Chloride Corrosion Process of Concrete with Different Water–Binder Ratios under Variable Temperature Drying–Wetting Cycles
Abstract
:1. Introduction
2. Experimental Program
2.1. Raw Materials
2.2. Mix Proportion and Specimen Preparation
2.3. Exposure Condition and Design of the Drying–Wetting Cycle System
2.4. Test Methods
2.4.1. Mechanical Properties Test
2.4.2. Porosity Test
2.4.3. Free Chloride Content
2.4.4. Chloride Ion Erosion Depth
2.4.5. Microstructure
3. Results and Discussion
3.1. Compressive Strength
3.2. Splitting Tensile Strength
3.3. Mass Loss Rate
3.4. RDEM
3.5. Porosity
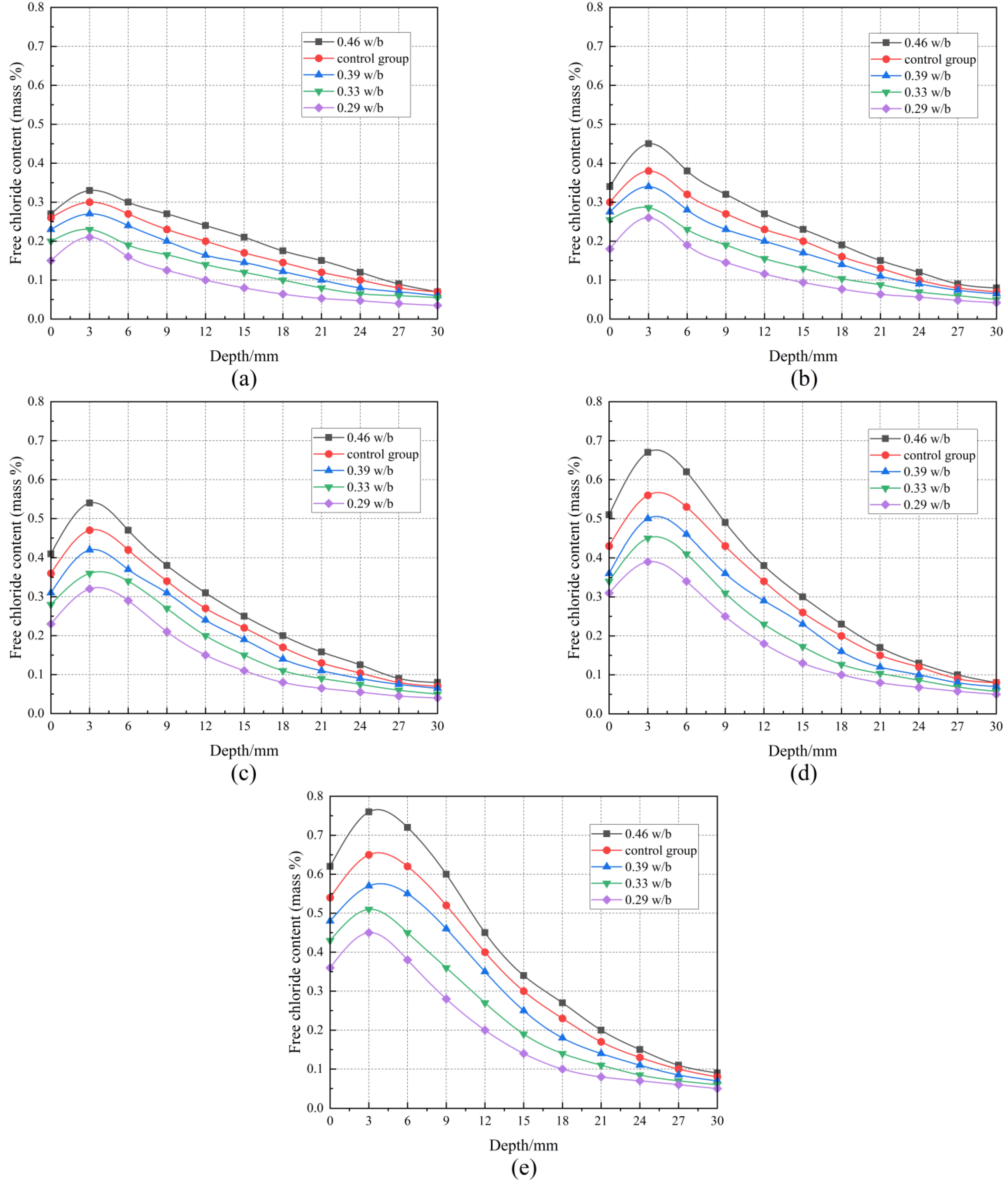
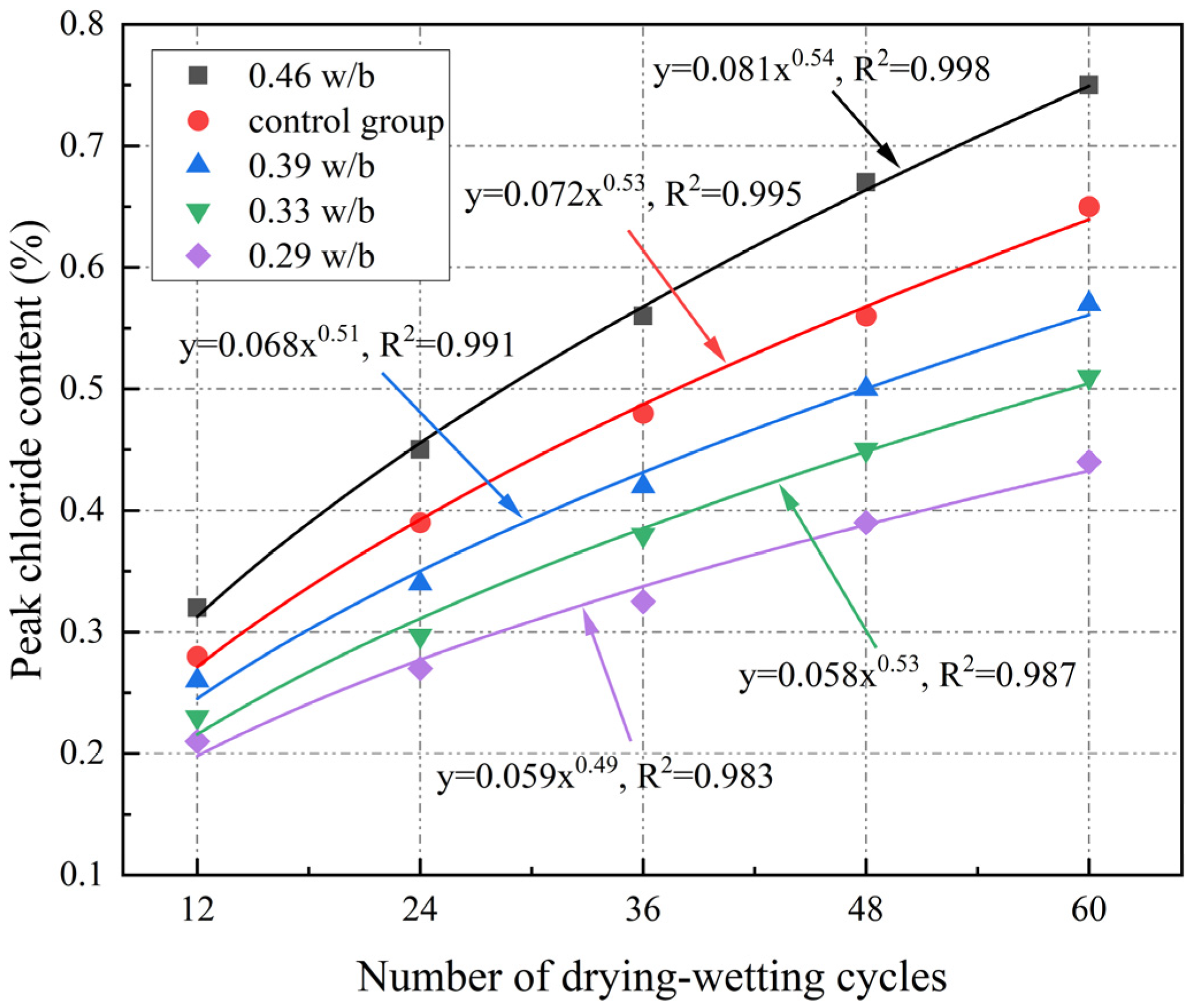
3.6. Free Chloride Content
3.7. Chloride Ion Erosion Depth
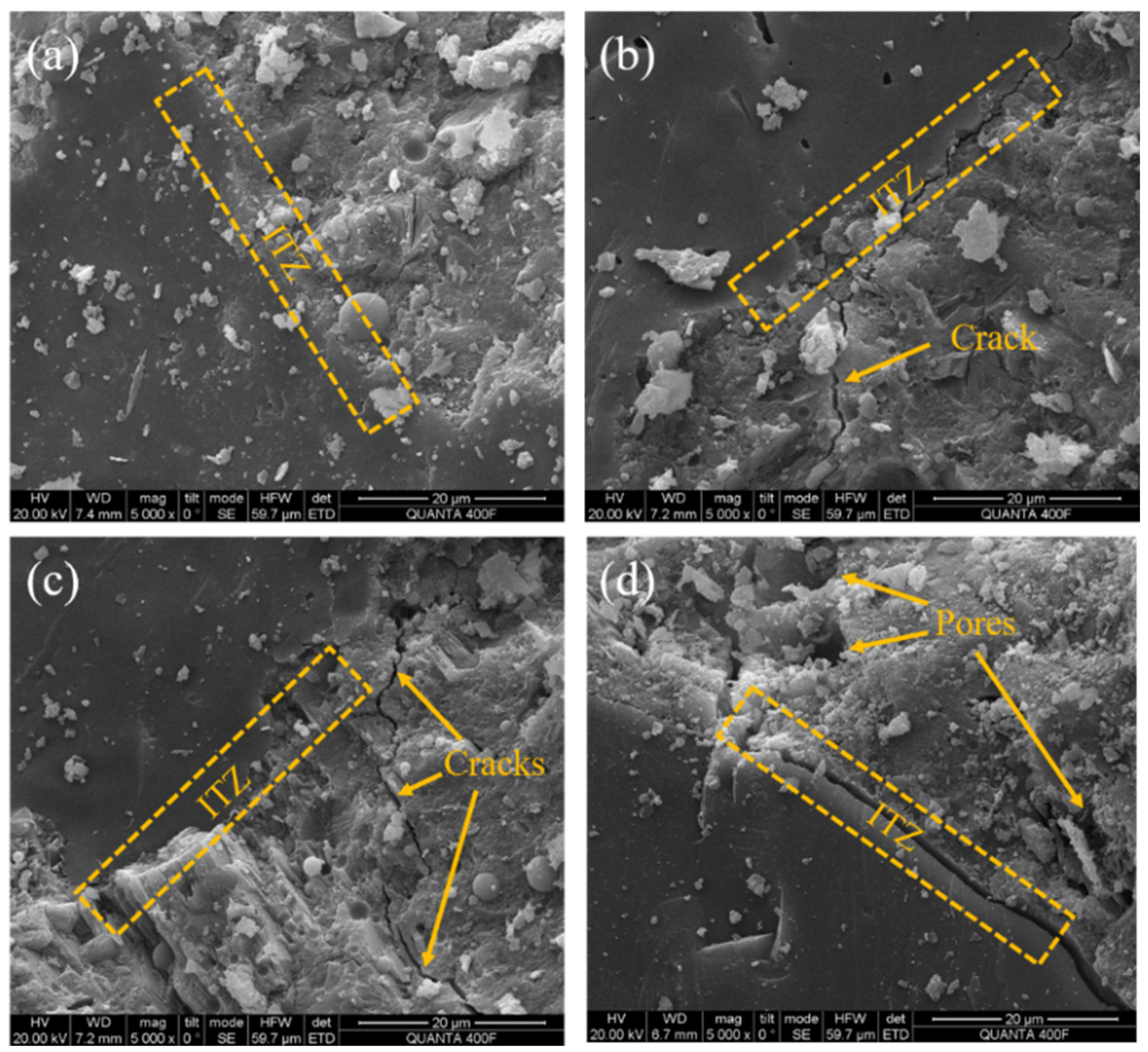
3.8. Microstructure
3.8.1. SEM
3.8.2. XRD
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, J.; Deng, P.; Li, X.; Zhang, J.; Wang, G. The vertical Non-uniform corrosion of Reinforced concrete exposed to the marine environments. Constr. Build. Mater. 2018, 183, 180–188. [Google Scholar] [CrossRef]
- Spiesz, P.; Brouwers, H.J.H. The apparent and effective chloride migration coefficients obtained in migration tests. Cem. Concr. Res. 2013, 48, 116–127. [Google Scholar] [CrossRef]
- Ting, M.Z.Y.; Wong, K.S.; Rahman, M.E.; Joo, M.S. Cyclic compressive behavior of limestone and silicomanganese slag concrete subjected to sulphate attack and wetting-drying action in marine environment. J. Build. Eng. 2021, 44, 103357. [Google Scholar] [CrossRef]
