Effects of Different Grazing Treatments on the Root System of Stipa krylovii Steppe
Abstract
:1. Introduction
2. Materials and Methods
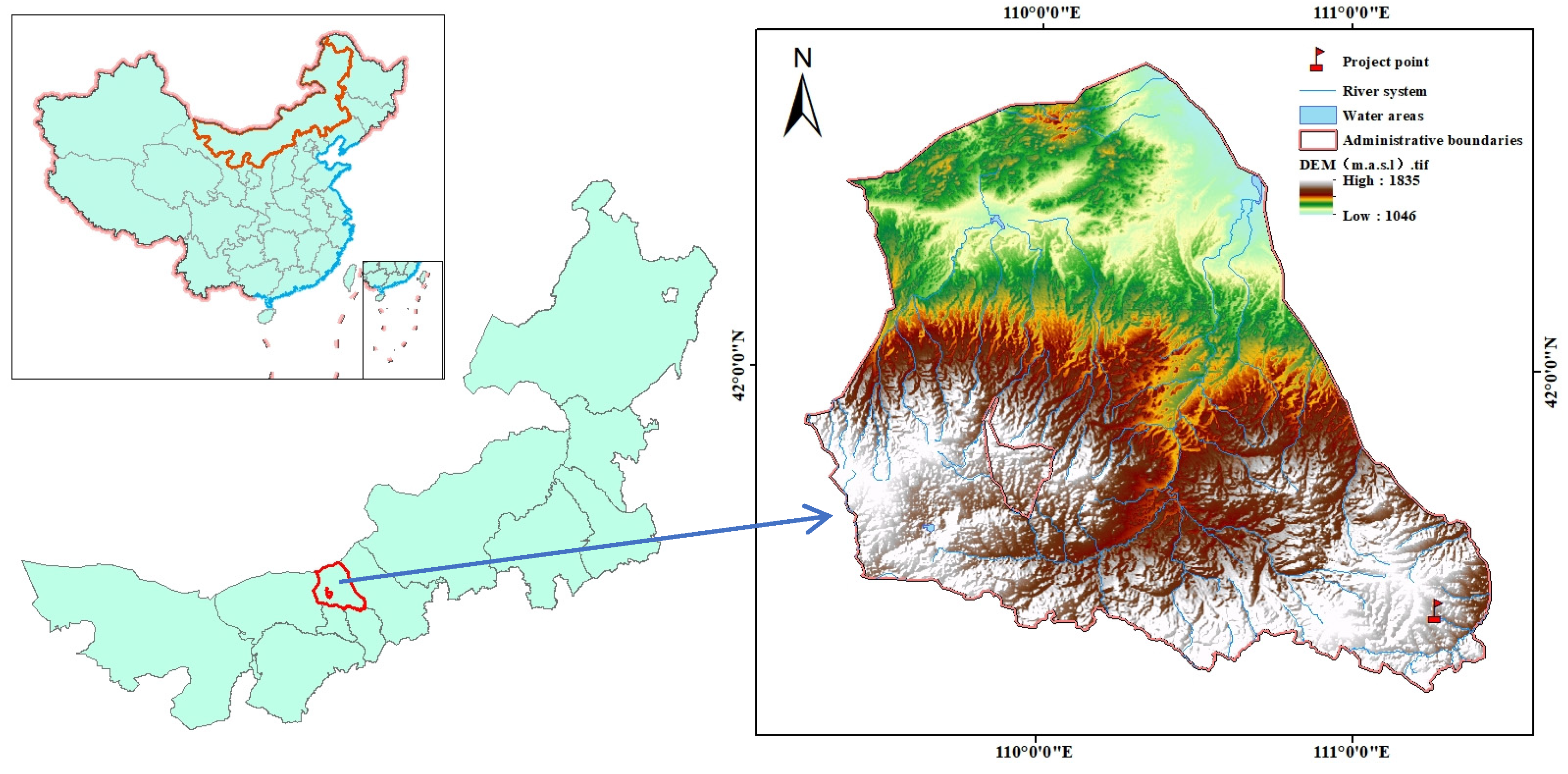
2.1. Study Area
2.2. Plot Settings
2.3. Sample Collection and Processing
2.4. Data Processing
3. Results
3.1. Differences in Root Biomass Density under Different Grazing Intensities
3.2. Differences in Root Distribution in Vertical Space under Different Grazing Intensities
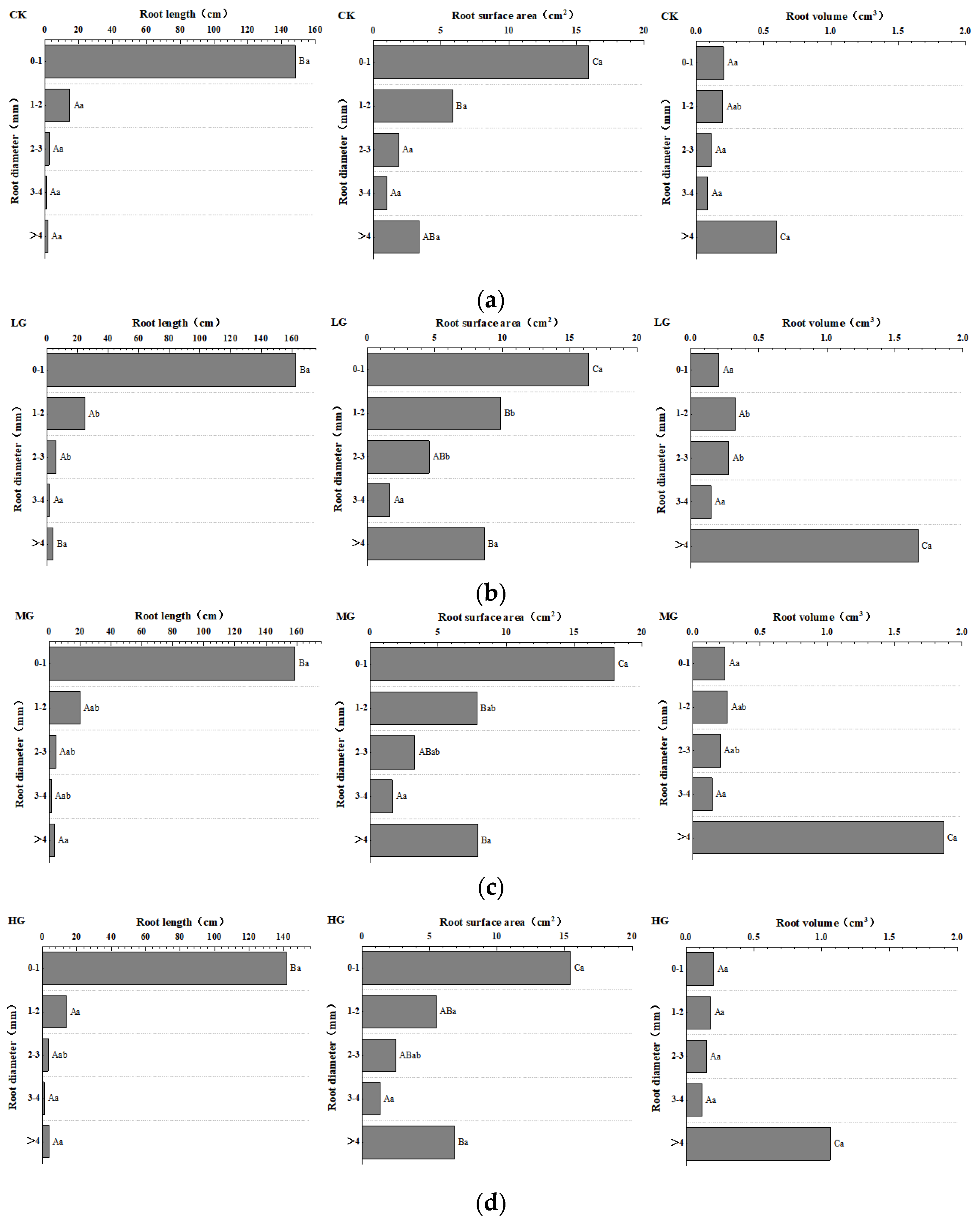
3.3. Differences in Diameter Class Distribution of Stipa krylovii under Different Grazing Intensities
3.4. Differences in Ecological Adaptability of Stipa krylovii under Different Grazing Intensities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bontti, E.E.; Decant, J.P.; Munson, S.M.; Gathany, M.A.; Przeszlowska, A.; Haddix, M.L.; Owens, S.; Burke, I.C.; Parton, W.J.; Harmon, M.E. Litter decomposition in grasslands of central north America (US great plains). Glob. Chang. Biol. 2009, 15, 1356–1363. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Jin, J.; Zhang, Y.N.; Liu, X.J.; Jin, Y.L.; Wang, C.J.; Han, G.D. Impacts of mixed-grazing on root biomass and belowground net primary production in a temperate desert steppe. R. Soc. Open Sci. 2019, 6, 180890. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Wei, Y.Q.; Liu, N.; Wang, Y.Q.; Manlike, A.; Zhang, Y.J.; Zhang, B. Mowing Facilitated Shoot and Root Litter Decomposition Compared with Grazing. Plants 2022, 11, 846. [Google Scholar] [CrossRef] [PubMed]
- Lodge, G.M.; Murphy, S.R. Root depth of native and sown perennial grass-based pastures, North-West Slopes, New South Wales. 1. Estimates from cores and effects of grazing treatments. Aust. J. Exp. Agric. 2006, 46, 337–345. [Google Scholar] [CrossRef]
- Golodets, C.; Kigel, J.; Sternberg, M. Recovery of plant species composition and ecosystem function after cessation of grazing in a Mediterranean grassland. Plant Soil 2010, 329, 365–378. [Google Scholar] [CrossRef]
- He, N.P.; Wu, L.; Wang, Y.S.; Han, X.G. Changes in carbon and nitrogen in soil particle-size fractions along a grassland restoration chronosequence in northern China. Geoderma 2009, 150, 302–308. [Google Scholar] [CrossRef]
- Shrestha, G.; Stahl, P.D. Carbon accumulation and storage in semi-arid sagebrush steppe: Effects of long-term grazing exclusion. Agric. Ecosyst. Environ. 2008, 125, 173–181. [Google Scholar] [CrossRef]
