Study on the Flow Field Distribution in Microfluidic Cells for Surface Plasmon Resonance Array Detection
Abstract
:1. Introduction
2. Theory, Methodology, and Results of Simulation Studies
2.1. Basic Simulation Methods
2.1.1. Simulation Model of Microfluidic Cells
2.1.2. Boundary Conditions and Numerical Model Conditions
2.2. Discussion of Simulation Results
2.2.1. Impact of Contact Angle on Flow Field Uniformity
2.2.2. Effects of Flow Velocity on Flow Field Uniformity
2.2.3. Effects of Micro-Pillar Structured Microfluidic Cell Detection Arrays
- (1)
- Impact of micro-pillar dimensions on the flow field distribution in the detection area
- (2)
- Impact of micro-pillar distribution on the flow field distribution in the detection area
- (1)
- Across different boundary configurations, changes in the flow dynamics within the detection zone are minimal; however, the flow velocity’s stability within the detection zone exhibits significant variability contingent on the differential length from the inlet to the outlet. This variability is closely related to the differential length from the inlet to the outlet and is influenced by the contact angle.
- (2)
- The integration of an optimally sized transition section within the microfluidic environment is conducive to enhancing the homogeneity of the fluid flow field.
- (3)
- Incorporating micro-structural arrays to induce flow turbulence within flow cells can improve flow uniformity, though it may lead to vortex formation near the inlet.
3. Experimental Study of Flow Distribution
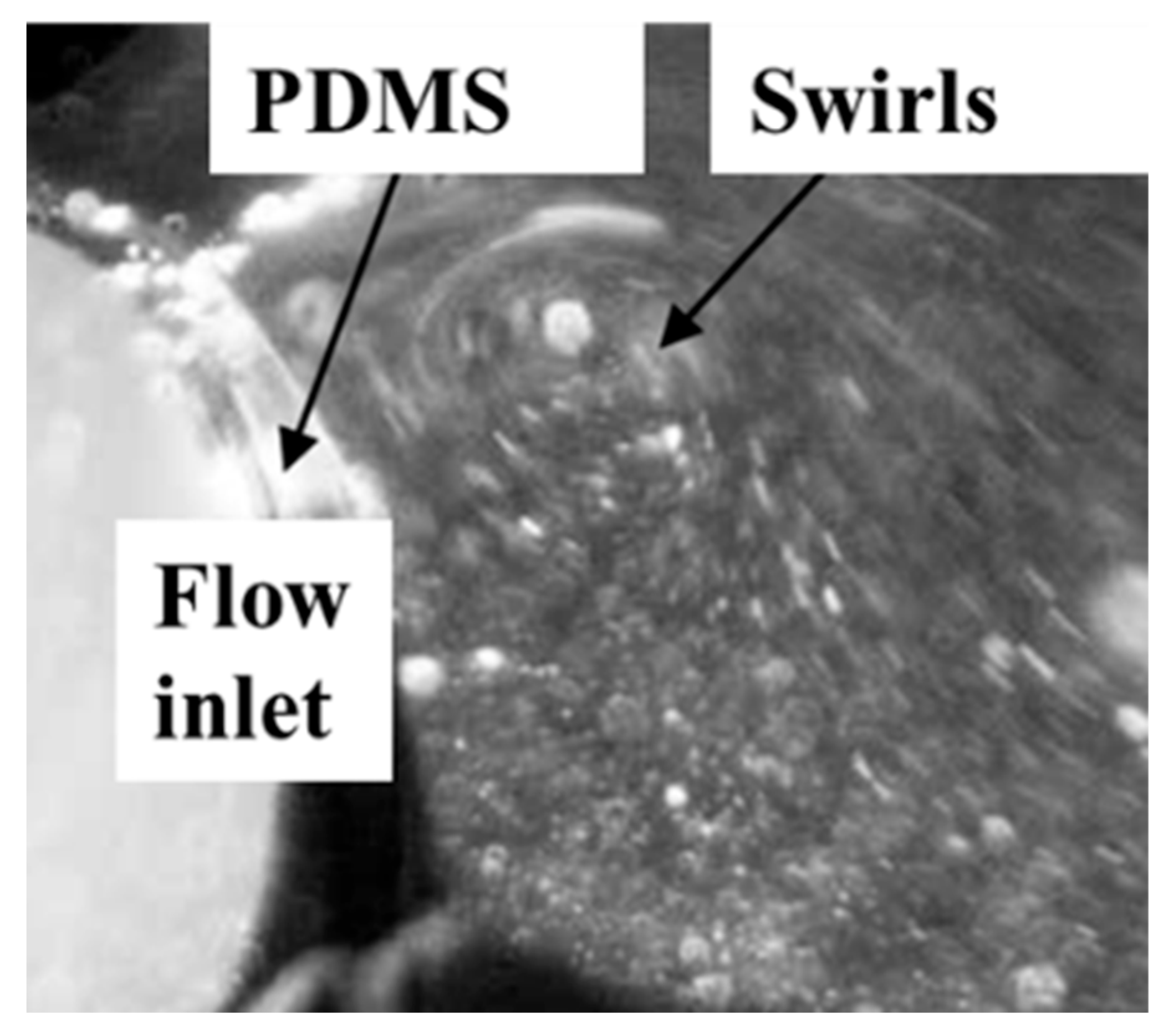
3.1. Mic-PIV Experiment
3.2. SPR Array Detection Experiments
3.2.1. Preliminary Experiment for Detection
- (1)
- Inconsistency in the inner diameter of the tubing, leading to the fragmentation of bubbles into several smaller segments;
- (2)
- A significant discrepancy between the set volume of the tubing and the actual volume, resulting in bubbles not being completely expelled through the bypass. The bubble issue can be resolved by replacing the tubing and adjusting its volume and tolerance appropriately.