- Medeiros, M.H.F.; Gobbi, A.; Reus, G.C.; Helene, P. Reinforced concrete in marine environment: Effect of wetting and drying cycles, height and positioning in relation to the sea shore. Constr. Build. Mater. 2013, 44, 452–457. [Google Scholar] [CrossRef]
- Shi, X.; Xie, N.; Fortune, K.; Gong, J. Durability of steel reinforced concrete in chloride environments: An overview. Constr. Build. Mater. 2012, 30, 125–138. [Google Scholar] [CrossRef]
- Zhong, C.; Lu, W.; Mao, W. Research on Capillary Water Absorption Characteristics of Modified Recycled Concrete under Different Freeze–Thaw Environments. Appl. Sci. 2024, 14, 1247. [Google Scholar] [CrossRef]
- Golewski, G.L. The effect of the addition of coal fly ash (CFA) on the control of water movement within the structure of the concrete. Materials 2023, 16, 5218. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, L.; Liu, R.; Zhu, P.; Liu, H.; Wang, X. Chloride penetration of concrete exposed to dry-wet cycle with various dry-wet ratios and temperature. Constr. Build. Mater. 2023, 400, 132883. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Liu, R.; Yu, J.; Liu, H. Chloride penetration of recycled fine aggregate concrete under drying–wetting cycles. Materials 2023, 16, 1306. [Google Scholar] [CrossRef] [PubMed]
- Nematollahzade, M.; Tajadini, A.; Afshoon, I.; Aslani, F. Influence of different curing conditions and water to cement ratio on properties of self-compacting concretes. Constr. Build. Mater. 2020, 237, 117570. [Google Scholar] [CrossRef]
- Ye, H.; Jin, N.; Jin, X.; Fu, C.; Chen, W. Chloride ingress profiles and binding capacity of mortar in cyclic drying-wetting salt fog environments. Constr. Build. Mater. 2016, 127, 733–742. [Google Scholar] [CrossRef]