- Soares, C.V.; Cecato, U.; Ribeiro, O.L.; Roma, C.F.D.; Jobim, C.C.; Beloni, T.; Perri, S.H.V. Root system and root and stem base organic reserves of pasture Tanzania grass fertilizer with nitrogen under grazing. Semin. Cemina Agrar. 2013, 34, 2415–2426. [Google Scholar] [CrossRef]
- Wilson, C.H.; Strickland, M.S.; Hutchings, J.A.; Bianchi, T.S.; Flory, S.L. Grazing enhances belowground carbon allocation, microbial biomass, and soil carbon in a subtropical grassland. Glob. Chang. Biol. 2018, 24, 2997–3009. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro, G.; Paruelo, J.M.; Oesterheld, M.; Jobbágy, E.G. Pathways of Grazing Effects on Soil Organic Carbon and Nitrogen. Rangel. Ecol. Manag. 2010, 63, 109–119. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Wardle, D.A.; Yeates, G.W. Linking above-ground and belowground interactions: How plant responses to foliar herbivory influence soil organisms. Soil Biol. Biochem. 1998, 30, 1867–1878. [Google Scholar] [CrossRef]
- Milchunas, D.G.; Lauenroth, W.K. Quantitative effects of grazing on vegetation and soils over a global range of environments. Ecol. Monogr. 1993, 63, 327–366. [Google Scholar] [CrossRef]
- Hafner, S.; Unteregelsbacher, S.; Seeber, E.; Lena, B.; Xu, X.L.; Li, X.G.; Guggenberger, G.; Miehe, G.; Kuzyakov, Y. Effect of grazing on carbon stocks and assimilate partitioning in a Tibetan montane pasture revealed by 13CO2 pulse labeling. Glob. Change Biol. 2012, 18, 528–538. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; Lilley, J.M.; Hunt, J.R.; Sprague, S.J.; Ytting, N.K.; Rasmussen, I.S.; Graham, J.M. Effect of defoliation by grazing or shoot removal on the root growth of field-grown wheat (Triticum aestivum L.). Crop Pasture Sci. 2015, 66, 249–259. [Google Scholar] [CrossRef]
- McInenly, L.E.; Merrill, E.H.; Cahill, J.F.; Juma, N.G. Festuca campestris alters root morphology and growth in response to simulated grazing and nitrogen form. Funct. Ecol. 2010, 24, 283–292. [Google Scholar] [CrossRef]
- Ribeiro, R.H.; Ibarr, M.A.; Besen, M.R.; Bayer, C.; Piva, J.T. Managing grazing intensity to reduce the global warming potential in integrated crop–livestock systems under no-till agriculture. Eur. J. Soil. Sci. 2020, 71, 1120–1131. [Google Scholar] [CrossRef]