- (1)
- Inject deionized water into the microfluidic cell; continue for 150 s to establish a baseline.
- (2)
- Inject 5% NaCl solution; continue for 180 s to measure its change in refractive index.
- (3)
- Switch back to deionized water; continue for 300 s.
- (4)
- Inject 0.9% NaCl solution; continue for 180 s.
- (5)
- Reinject deionized water.
3.2.2. Repeatability Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Das, S.; Devireddy, R.; Gartia, M.R. Surface Plasmon Resonance (SPR) Sensor for Cancer Biomarker Detection. Biosensors 2023, 13, 396. [Google Scholar] [CrossRef] [PubMed]
- Jebelli, A.; Oroojalian, F.; Fathi, F.; Mokhtarzadeh, A.; Guardia, M.D.L. Recent Advances in Surface Plasmon Resonance Biosensors for microRNAs Detection. Biosens. Bioelectron. 2020, 169, 112599. [Google Scholar] [CrossRef] [PubMed]
- Konoplev, G.; Agafonova, D.; Bakhchova, L.; Mukhin, N.; Kurachkina, M.; Schmidt, M.-P.; Verlov, N.; Sidorov, A.; Oseev, A.; Stepanova, O.; et al. Label-Free Physical Techniques and Methodologies for Proteins Detection in Microfluidic Biosensor Structures. Biomedicines 2022, 10, 207. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-M.; Lv, S.; Zhang, W.; Cui, Y. Microfluidic Point-of-Care (POC) Devices in Early Diagnosis: A Review of Opportunities and Challenges. Sensors 2022, 22, 1620. [Google Scholar] [CrossRef] [PubMed]
- Homola, J. Surface Plasmon Resonance Sensors for Detection of Chemical and Biological Species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef] [PubMed]
- Souto, D.E.P.; Volpe, J.; Gonçalves, C.D.C.; Ramos, C.H.I.; Kubota, L.T. A Brief Review on the Strategy of Developing SPR-Based Biosensors for Application to the Diagnosis of Neglected Tropical Diseases. Talanta 2019, 205, 120122. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, P.; Chen, Y. Surface Plasmon Resonance Biosensors: A Review of Molecular Imaging with High Spatial Resolution. Biosensors 2024, 14, 84. [Google Scholar] [CrossRef] [PubMed]
- Macchia, E.; Sarcina, L.; Picca, R.A.; Manoli, K.; Di Franco, C.; Scamarcio, G.; Torsi, L. Ultra-Low HIV-1 P24 Detection Limits with a Bioelectronic Sensor. Anal. Bioanal. Chem. 2020, 412, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Macchia, E.; Picca, R.A.; Manoli, K.; Di Franco, C.; Blasi, D.; Sarcina, L.; Ditaranto, N.; Cioffi, N.; Österbacka, R.; Scamarcio, G.; et al. About the Amplification Factors in Organic Bioelectronic Sensors. Mater. Horiz. 2020, 7, 999–1013. [Google Scholar] [CrossRef]
- Wang, C.; Liu, M.; Wang, Z.; Li, S.; Deng, Y.; He, N. Point-of-Care Diagnostics for Infectious Diseases: From Methods to Devices. Nano Today 2021, 37, 101092. [Google Scholar] [CrossRef]
