Effect of Pseudomonas aeruginosa on Corrosion Behavior of X65 Carbon Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacteria and Culture
2.3. Immersion Experiment
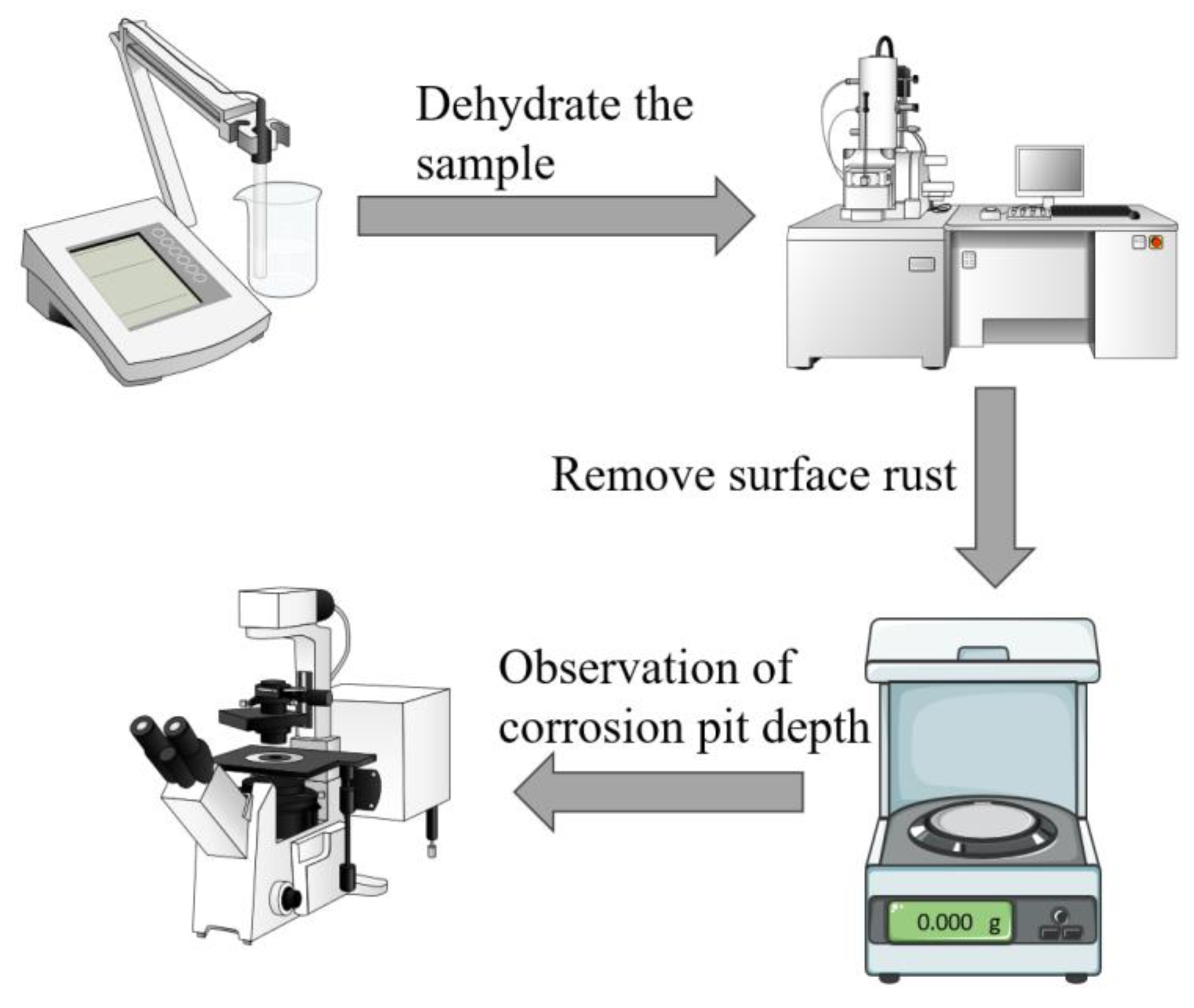
2.4. Weightlessness Testing and Surface Analysis
- (1)
- After the samples were removed from different cycles of immersion, the samples were rinsed with phosphate-buffered saline (PBS) to remove the floating P. aeruginosa cells and impurities on the sample surface. The samples were then immersed in 2.5% glutaraldehyde solution for 24 h to immobilize the formed biofilm on the specimen surface, followed by gradual dehydration with ethanol solutions at 50%, 60%, 70%, 80%, 90% and 100% concentrations. To improve the electrical conductivity of the specimen surface, a gold film was sprayed on the surface of the specimen, and then the biofilm was observed by field emission scanning electron microscope (SEM, Quanta FEG 250, FEI, Hillsborough, OR, USA).