- Sun, R.; Hu, X.; Ling, Y.; Zuo, Z.; Zhuang, P.; Wang, F. Chloride diffusion behavior of engineered cementitious composite under dry-wet cycles. Constr. Build. Mater. 2020, 260, 119943. [Google Scholar] [CrossRef]
- Wang, J.; Xu, S.; Tian, M.; Li, Q.; Wang, Y. Study on the transport properties of chloride ions in concrete under dry-wet-temperature double cycling. China Concr. Cem. Prod. 2019, 2, 6–9. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhong, Z.; Ma, C.; Liu, J.; Zhou, H. Effects of discontinuous dry-wet cycles on the migration of chloride ions in concrete cover. J. Build. Mater. 2023, 26, 137–142. [Google Scholar]
- Liu, J.; Yu, H.; Sun, W.; Chen, S. Research on Influecning Factors of Chloride Ion Binding Capability of Concrete. Bull. Chin. Ceram. Soc. 2011, 30, 172–176. [Google Scholar] [CrossRef]
- Zibara, H.; Hooton, R.D.; Thomas, M.D.A.; Stanish, K. Influence of the C/S and C/A ratios of hydration products on the chloride ion binding capacity of lime-SF and lime-MK mixtures. Cem. Concr. Res. 2008, 38, 422–426. [Google Scholar] [CrossRef]
- Yu, L.B.; Jiang, L.H.; Chu, H.Q.; Guo, M.Z.; Zhu, Z.Y.; Dong, H. Effect of electrochemical chloride removal and ground granulated blast furnace slag on the chloride binding of cement paste subjected to NaCl and Na2SO4 attack. Constr. Build. Mater. 2019, 220, 538–546. [Google Scholar] [CrossRef]
- Song, Z.; Jiang, L.; Zhang, Z.; Xiong, C. Distance-associated chloride binding capacity of cement paste subjected to natural diffusion. Constr. Build. Mater. 2016, 112, 925–932. [Google Scholar] [CrossRef]