- Mejía-Salazar, J.R.; Oliveira, O.N. Plasmonic Biosensing: Focus Review. Chem. Rev. 2018, 118, 10617–10625. [Google Scholar] [CrossRef] [PubMed]
- Güvener, N.; Oguzhan Caglayan, M.; Altintas, Z. Surface Plasmon Resonance Sensors. In Fundamentals of Sensor Technology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 163–196. ISBN 978-0-323-88431-0. [Google Scholar]
- Bell, N.A.W.; Molloy, J.E. Microfluidic Flow-Cell with Passive Flow Control for Microscopy Applications. PLoS ONE 2020, 15, e0244103. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Kushwaha, A.S.; Dubey, S.K.; Srivastava, S.K. A Comparative Study of Different Types of Sandwiched Structures of SPR Biosensor for Sensitive Detection of ssDNA. Photonics Nanostruct.-Fundam. Appl. 2022, 48, 100984. [Google Scholar] [CrossRef]
- Thadson, K.; Sasivimolkul, S.; Suvarnaphaet, P.; Visitsattapongse, S.; Pechprasarn, S. Measurement Precision Enhancement of Surface Plasmon Resonance Based Angular Scanning Detection Using Deep Learning. Sci. Rep. 2022, 12, 2052. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-S.; Fan, S.-K. Microfluidic Surface Plasmon Resonance Sensors: From Principles to Point-of-Care Applications. Sensors 2016, 16, 1175. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, R.; Zhu, C.; Ge, B.; Zhu, X.; Mi, L.; Ma, J.; Han, C.; Chen, H.; Fei, Y. Label-Free Microarray-Based Binding Affinity Constant Measurement with Modified Fluidic Arrangement. BioChip J. 2018, 12, 11–17. [Google Scholar] [CrossRef]
- Kumar, A.S.; Venkatesalu, S.; Dilliyappan, S.; Pasupulla, A.P.; Prathap, L.; Palaniyandi, T.; Baskar, G.; Ravi, M.; Sugumaran, A. Microfluidics as Diagnostic Tools. Clin. Chim. Acta 2024, 556, 117841. [Google Scholar] [CrossRef] [PubMed]
- Sathish, S.; Shen, A.Q. Toward the Development of Rapid, Specific, and Sensitive Microfluidic Sensors: A Comprehensive Device Blueprint. JACS Au 2021, 1, 1815–1833. [Google Scholar] [CrossRef]
- Lakayan, D.; Tuppurainen, J.; Eemeli Suutari, T.; Van Iperen, D.J.; Somsen, G.W.; Kool, J. Design and Evaluation of a Multiplexed Angular-Scanning Surface Plasmon Resonance System Employing Line-Laser Optics and CCD Detection in Combination with Multi-Ligand Sensor Chips. Sens. Actuators B Chem. 2019, 282, 243–250. [Google Scholar] [CrossRef]
- He, Y.; Li, Y.; Hao, X.; Zhou, J.; Liu, S. Micro-Flow Sensor for Continuous Resin Fluidity Monitoring between Fibers. Sens. Actuators B Chem. 2019, 282, 177–186. [Google Scholar] [CrossRef]