- (2)
- Nitric acid, hexamethylenetetramine and deionized water were used to prepare a rust remover to remove biofilm and corrosion products from the surface of X65 steel. Then the samples were washed with deionized water and anhydrous ethanol, and finally, the samples were dried. The weight of the dried samples was weighed and recorded.
- (3)
- The diameter, depth and number of corrosion pits on the surface of the specimens were measured using a confocal laser scanning microscope (CLSM, OLS5000, Olympus, Tokyo, Japan).
2.5. Electrochemical Tests
3. Results
3.1. Weightlessness Analysis
3.2. Surface Morphology
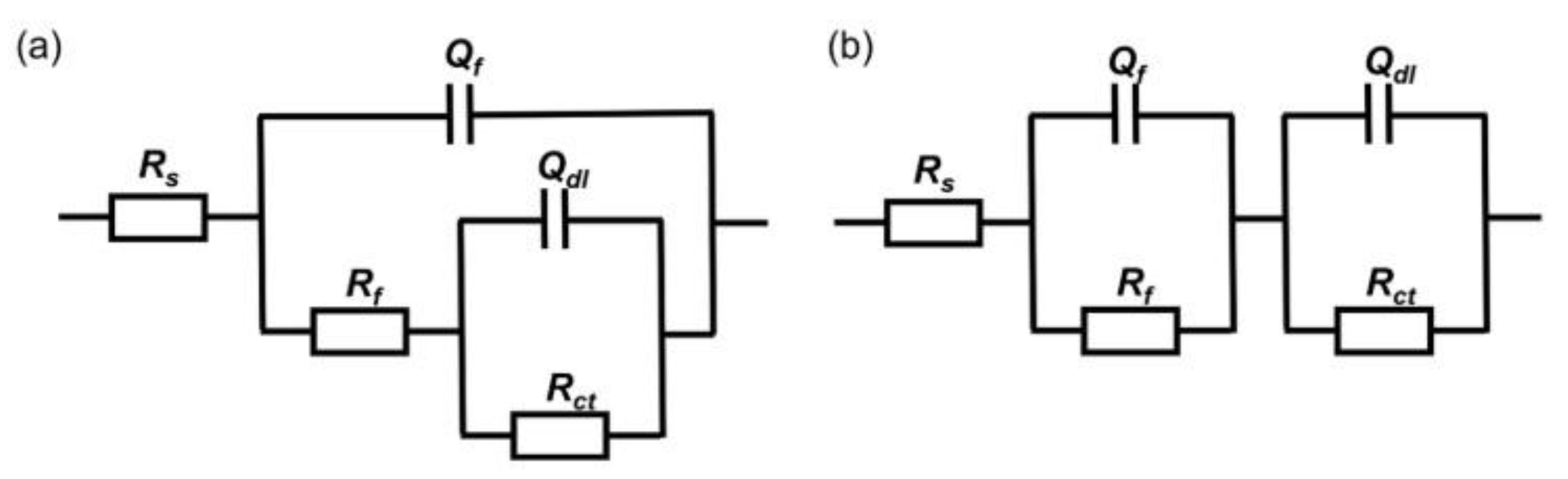
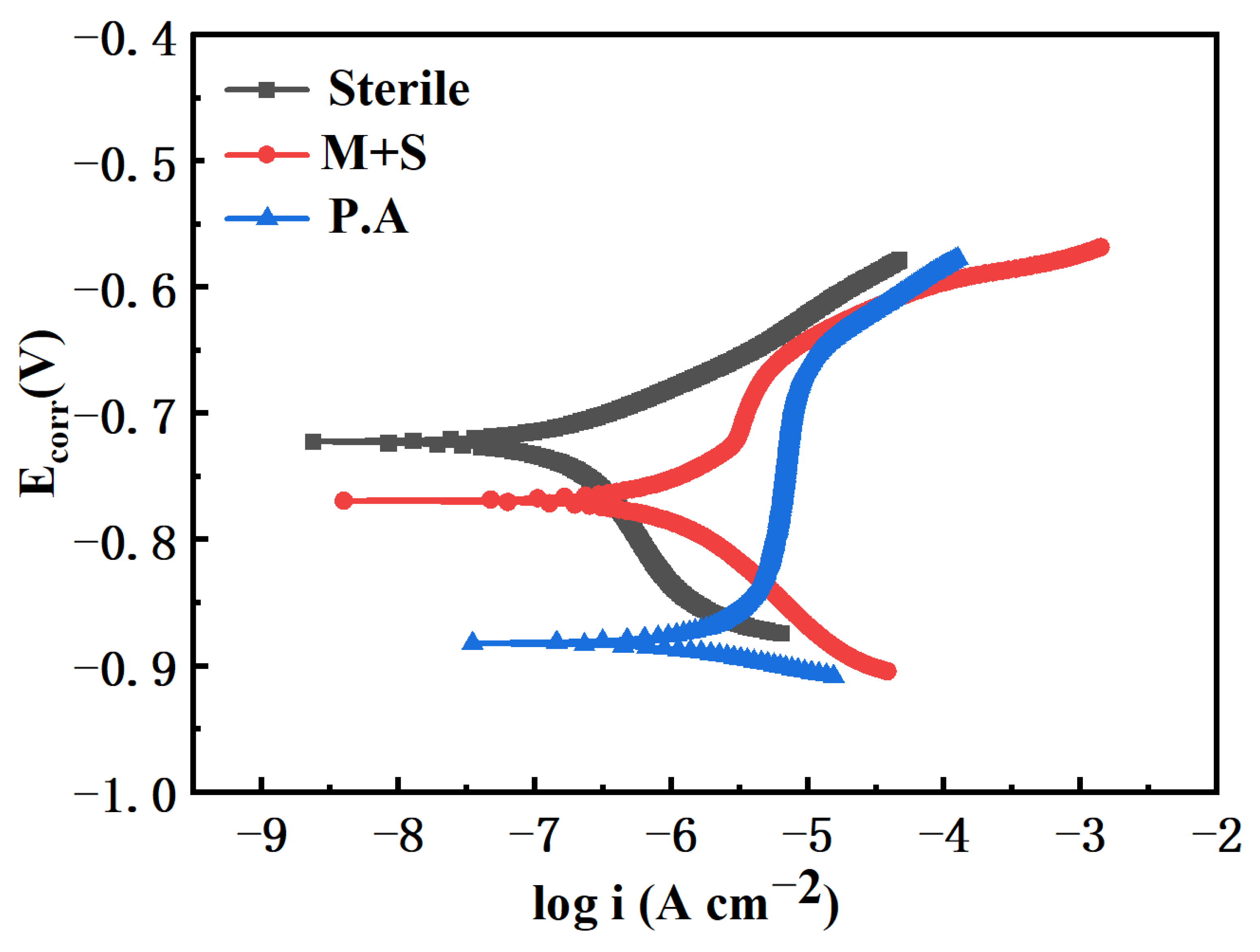
3.3. Electrochemical Analysis
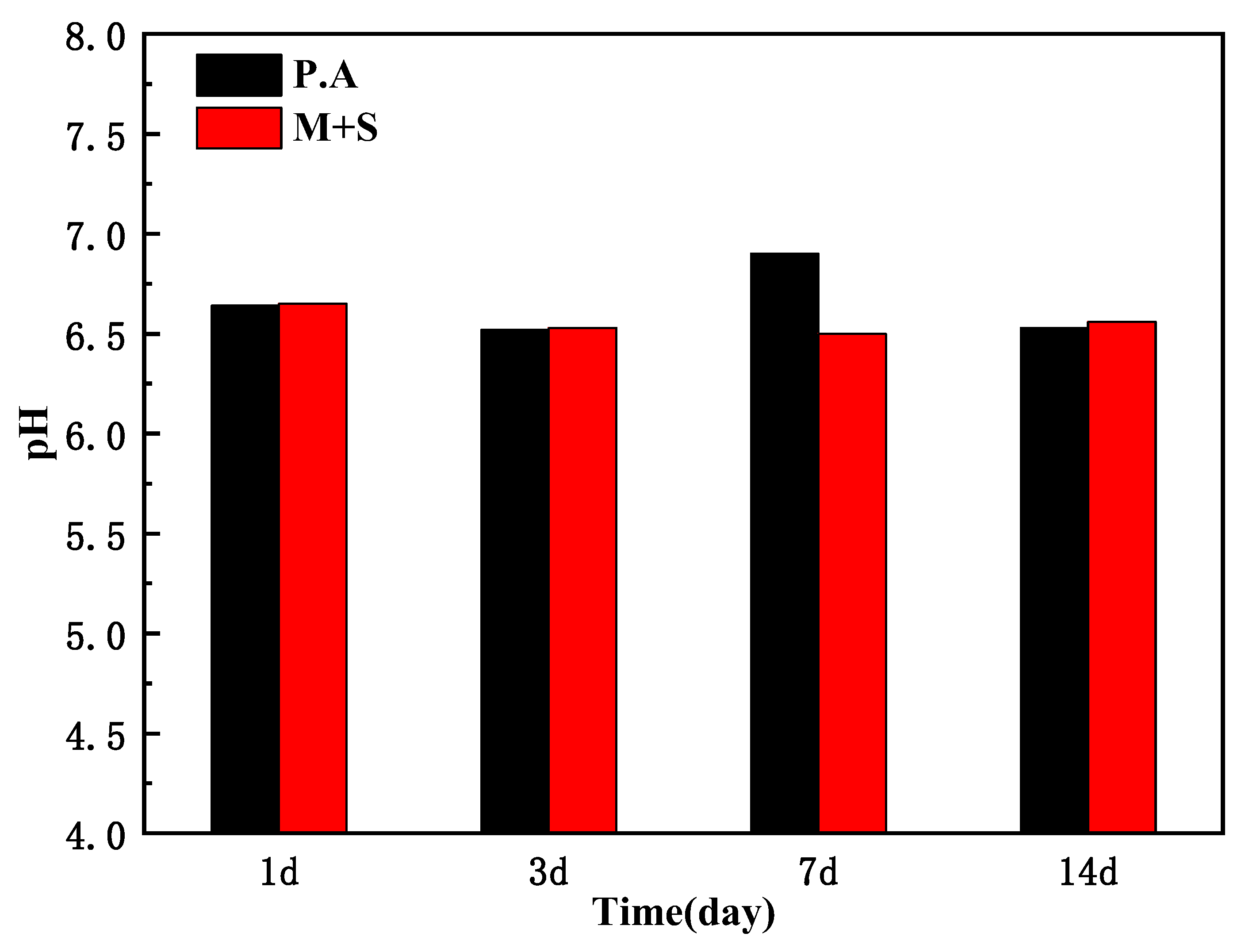
3.4. pH Analysis of Medium
4. Conclusions
- From the morphology of the biofilm, the biofilm morphology of the knockout P. aeruginosa was consistent with that produced by the wild-type P. aeruginosa, indicating that the knockout of the phzM and phzS genes did not have any particular effect on the biofilm produced by P. aeruginosa on the surface of X65 pipeline steel.