- Chang, H. Chloride binding capacity of pastes influenced by carbonation under three conditions. Cem. Concr. Compos. 2017, 84, 1–9. [Google Scholar] [CrossRef]
- Thomas, M.D.A.; Hooton, R.D.; Scott, A.; Zibara, H. The effect of supplementary cementitious materials on chloride binding in hardened cement paste. Cem. Concr. Res. 2012, 42, 1–7. [Google Scholar] [CrossRef]
- Boddy, A.; Bentz, E.; Thomas, M.D.A.; Hooton, R.D. An overview and sensitivity study of a multimechanistic chloride transport model. Cem. Concr. Res. 1999, 29, 827–837. [Google Scholar] [CrossRef]
- Nguyen, T.S.; Lorente, S.; Carcasses, M. Effect of the environment temperature on the chloride diffusion through CEM-I and CEM-V mortars: An experimental study. Constr. Build. Mater. 2009, 23, 795–803. [Google Scholar] [CrossRef]
- Dousti, A.; Rashetnia, R.; Ahmadi, B.; Shekarchi, M. Influence of exposure temperature on chloride diffusion in concretes incorporating silica fume or natural zeolite. Constr. Build. Mater. 2013, 49, 393–399. [Google Scholar] [CrossRef]
- AlKhaja, W.A. Influence of temperature, cement type and level of concrete consolidation on chloride ingress in conventional and high-strength concretes. Constr. Build. Mater. 1997, 11, 9–13. [Google Scholar] [CrossRef]
- Oh, B.H.; Jang, S.Y. Effects of material and environmental parameters on chloride penetration profiles in concrete structures. Cem. Concr. Res. 2007, 37, 47–53. [Google Scholar] [CrossRef]
- Song, H.W.; Lee, C.H.; Ann, K.Y. Factors influencing chloride transport in concrete structures exposed to marine environments. Cem. Concr. Compos. 2008, 30, 113–121. [Google Scholar] [CrossRef]