- Jackson, R.B.; Schenk, H.J.; Jobbágy, E.G.; Canadell, J.; Colello, G.D.; Dickinson, R.E.; Field, C.B.; Friedlingstein, P.; Heimann, M.; Hibbard, K.; et al. Belowground consequences of vegetation change and their treatment in models. Ecol. Appl. 2000, 10, 70–483. [Google Scholar] [CrossRef]
- Schenk, H.J.; Jackson, R.B. The global biogeography of roots. Ecol. Monogr. 2002, 72, 311–328. [Google Scholar] [CrossRef]
- Zhu, L.; Qin, F.; Yang, C.; Ma, X. Drive-Mechanism of Land Erosion in the Cross Redion between Farmland and Grassland in Vinshan Mountain. Res. Soil. Water Conserv. 2008, 15, 34–37. [Google Scholar]
- Xu, M. A review of grassland carrying capacity: Perspective and dilemma for research in China on “forage-livestock balance”. Acta Pratacult. Sin. 2014, 23, 321–329. [Google Scholar]
- Yang, Z.Q.; Miao, P.; Zheng, Y.F.; Guo, J.Y.; Li, Y.; Liu, T.; He, X.X. Impacts of Grazing on Vegetation and Soil Physicochemical Properties in Northern Yinshan Mountain Grasslands. Sustainability 2023, 15, 16028. [Google Scholar] [CrossRef]
- Fransen, B.; Blijjenberg, J.; de Kroon, H. Root morphological and physiological plasticity of perennial grass species and the exploitation of spatial and temporal heterogeneous nutrient patches. Plant Soil. 1999, 211, 179–189. [Google Scholar] [CrossRef]
- Zhang, T.R.; Li, F.Y.; Wu, L.; Wang, H.; Li, Y.L.; Shi, C.J. Seasonal grazing alters nutrient resorption and conservation, and affects spring growth of Stipa grandis. J. Plant Ecol. 2023, 16, rtac083. [Google Scholar] [CrossRef]
- Ren, W.; Xie, J.; Hou, X.; Li, X.; Guo, H.; Hu, N.; Kong, L.; Zhang, J.; Chang, C.; Wu, Z. Potential molecular mechanisms of overgrazing-induced dwarfism in sheepgrass (Leymus chinensis) analyzed using proteomic data. BMC Plant Biol. 2018, 18, 81. [Google Scholar] [CrossRef]
- Sarquis, A.; Pestoni, S.; Cingolani, A.M.; Harguindeguy, N.P. Physiognomic changes in response to herbivory increase carbon allocation to roots in a temperate grassland of central Argentina. Plant Ecol. 2019, 220, 699–709. [Google Scholar] [CrossRef]
- Du, B.H.; Gao, C.P.; Hadachaolu. Effect of Grazing on Plant Biomass and Carbon Storage of Typical Grassland in Xilinguole. J. Res. Soil. Water Conserv. 2018, 25, 139–146+152. [Google Scholar]
- Cui, S.; Zhu, X.; Wang, S.; Zhang, Z.; Xu, B.; Luo, C.; Zhao, L.; Zhao, X. Effects of seasonal grazing on soil respiration in alpine meadow on the Tibetan plateau. Soil. Use Manag. 2014, 30, 435–443. [Google Scholar] [CrossRef]