- According to the weight loss and electrochemical results, the weight loss in the wild medium was nearly 8 times greater than that in the knockout environment, and the Rct value decreased from 1.7 × 102 ± 8.9 Ω∙cm2 to 3.8 × 104 ± 2.4 × 104 Ω∙cm2 after 14 days of immersion. The corrosion of X65 pipeline steel by P. aeruginosa after knockdown of the phzM and phzS genes was attenuated, suggesting that the phzM and phzS genes, which regulate the synthesis of PYO, play an important role in accelerating the microbial corrosion of P. aeruginosa.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, M.Z.; Liu, Y.; Li, M.J.; Qiu, J. Current situation and trends in the development and utilization of marine resources in China. Ocean Dev. Manag. 2013, 12, 13–16. [Google Scholar]
- Sun, X.J. Research and Development of High-Performance Steel for Offshore Engineering. Ph.D. Dissertation, University of Science and Technology Beijing, Beijing, China, 2018. [Google Scholar]
- Golchinvafa, A.; Mousavi Anijdan, S.H.; Sabzi, M.; Sadeghi, M. The effect of natural inhibitor concentration of Fumaria officinalis and temperature on corrosion protection mechanism in API X80 pipeline steel in 1 MH2SO4 solution. Int. J. Press. Vessels Pip. 2020, 188, 104241. [Google Scholar] [CrossRef]
- Asadian, M.; Sabzi, M.; Mousavi Anijdan, S.H. The effect of temperature, CO2, H2S gases and the resultant iron carbonate and iron sulfide compounds on the sour corrosion behaviour of ASTM A-106 steel for pipeline transportation. Int. J. Press. Vessels Pip. 2019, 171, 184–193. [Google Scholar] [CrossRef]
- Deng, C.Y.; Zhang, Y.F.; Huo, L.X.; Bai, B.R.; Li, X.W.; Cao, J. CTOD fracture toughness of welded joints of X65 pipeline steel. Trans. China Weld. Inst. 2003, 3, 13–16. [Google Scholar]
- Lv, M.Y.; Du, M.; Li, Z.X. Investigation of mixed species biofilm on corrosion of X65 steel in seawater environment. Bioelectrochemistry 2021, 143, 107951. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.Y.; Xu, D.K. Demystifying MIC mechanisms. In Proceedings of the Corrosion Conference and Expo, San Antonio, TX, USA, 14–18 March 2010. [Google Scholar]
- Gu, T.Y.; Xu, D.K. Why are some microbes corrosive and some not? In Proceedings of the Corrosion Conference and Expo, San Antonio, TX, USA, 17–21 March 2013. [Google Scholar]
- Gu, T.Y. New understandings of biocorrosion mechanisms and their classification. J. Microb. Biochem. Technol. 2012, 4, 3–6. [Google Scholar] [CrossRef]
- Xu, D.K.; Gu, T.Y. Carbon source starvation triggered more aggressive carbon steel by the Desulfovibrio vulgaris biofilm. Int. Biodeterior. Biodegrad. 2014, 91, 74–81. [Google Scholar] [CrossRef]
