Bordeleau, E.; Purcell, E.B.; Lafontaine, D.A.; Fortier, L.C.; Tamayo, R.; Burrus, V. Cyclic di-GMP riboswitch-regulated type IV pili contribute to aggregation of Clostridium difficile. J. Bacteriol. 2015, 197, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.J.; Nguyen, V.; O’Brien, D.K.; Rodgers, K.; Walker, R.A.; Melville, S.B. Type IV pili-dependent gliding motility in the Gram-positive pathogen Clostridium perfringens and other Clostridia. Mol. Microbiol. 2006, 62, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Chiang, Y.C.; Tseng, T.Y.; Wu, H.Y.; Chen, Y.Y.; Wu, C.H.; Chiu, C.H. Molecular and functional analysis of the type IV pilus gene cluster in Streptococcus sanguinis SK36. Appl. Environ. Microbiol. 2019, 85, e02788-18. [Google Scholar] [CrossRef] [PubMed]
- Gurung, I.; Spielman, I.; Davies, M.R.; Lala, R.; Gaustad, P.; Biais, N.; Pelicic, V. Functional analysis of an unusual type IV pilus in the Gram-positive Streptococcus sanguinis. Mol. Microbiol. 2015, 99, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Hospenthal, M.K.; Costa, T.R.D.; Waksman, G. A comprehensive guide to pilus biogenesis in Gram-negative bacteria. Nat. Rev. Microbiol. 2017, 15, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Barnier, J.P.; Meyer, J.; Kolappan, S.; Bouzinba-Segard, H.; Gesbert, G.; Jamet, A.; Frapy, E.; Schonherr-Hellec, S.; Capel, E.; Virion, Z.; et al. The minor pilin PilV provides a conserved adhesion site throughout the antigenically variable meningococcal type IV pilus. Proc. Natl. Acad. Sci. USA 2021, 118, e2109364118. [Google Scholar] [CrossRef]
- Nguyen, Y.; Sugiman-Marangos, S.; Harvey, H.; Bell, S.D.; Charlton, C.L.; Junop, M.S.; Burrows, L.L. Pseudomonas aeruginosa minor pilins prime type IVa pilus assembly and promote surface display of the PilY1 adhesin. J. Biol. Chem. 2015, 290, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, T.; Bardiaux, B.; Francetic, O.; Izadi-Pruneyre, N.; Nilges, M. Structure and function of minor pilins of type IV pili. Med. Microbiol. Immunol. 2020, 209, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Wang, H.Y.; Wu, C.H.; Lin, Y.J.; Chiu, C.H. Prevalence of type IV pili-mediated twitching motility in Streptococcus sanguinis strains and its impact on biofilm formation and host adherence. Appl. Environ. Microbiol. 2022, 88, e0140322. [Google Scholar] [CrossRef] [PubMed]
- Maier, B.; Wong, G.C.L. How bacteria use type IV pili machinery on surfaces. Trends Microbiol. 2015, 23, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Ellison, C.K.; Whitfield, G.B.; Brun, Y.V. Type IV Pili: Dynamic bacterial nanomachines. FEMS Microbiol. Rev. 2022, 46, fuab053. [Google Scholar] [CrossRef]
- Dos Santos Souza, I.; Maissa, N.; Ziveri, J.; Morand, P.C.; Coureuil, M.; Nassif, X.; Bourdoulous, S. Meningococcal disease: A paradigm of type-IV pilus dependent pathogenesis. Cell. Microbiol. 2020, 22, e13185. [Google Scholar] [CrossRef]
- Leighton, T.L.; Buensuceso, R.N.; Howell, P.L.; Burrows, L.L. Biogenesis of Pseudomonas aeruginosa type IV pili and regulation of their function. Environ. Microbiol. 2015, 17, 4148–4163. [Google Scholar] [CrossRef] [PubMed]
- Nieto, V.; Kroken, A.R.; Grosser, M.R.; Smith, B.E.; Metruccio, M.M.E.; Hagan, P.; Hallsten, M.E.; Evans, D.J.; Fleiszig, S.M.J. Type IV pili can mediate bacterial motility within epithelial cells. mBio 2019, 10, e02880-18. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.L.; Gurung, I.; Anonsen, J.H.; Spielman, I.; Harper, E.; Hall, A.M.J.; Goosens, V.J.; Raynaud, C.; Koomey, M.; Biais, N.; et al. Global biochemical and structural analysis of the type IV pilus from the Gram-positive bacterium Streptococcus sanguinis. J. Biol. Chem. 2019, 294, 6796–6808. [Google Scholar] [CrossRef] [PubMed]
- Martini, A.M.; Moricz, B.S.; Woods, L.J.; Jones, B.D. Type IV pili of Streptococcus sanguinis contribute to pathogenesis in experimental infective endocarditis. Microbiol. Spectr. 2021, 9, e0175221. [Google Scholar] [CrossRef] [PubMed]