- Ribeiro, R.H.; Ibarr, M.A.; Bratti, F.; Pinheiro, D.; Auler, A.C.; Chiavegato, M.B.; Piva, J.T.; Dieckow, J. Grazing intensity and nitrogen fertilization effects on biomass and morphology of black oat roots in an integrated crop–livestock system. Agron. J. 2023, 115, 512–525. [Google Scholar] [CrossRef]
- Auler, A.C.; Galetto, S.L.; Hennipman, F.S.; Guntzel, E.D.; Giarola, N.F.; Fonseca, A.F.D. Soil structural quality degradation by the increase in grazing intensity in integrated crop-livestock system. Bragantia 2017, 76, 550–556. [Google Scholar] [CrossRef]
- Bonetti, J.D.A.; Anghinoni, I.; Gubiani, P.I.; Cecagno, D.; de Moraes, M.T. Impact of a long-term crop–livestock system on the physical and hydraulic properties of an Oxisol. Soil. Tillage Res. 2019, 186, 280–291. [Google Scholar] [CrossRef]
- Silva, F.D.D.; Amado, T.J.C.; Ferreira, A.O.; Assmann, J.M.; Anghinoni, I.; Carvalho, P.C.D.F. Soil carbon indices as affected by 10 years of integrated crop-livestock production with different pasture grazing intensities in southern Brazil. Agric. Ecosyst. Environ. 2014, 190, 60–69. [Google Scholar] [CrossRef]
- Ribeiro, R.H.; Dieckow, J.; Piva, J.T.; Bratti, F. Roots and aboveground carbon and nitrogen inputs by black oats (Avena strigosa Schreb.) as affected by grazing and nitrogen in integrated croplivestock system in subtropical Brazil. Plant Soil 2020, 451, 447–458. [Google Scholar] [CrossRef]
- Hoogsteen, M.J.J.; Bakker, E.J.; van Eekeren, N.; Tittonell, P.A.; Groot, J.C.J.; van Ittersum, M.K.; Lantinga, E.A. Do Grazing Systems and Species Composition Affect Root Biomass and Soil Organic Matter Dynamics in Temperate Grassland Swards? Sustainability 2020, 12, 1260. [Google Scholar] [CrossRef]
- Wu, G.; Wang, D.; Liu, Y.; Ding, L.-M.; Liu, Z.H. Warm-season grazing benefits species diversity conservation and topsoil nutrient sequestration in alpine meadow. Land. Degrad. Dev. 2016, 28, 1311–1319. [Google Scholar]
- Zhang, T.; Li, F.Y.; Wang, H.; Wu, L.; Shi, C.; Li, Y.; Hu, J. Effects of defoliation timing on plant nutrient resorption and hay production in a semi-arid steppe. J. Plant Ecol. 2021, 14, 44–57. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Houlton, B.Z.; Smith, W.K.; Marklein, A.R.; Reed, S.C.; Parton, W.; Del Grosso, S.J.; Running, S.W. Patterns of new versus recycled primary production in the terrestrial biosphere. Proc. Natl. Acad. Sci. USA 2013, 110, 12733–12737. [Google Scholar] [CrossRef]