- Kakooei, S.; Ismail, M.C.; Ariwahjoedi, B. Mechanisms of microbiologically influenced corrosion: A review. World Appl. Sci. J. 2012, 17, 524–531. [Google Scholar]
- Yuan, S.J.; Choong, A.M.F.; Pehkonen, S.O. The influence of the marine aerobic Pseudomonas strain on the corrosion of 70/30 Cu-Ni alloy. Corros. Sci. 2007, 49, 4352–4385. [Google Scholar] [CrossRef]
- Morales, J.; Esparza, P.; González, S.; Salvarezza, R.; Arévalo, M.P. The role of Pseudomonas aeruginosa on the localized corrosion of 304 stainless steel. Corros. Sci. 1993, 34, 1531–1540. [Google Scholar] [CrossRef]
- Glasser, N.R.; Saunders, S.H.; Newman, D.K. The colorful world of extracellular electron shuttles. Annu. Rev. Microbiol. 2017, 71, 731–751. [Google Scholar] [CrossRef] [PubMed]
- Köhler, T.; Van Delden, C.; Curty, L.K.; Hamzehpour, M.M.; Pechere, J.C. Overexpression of the MexEF-OprN multidrug efflux system affects cell-to-cell signaling in Pseudomonas aeruginosa. J. Bacteriol. 2001, 183, 5213–5222. [Google Scholar] [CrossRef] [PubMed]
- Hamzah, E.; Hussain, M.Z.; Ibrahim, Z.; Abdolahi, A. Influence of Pseudomonas aeruginosa bacteria on corrosion resistance of 304 stainless steel. Corros. Eng. Sci. Technol. 2013, 48, 116–120. [Google Scholar] [CrossRef]
- Xu, Y.Y. Pathogenicity of Pseudomonas aeruginosa Is Regulated by Signaling Molecules and Its Mechanisms. Ph.D. Dissertation, Northwest University, Xi’an, China, 2013. [Google Scholar]
- Mavrodi, D.V.; Bonsall, R.F.; Delaney, S.M.; Soule, M.J.; Thomashow, L.S. Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J. Bacteriol. 2001, 183, 6454–6465. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.L. Screening and Study of Regulatory Genes Affecting the Expression of phzA2 in the Genome of Pseudomonas aeruginosa. Master’s Dissertation, Northwest University, Xi’an, China, 2012. [Google Scholar]
- Parsons, J.F.; Greenhagen, B.T.; Shi, K.; Calabrese, K.; Robinson, H.; Ladner, J.E. Structural and functional analysis of the pyocyanin biosynthetic protein PhzM from Pseudomonas aeruginosa. Biochemistry 2007, 46, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Greenhagen, B.T.; Shi, K.; Robinson, H.; Gamage, S.; Bera, A.K.; Ladner, J.E.; Parsons, J.F. Crystal structure of the pyocyanin biosynthetic protein PhzS. Biochemistry 2008, 47, 5281–5289. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kern, S.E.; Newman, D.K. Endogenous phenazine antibiotics promote anaerobic survival of Pseudomonas aeruginosa via extracellular electron transfer. J. Bacteriol. 2010, 192, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Winstanley, C.; Fothergill, J.L. The role of quorum sensing in chronic cystic fibrosis Pseudomonas aeruginosa infections. FEMS Microbiol. Lett. 2009, 290, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bushell, F.M.L.; Tonner, P.D.; Jabbari, S.; Schmid, A.K.; Lund, P.A. Synergistic Impacts of Organic Acids and pH on Growth of Pseudomonas aeruginosa: A Comparison of Parametric and Bayesian Non-parametric Methods to Model Growth. Front. Microbiol. 2019, 9, 3196. [Google Scholar] [CrossRef] [PubMed]