- Matsumura, T.; Shirai, K.; Saegusa, T. Verification method for durability of reinforced concrete structures subjected to salt attack under high temperature conditions. Nucl. Eng. Des. 2008, 238, 1181–1188. [Google Scholar] [CrossRef]
- So, H.-S.; Choi, S.-H.; Seo, K.-S.; Seo, C.-S.; So, S.-Y. The Properties of Chloride Ion Diffusion of Concrete Under High Temperature Conditions, with Implications for the Storage of Spent Nuclear Fuel in Concrete Casks. Ksce J. Civ. Eng. 2014, 18, 2227–2233. [Google Scholar] [CrossRef]
- Chen, J.; Wang, D. New mix design method for HPC–overall calculation method. Chin. Ceram. Soc. 2000, 28, 194–198. [Google Scholar] [CrossRef]
- Tam, V.W.Y.; Tam, C.M.; Wang, Y. Optimization on proportion for recycled aggregate in concrete using two-stage mixing approach. Constr. Build. Mater. 2007, 21, 1928–1939. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, Y.; Deng, Y. Island ecosystem evaluation and sustainable development strategies: A case study of the Zhoushan Archipelago. Glob. Ecol. Conserv. 2021, 28, e01603. [Google Scholar] [CrossRef]
- Amey, S.L.; Johnson, D.A.; Miltenberger, M.A.; Farzam, H. Predicting the service life of concrete marine structures: An environmental methodology. ACI Struct. J. 1998, 95, 205–214. [Google Scholar]
- Wu, J.; Faye, P.N.; Zhang, W.; Diao, B. Chloride diffusivity and service life prediction of RC columns with sustained load under chloride environment. Constr. Build. Mater. 2018, 158, 97–107. [Google Scholar] [CrossRef]
- GB/T 14685-2022; Pebble and Crushed Stone for Construction. China Standards Press: Beijing, China, 2022.