- Laurenceau, R.; Pehau-Arnaudet, G.; Baconnais, S.; Gault, J.; Malosse, C.; Dujeancourt, A.; Campo, N.; Chamot-Rooke, J.; Le Cam, E.; Claverys, J.P.; et al. A type IV pilus mediates DNA binding during natural transformation in Streptococcus pneumoniae. PLoS Pathog. 2013, 9, e1003473. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Alves, J.M.; Kitten, T.; Brown, A.; Chen, Z.; Ozaki, L.S.; Manque, P.; Ge, X.; Serrano, M.G.; Puiu, D.; et al. Genome of the opportunistic pathogen Streptococcus sanguinis. J. Bacteriol. 2007, 189, 3166–3175. [Google Scholar] [CrossRef] [PubMed]
- Maldarelli, G.A.; Piepenbrink, K.H.; Scott, A.J.; Freiberg, J.A.; Song, Y.; Achermann, Y.; Ernst, R.K.; Shirtliff, M.E.; Sundberg, E.J.; Donnenberg, M.S.; et al. Type IV pili promote early biofilm formation by Clostridium difficile. Pathog. Dis. 2016, 74, ftw061. [Google Scholar] [CrossRef] [PubMed]
- Purcell, E.B.; McKee, R.W.; Bordeleau, E.; Burrus, V.; Tamayo, R. Regulation of type IV pili contributes to surface behaviors of historical and epidemic strains of Clostridium difficile. J. Bacteriol. 2016, 198, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.J.; Therit, B.; Melville, S.B. Type IV pili and the CcpA protein are needed for maximal biofilm formation by the gram-positive anaerobic pathogen Clostridium perfringens. Infect. Immun. 2008, 76, 4944–4951. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, R.J.; Houte, J.V. Bacterial adherence in oral microbial ecology. Annu. Rev. Microbiol. 1975, 29, 19–44. [Google Scholar] [CrossRef]
- McCallum, M.; Burrows, L.L.; Howell, P.L. The dynamic structures of the type IV pilus. Microbiol. Spectr. 2019, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.; Burne, R.A.; Chen, Y.Y. The pH-dependent expression of the urease operon in Streptococcus salivarius is mediated by CodY. Appl. Environ. Microbiol. 2014, 80, 5386–5393. [Google Scholar] [CrossRef]
- Kolappan, S.; Coureuil, M.; Yu, X.; Nassif, X.; Egelman, E.H.; Craig, L. Structure of the Neisseria meningitidis type IV pilus. Nat. Commun. 2016, 7, 13015. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Coureuil, M.; Osinski, T.; Orlova, A.; Altindal, T.; Gesbert, G.; Nassif, X.; Egelman, E.H.; Craig, L. Cryoelectron microscopy reconstructions of the Pseudomonas aeruginosa and Neisseria gonorrhoeae type IV pili at sub-nanometer resolution. Structure 2017, 25, 1423–1435.e1424. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fei, N.; Ji, W.; Qiao, P.; Yang, L.; Liu, D.; Guan, W.; Zhao, T. pilA gene contributes to virulence, motility, biofilm formation, and interspecific competition of bacteria in Acidovorax citrulli. Microorganisms 2023, 11, 1806. [Google Scholar] [CrossRef] [PubMed]
- Odermatt, P.D.; Nussbaum, P.; Monnappa, S.; Tala, L.; Li, Z.; Sivabalasarma, S.; Albers, S.V.; Persat, A. Archaeal type IV pili stabilize Haloferax volcanii biofilms in flow. Curr. Biol. 2023, 33, 3265–3271.e3264. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, S.D.; Henrichsen, J. Further studies of twitching Streptococcus sanguis isolated from the human throat. Isolation of strains with a new antigen. Acta Pathol. Microbiol. Scand. B 1976, 84B, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Palalay, J.S.; Simsek, A.N.; Reed, J.L.; Koch, M.D.; Sabass, B.; Sanfilippo, J.E. Shear force enhances adhesion of Pseudomonas aeruginosa by counteracting pilus-driven surface departure. Proc. Natl. Acad. Sci. USA 2023, 120, e2307718120. [Google Scholar] [CrossRef]
- Liang, X.; Chen, Y.Y.; Ruiz, T.; Wu, H. New cell surface protein involved in biofilm formation by Streptococcus parasanguinis. Infect. Immun. 2011, 79, 3239–3248. [Google Scholar] [CrossRef] [PubMed]