| Element | Mn | Mo | Si | Cr | Cu | Nb | C | V | Ti | Al | Ni | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X65 | 1.56 | 0.39 | 0.23 | 0.18 | 0.16 | 0.05 | 0.1 | 0.03 | 0.03 | 0.03 | 0.01 | Bal. |
| Duration/Day | Rs (Ω∙cm2) | Qf (Ω−1 cm−2 sn) | Rf (Ω∙cm2) | Qdl (Ω−1 cm−2 sn) | Rct (Ω∙cm2) |
|---|---|---|---|---|---|
| Sterile | |||||
| 1 | 6.7 ± 0.2 | 1.8 × 10−5 ± 3.9 × 10−6 | 1.8 × 103 ± 0.6 × 102 | 5.5 × 10−4 ± 1.3 × 10−5 | 7.5 × 103 ± 3.6 × 103 |
| 3 | 6.7 ± 0.3 | 4.2 × 10−5 ± 3.7 × 10−6 | 2.5 × 103 ± 0.4 × 102 | 4.5 × 10−4 ± 2.6 × 10−5 | 8.3 × 104 ± 1.0 × 102 |
| 7 | 6.4 ± 0.8 | 1.8 × 10−4 ± 5.1 × 10−5 | 2.8 × 103 ± 8.2 × 102 | 3.1 × 10−5 ± 2.8 × 10−6 | 7.1 × 104 ± 1.1 × 102 |
| 14 | 6.4 ± 0.6 | 1.9 × 10−4 ± 5.2 × 10−5 | 3.6 × 103 ± 5.3 × 102 | 2.9 × 10−5 ± 8.9 × 10−6 | 6.2 × 104 ± 8.3 × 102 |
| P. aeruginosa | |||||
| 1 | 5.9 ± 0.2 | 4.4 × 10−5 ± 1.2 × 10−5 | 1.8 × 103 ± 2.4 × 102 | 6.9 × 10−4 ± 1.6 × 10−5 | 3.1 × 102 ± 2.1 × 101 |
| 3 | 5.6 ± 0.4 | 7.4 × 10−5 ± 2.1 × 10−6 | 2.1 × 103 ± 1.3 × 102 | 8.7 × 10−4 ± 2.2 × 10−5 | 3.5 × 102 ± 8.3 × 100 |
| 7 | 6.0 ± 0.2 | 7.4 × 10−5 ± 8.1 × 10−6 | 2.2 × 103 ± 3.3 × 102 | 1.3 × 10−3 ± 9.3 × 10−6 | 1.9 × 102 ± 2.4 × 100 |
| 14 | 5.6 ± 0.2 | 1.2 × 10−4 ± 5.6 × 10−5 | 2.9 × 103 ± 2.0 × 102 | 1.6 × 10−3 ± 1.4 × 10−5 | 1.7 × 102 ± 8.9 × 100 |
| M+S | |||||
| 1 | 4.5 ± 3.9 | 2.8 × 10−1 ± 4.9 × 10−1 | 3.9 × 102 ± 3.4 × 102 | 3.3 × 10−1 ± 5.8 × 10−1 | 2.2 × 104 ± 1.4 × 103 |
| 3 | 6.5 ± 1.2 × 10−1 | 1.1 × 10−4 ± 2.1 × 10−6 | 1.2 × 103 ± 4.9 × 100 | 1.8 × 10−5 ± 9.3 × 10−8 | 3.5 × 104 ± 3.1 × 103 |
| 7 | 6.7 ± 1.5 × 10−1 | 1.2 × 10−4 ± 2.1 × 10−6 | 5.9 × 102 ± 5.7 × 100 | 1.6 × 10−5 ± 2.1 × 10−7 | 3.8 × 104 ± 4.3 × 103 |
| 14 | 6.4 ± 1.1 × 10−1 | 2.2 × 100 ± 3.8 × 100 | 1.8 × 102 ± 1.5 × 102 | 8.9 × 101 ± 1.6 × 102 | 3.8 × 104 ± 2.4 × 104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Z.; Guo, R.; Tang, J.; Zhang, X. Effect of Pseudomonas aeruginosa on Corrosion Behavior of X65 Carbon Steel. Materials 2024, 17, 2428. https://doi.org/10.3390/ma17102428
Shao Z, Guo R, Tang J, Zhang X. Effect of Pseudomonas aeruginosa on Corrosion Behavior of X65 Carbon Steel. Materials. 2024; 17(10):2428. https://doi.org/10.3390/ma17102428
Chicago/Turabian StyleShao, Zixuan, Ruiqi Guo, Jianhua Tang, and Xin Zhang. 2024. "Effect of Pseudomonas aeruginosa on Corrosion Behavior of X65 Carbon Steel" Materials 17, no. 10: 2428. https://doi.org/10.3390/ma17102428