- GB/T 50081-2019; Standard for Test Methods of Concrete Physical and Mechanical Properties. China Architecture and Building Press: Beijing, China, 2019.
- GB/T 50082-2009; Standard for Test Methods of Long-Term Performance and Durability of Ordinary Concrete. China Architecture and Building Press: Beijing, China, 2009.
- ASTM C642; Standard Test Method for Density, Absorption, and Voids in Hardened Concrete. ASTM International: West Conshohocken, PA, USA, 2013.
- JGJ/T 322–2013; Technical Specifications for Test of Chloride Ion Content in Concrete. China Architecture and Building Press: Beijing, China, 2013.
- Zhang, G.; Li, Z.; Zhang, L.; Shang, Y.; Wang, H. Experimental research on drying control condition with minimal effect on concrete strength. Constr. Build. Mater. 2017, 135, 194–202. [Google Scholar] [CrossRef]
- Wang, K.; Guo, J.; Wu, H.; Yang, L. Influence of dry-wet ratio on properties and microstructure of concrete under sulfate attack. Constr. Build. Mater. 2020, 263, 120635. [Google Scholar] [CrossRef]
- Birnin-Yauri, U.A.; Glasser, F.P. Friedel’s salt, Ca2Al(OH)6(Cl,OH)·2H2O: Its solid solutions and their role in chloride binding. Cem. Concr. Res. 1998, 28, 1713–1723. [Google Scholar] [CrossRef]
- Du, X.; Li, Z.; Han, J.; Tan, C. Effect of Different Humidity-Controlling Modes on Microstructure and Compressive Behavior of Ordinary Concrete. J. Mater. Civ. Eng. 2020, 32, 04019337. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, M.; Cui, J. A review on the deterioration of mechanical and durability performance of marine-concrete under the scouring action. J. Build. Eng. 2023, 66, 105924. [Google Scholar] [CrossRef]
- Ma, H.; Gong, W.; Yu, H.; Sun, W. Durability of concrete subjected to dry-wet cycles in various types of salt lake brines. Constr. Build. Mater. 2018, 193, 286–294. [Google Scholar] [CrossRef]
- Abelha Rocha, C.A.; Cordeiro, G.C.; Toledo Filho, R.D. Use of thermal analysis to determine the hydration products of oil well cement pastes containing NaCl and KCl. J. Therm. Anal. Calorim. 2015, 122, 1279–1288. [Google Scholar] [CrossRef]
- Silva, Y.F.; Delvasto, S. Durability of self-compacting concrete with addition of residue of masonry when exposed to carbonation and chlorides mediums. Constr. Build. Mater. 2021, 297, 123817. [Google Scholar] [CrossRef]
- Cheewaket, T.; Jaturapitakkul, C.; Chalee, W. Concrete durability presented by acceptable chloride level and chloride diffusion coefficient in concrete: 10-year results in marine site. Mater. Struct. 2014, 47, 1501–1511. [Google Scholar] [CrossRef]
- Cao, J.; Jin, Z.; Ding, Q.; Xiong, C.; Zhang, G. Influence of the dry/wet ratio on the chloride convection zone of concrete in a marine environment. Constr. Build. Mater. 2022, 316, 125794. [Google Scholar] [CrossRef]
- Bao, J.; Zheng, R.; Wei, J.; Zhang, P.; Xue, S.; Liu, Z. Numerical and experimental investigation of coupled capillary suction and chloride penetration in unsaturated concrete under cyclic drying-wetting condition. J. Build. Eng. 2022, 51, 104273. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, L.; Wang, Y.; Liu, C.; Li, Q. Effects of coarse aggregates on chloride diffusion coefficients of concrete and interfacial transition zone under experimental drying-wetting cycles. Constr. Build. Mater. 2018, 185, 230–245. [Google Scholar] [CrossRef]