- Craig, L.; Volkmann, N.; Arvai, A.S.; Pique, M.E.; Yeager, M.; Egelman, E.H.; Tainer, J.A. Type IV pilus structure by cryo-electron microscopy and crystallography: Implications for pilus assembly and functions. Mol. Cell 2006, 23, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Harvey, H.; Bondy-Denomy, J.; Marquis, H.; Sztanko, K.M.; Davidson, A.R.; Burrows, L.L. Pseudomonas aeruginosa defends against phages through type IV pilus glycosylation. Nat. Microbiol. 2018, 3, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Kilian, M.; Mikkelsen, L.; Henrichsen, J. Taxonomic study of viridans streptococci: Description of Streptococcus gordonii sp. nov. and emended descriptions of Streptococcus sanguis (White and Niven 1946), Streptococcus oralis (Bridge and Sneath 1982), and Streptococcus mitis (Andrewes and Horder 1906). Int. J. Syst. Evol. Microbiol. 1989, 39, 471–484. [Google Scholar]
- Lau, P.C.; Sung, C.K.; Lee, J.H.; Morrison, D.A.; Cvitkovitch, D.G. PCR ligation mutagenesis in transformable streptococci: Application and efficiency. J. Microbiol. Methods 2002, 49, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Rubens, C.E.; Heggen, L.M. Tn916 delta E: A Tn916 transposon derivative expressing erythromycin resistance. Plasmid 1988, 20, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Perez-Casal, J.; Caparon, M.G.; Scott, J.R. Mry, a trans-acting positive regulator of the M protein gene of Streptococcus pyogenes with similarity to the receptor proteins of two-component regulatory systems. J. Bacteriol. 1991, 173, 2617–2624. [Google Scholar] [CrossRef] [PubMed]
- Paik, S.; Senty, L.; Das, S.; Noe, J.C.; Munro, C.L.; Kitten, T. Identification of virulence determinants for endocarditis in Streptococcus sanguinis by signature-tagged mutagenesis. Infect. Immun. 2005, 73, 6064–6074. [Google Scholar] [CrossRef] [PubMed]
- Loo, C.Y.; Corliss, D.A.; Ganeshkumar, N. Streptococcus gordonii biofilm formation: Identification of genes that code for biofilm phenotypes. J. Bacteriol. 2000, 182, 1374–1382. [Google Scholar] [CrossRef] [PubMed]





| Strains | Relevant Phenotype a | Description | Source or Reference |
|---|---|---|---|
| S. sanguinis strains | |||
| CGMH010 | Clinical isolate | [14] | |
| ΔpilA1 | EmR, PilA1− | pilA1 is replaced with erm in CGMH010 | This study |
| ΔpilA2 | EmR, PilA2− | pilA2 is replaced with erm in CGMH010 | This study |
| ΔpilA3 | EmR, PilA3− | pilA3 is replaced with erm in CGMH010 | This study |
| ΔpilA12 | EmR, PilA1−,PilA2− | pilA1 and pilA2 are replaced with erm in CGMH010 | This study |
| ΔpilA13 | EmR, KmR, PilA1−, PilA3− | pilA1 and pilA3 are replaced with erm and kan, respectively, in CGMH010 | This study |
| ΔpilA23 | EmR, PilA2−, PilA3− | pilA2 and pilA3 are replaced with erm in CGMH010 | This study |
| ΔpilA123 | EmR, PilA1−, PilA2−, PilA3− | All three pilA genes are replaced with erm in CGMH010 | This study |
| ΔpilT | EmR, PilT− | Also known as CHW01, pilT is replaced with erm in CGMH010 | [14] |
| SK36 | oral isolate | [39] | |
| Plasmids | |||
| pET28a(+) | KmR | Expression vector for N-terminal His-tagged proteins | Novagen |
| pET28a/pilA1 | KmR | pET28a(+) harboring the coding sequence of pilA1 | This study |
| pET28a/pilA2 | KmR | pET28a(+) harboring the coding sequence of pilA2 | This study |
| pET28a/pilA3 | KmR | pET28a(+) harboring the coding sequence of pilA3 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-Y.M.; Yang, Y.-C.; Shieh, H.-R.; Lin, Y.-J.; Ke, W.-J.; Chiu, C.-H. Functional Analysis of the Major Pilin Proteins of Type IV Pili in Streptococcus sanguinis CGMH010. Int. J. Mol. Sci. 2024, 25, 5402. https://doi.org/10.3390/ijms25105402
Chen Y-YM, Yang Y-C, Shieh H-R, Lin Y-J, Ke W-J, Chiu C-H. Functional Analysis of the Major Pilin Proteins of Type IV Pili in Streptococcus sanguinis CGMH010. International Journal of Molecular Sciences. 2024; 25(10):5402. https://doi.org/10.3390/ijms25105402
Chicago/Turabian StyleChen, Yi-Ywan M., Yuan-Chen Yang, Hui-Ru Shieh, Yu-Juan Lin, Wan-Ju Ke, and Cheng-Hsun Chiu. 2024. "Functional Analysis of the Major Pilin Proteins of Type IV Pili in Streptococcus sanguinis CGMH010" International Journal of Molecular Sciences 25, no. 10: 5402. https://doi.org/10.3390/ijms25105402