- Rampazzi, S.; Danese, G.; Leporati, F.; Marabelli, F. A Localized Surface Plasmon Resonance-Based Portable Instrument for Quick On-Site Biomolecular Detection. IEEE Trans. Instrum. Meas. 2016, 65, 317–327. [Google Scholar] [CrossRef]
- Wang, Q.; Song, H.; Zhu, A.; Qiu, F. A Label-Free and Anti-Interference Dual-Channel SPR Fiber Optic Sensor With Self-Compensation for Biomarker Detection. IEEE Trans. Instrum. Meas. 2021, 70, 1–7. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Zhang, H.; Huang, Y.; Zhang, N.; Li, W.; Qiu, X.; Yu, D.; Zhang, L. Integration of a Multichannel Surface Plasmon Resonance Sensor Chip and Refractive Index Matching Film Array for Protein Detection in Human Urine. Talanta 2022, 246, 123533. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Huang, Y.; Zhang, H.; Fu, Y.; Yang, Z.; Chen, Y.; Qiu, X.; Yu, D.; Zhang, L. Multi-Parameter Surface Plasmon Resonance Instrument for Multiple Nucleic Acid Quantitative Detection. Biomed. Microdevices 2023, 25, 24. [Google Scholar] [CrossRef] [PubMed]
- Pattanayak, P.; Singh, S.K.; Gulati, M.; Vishwas, S.; Kapoor, B.; Chellappan, D.K.; Anand, K.; Gupta, G.; Jha, N.K.; Gupta, P.K.; et al. Microfluidic Chips: Recent Advances, Critical Strategies in Design, Applications and Future Perspectives. Microfluid. Nanofluid 2021, 25, 99. [Google Scholar] [CrossRef]
- Keshavarz, M.; Chowdhury, A.K.M.R.H.; Kassanos, P.; Tan, B.; Venkatakrishnan, K. Self-Assembled N-Doped Q-Dot Carbon Nanostructures as a SERS-Active Biosensor with Selective Therapeutic Functionality. Sens. Actuators B Chem. 2020, 323, 128703. [Google Scholar] [CrossRef]
- Borg, K.N.; Jaffiol, R.; Ho, Y.-P.; Zeng, S. Enhanced Biosensing of Tumor Necrosis Factor-Alpha Based on Aptamer-Functionalized Surface Plasmon Resonance Substrate and Goos–Hänchen Shift. Analyst 2024. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Z.; Zhang, M.; Guo, W.; Li, N.; Deng, Y.; Shi, Q. A Microfluidic Chip with Double-Sided Herringbone Microstructures for Enhanced Capture of Rare Tumor Cells. J. Mater. Chem. B 2017, 5, 9114–9120. [Google Scholar] [CrossRef] [PubMed]
- Lynn, N.S.; Martínez-López, J.-I.; Bocková, M.; Adam, P.; Coello, V.; Siller, H.R.; Homola, J. Biosensing Enhancement Using Passive Mixing Structures for Microarray-Based Sensors. Biosens. Bioelectron. 2014, 54, 506–514. [Google Scholar] [CrossRef]
- Liu, H.; Koch, C.; Haller, A.; Joosse, S.A.; Kumar, R.; Vellekoop, M.J.; Horst, L.J.; Keller, L.; Babayan, A.; Failla, A.V.; et al. Evaluation of Microfluidic Ceiling Designs for the Capture of Circulating Tumor Cells on a Microarray Platform. Adv. Biosyst. 2020, 4, 1900162. [Google Scholar] [CrossRef]