- Ye, H.; Jin, N.; Jin, X.; Fu, C. Model of chloride penetration into cracked concrete subject to drying-wetting cycles. Constr. Build. Mater. 2012, 36, 259–269. [Google Scholar] [CrossRef]










| Components | Cement | Fly Ash | Silica Fume |
|---|---|---|---|
| Chemical composition (%) | |||
| SiO2 | 22.16 | 43.86 | 95.4 |
| Al2O3 | 6.67 | 31.23 | 0.84 |
| Fe2O3 | 4.23 | 9.18 | 0.18 |
| CaO | 60.13 | 9.11 | 1.96 |
| MgO | 1.33 | 1.04 | 0.75 |
| SO3 | 1.76 | 0.75 | – |
| TiO2 | 0.23 | 1.14 | – |
| K2O | 0.42 | 2.07 | – |
| Loss on ignition | 2.56 | 1.87 | 1.79 |
| Aggregate Type | Apparent Density/ kg/m3 | Bulk Density/ kg/m3 | Water Absorption/ % | Crush Value/ % |
|---|---|---|---|---|
| NCA | 2718 | 1576 | 0.7 | 4.2 |
| NFA | 2636 | 1549 | 1.3 | 8.7 |
| Mixture | w/b | NCA | NFA | Cement | FLY ASH | Silica Fume | Water | SP |
|---|---|---|---|---|---|---|---|---|
| Control group | 0.46 | 1084.42 | 661.69 | 289.65 | 57.93 | 38.62 | 180.86 | 1.93 |
| A | 0.46 | 1084.42 | 661.69 | 289.65 | 57.93 | 38.62 | 180.86 | 1.93 |
| B | 0.39 | 1121.86 | 625.38 | 320.54 | 64.11 | 42.74 | 167.08 | 2.14 |
| C | 0.33 | 1158.86 | 589.50 | 351.07 | 70.21 | 46.81 | 153.47 | 2.34 |
| D | 0.29 | 1183.90 | 565.21 | 371.74 | 74.35 | 49.56 | 144.26 | 2.48 |
| Months | Mean Temperature/°C | Test Temperature/°C |
|---|---|---|
| 1, 2, 3, 11, 12 | 4.2 | 24 |
| 4, 10 | 12.4 | 35 |
| 5, 6, 9 | 21.2 | 43 |
| 7, 8 | 28.0 | 49 |
| Drying–Wetting Cycle | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature/°C | 24 | 24 | 24 | 35 | 43 | 43 | 49 | 49 | 43 | 35 | 24 | 24 |
| Depth (mm) | Δ(%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 27 | 30 | |
| 0.46 w/b | 0.35 | 0.43 | 0.42 | 0.33 | 0.21 | 0.13 | 0.10 | 0.05 | 0.03 | 0.02 | 0.02 |
| Control group | 0.28 | 0.35 | 0.34 | 0.29 | 0.20 | 0.12 | 0.09 | 0.04 | 0.03 | 0.02 | 0.01 |
| 0.39 w/b | 0.25 | 0.30 | 0.29 | 0.26 | 0.18 | 0.11 | 0.08 | 0.04 | 0.03 | 0.02 | 0.01 |
| 0.33 w/b | 0.23 | 0.28 | 0.26 | 0.20 | 0.13 | 0.07 | 0.06 | 0.04 | 0.03 | 0.02 | 0.01 |
| 0.29 w/b | 0.21 | 0.24 | 0.22 | 0.16 | 0.10 | 0.06 | 0.05 | 0.03 | 0.02 | 0.01 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Chen, C.; Liu, R.; Zhu, P.; Liu, H.; Jiang, H.; Yu, J. Chloride Corrosion Process of Concrete with Different Water–Binder Ratios under Variable Temperature Drying–Wetting Cycles. Materials 2024, 17, 2263. https://doi.org/10.3390/ma17102263
Wang L, Chen C, Liu R, Zhu P, Liu H, Jiang H, Yu J. Chloride Corrosion Process of Concrete with Different Water–Binder Ratios under Variable Temperature Drying–Wetting Cycles. Materials. 2024; 17(10):2263. https://doi.org/10.3390/ma17102263
Chicago/Turabian StyleWang, Lei, Chunhong Chen, Ronggui Liu, Pinghua Zhu, Hui Liu, Hongwei Jiang, and Jiang Yu. 2024. "Chloride Corrosion Process of Concrete with Different Water–Binder Ratios under Variable Temperature Drying–Wetting Cycles" Materials 17, no. 10: 2263. https://doi.org/10.3390/ma17102263