- Mejía-Salazar, J.R.; Rodrigues Cruz, K.; Materón Vásques, E.M.; Novais De Oliveira, O., Jr. Microfluidic Point-of-Care Devices: New Trends and Future Prospects for eHealth Diagnostics. Sensors 2020, 20, 1951. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liang, Z.; Huang, Y.; Kim, K.; Vandeventer, P.; Fan, D. Acceleration of Biomolecule Enrichment and Detection with Rotationally Motorized Opto-Plasmonic Microsensors and the Working Mechanism. ACS Nano 2020, 14, 15204–15215. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jiang, Z.; Wang, J.; Ren, Y.; Wu, A. Microfluidic Applications on Circulating Tumor Cell Isolation and Biomimicking of Cancer Metastasis. Electrophoresis 2020, 41, 933–951. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dallmann, R.; Lu, R.; Yan, J.; Charmet, J. Flow Rate-Independent Multiscale Liquid Biopsy for Precision Oncology. ACS Sens. 2023, 8, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Afzal, A.; Kim, K.-Y. Design Optimization of Micromixers. In Analysis and Design Optimization of Micromixers; SpringerBriefs in Applied Sciences and Technology; Springer: Singapore, 2021; pp. 45–61. ISBN 978-981-334-290-3. [Google Scholar]
- Chen, X.; Li, T.; Zeng, H.; Hu, Z.; Fu, B. Numerical and Experimental Investigation on Micromixers with Serpentine Microchannels. Int. J. Heat Mass Transf. 2016, 98, 131–140. [Google Scholar] [CrossRef]
- Shanbhag, V.V.; Mukherjee, J.; Pandit, A.B. Analytical and Numerical Investigations of Mixing Fluids in Microchannel Systems of Different Geometrical Configurations. Can. J. Chem. Eng. 2022, 100, 2217–2229. [Google Scholar] [CrossRef]




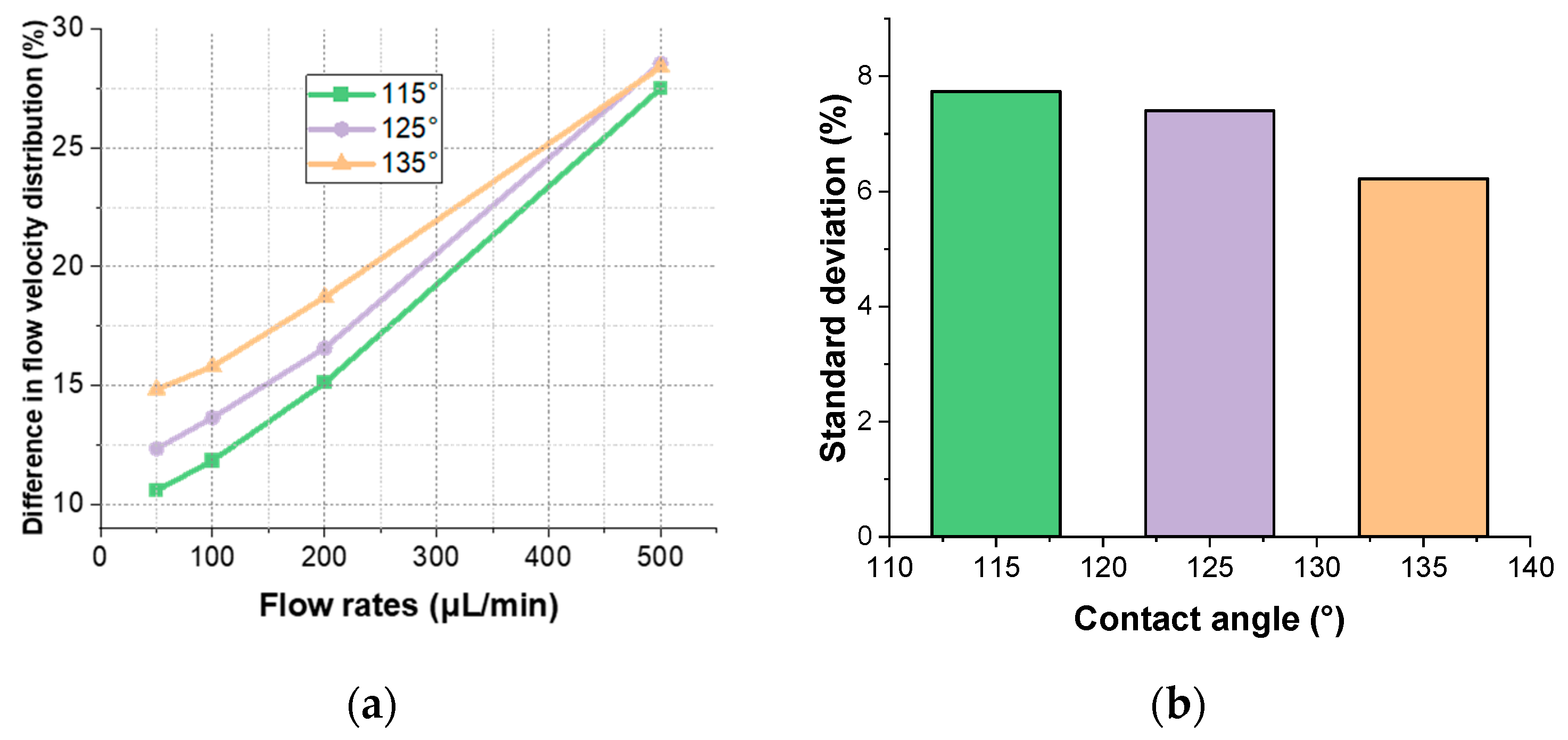






| Contact Angle (°) | 135 | 125 | 115 |
|---|---|---|---|
| Microfluidic cell area (mm2) | 30.50 | 29.23 | 27.61 |
| Flow velocity distribution (mm/s) | 0.757~0.638 | 0.726~0.627 | 0.710~0.626 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Li, J.; Wang, P.; Ma, S.; Li, B. Study on the Flow Field Distribution in Microfluidic Cells for Surface Plasmon Resonance Array Detection. Materials 2024, 17, 2426. https://doi.org/10.3390/ma17102426
Chen W, Li J, Wang P, Ma S, Li B. Study on the Flow Field Distribution in Microfluidic Cells for Surface Plasmon Resonance Array Detection. Materials. 2024; 17(10):2426. https://doi.org/10.3390/ma17102426
Chicago/Turabian StyleChen, Wanwan, Jing Li, Peng Wang, Shuai Ma, and Bin Li. 2024. "Study on the Flow Field Distribution in Microfluidic Cells for Surface Plasmon Resonance Array Detection" Materials 17, no. 10: 2426. https://doi.org/10.3390/ma17102426