- Zhou, L.; Addo-Danso, S.D.; Wu, P.; Li, S.; Zou, X.; Zhang, Y.; Ma, X. Leaf resorption efficiency in relation to foliar and soil nutrient concentrations and stoichiometry of Cunninghamia lanceolata with stand development in southern China. J. Soils Sediments 2016, 16, 1448–1459. [Google Scholar] [CrossRef]
- Kumar, S.; Udawatta, R.P.; Anderson, S.H. Root length density and carbon content of agroforestry and grass buffers under grazed pasture systems in a Hapludalf. Agrofor. Syst. 2010, 80, 85–96. [Google Scholar] [CrossRef]
- Li, T.L.; Huo, G.W.; Wu, Y.N. Comparison of root traits of Stipa krylovii and Allium polyrhizum under grazing in typical steppe. J. Chin. J. Appl. Ecol. 2022, 33, 360–368. [Google Scholar]
- Larreguy, C.; Carrera, A.L.; Bertiller, M.B. Production and turnover rates of shallow fine roots in rangelands of the Patagonian monte, Argentina. Ecol. Res. 2012, 27, 61–68. [Google Scholar] [CrossRef]
- Pucheta, E.; Bonamici, I.; Cabido, M.; Díaz, S. Below-ground biomass and productivity of a grazed site and a neighbouring ungrazed exclosure in a grassland in central Argentina. Austral Ecol. 2004, 29, 201–208. [Google Scholar] [CrossRef]
- Piazzetta, H.V.L.; Moraes, A.D.; Ribeiro, T.M.D.; Sandini, I.E.; Lustosa, S.B.C.; Pelissari, A. Pastejo e nitrogênio sobre o crescimento de raízes na mistura de aveia preta e azevém. Semin. Ciênc. Agrár. 2014, 35, 2749. [Google Scholar] [CrossRef]



| Vegetation | Root Length | Root Surface Area | Root Volume | Root Tips | Specific Root Length | |
|---|---|---|---|---|---|---|
| Stipa krylovii | Root surface area | 0.872 ** | ||||
| Root volume | 0.588 ** | 0.873 ** | ||||
| Root tips | 0.913 ** | 0.788 ** | 0.547 ** | |||
| Specific root length | 0.261 | 0.018 | −0.105 | 0.325 * | ||
| Specific root surface area | 0.221 | 0.191 | 0.179 | −0.280 | 0.886 ** |
| Vegetation | Principal Component | Total | Variability (%) | Cumulative (%) |
|---|---|---|---|---|
| Stipa krylovii | 1 | 3.462 | 57.70 | 57.70 |
| 2 | 1.798 | 29.97 | 87.67 |
| Vegetation | Principal Component | Root Length | Root Surface Area | Root Volume | Root Tips | Specific Root Length | Specific Root Surface Area |
|---|---|---|---|---|---|---|---|
| Stipa krylovii | 1 | 0.498 | 0.499 | 0.414 | 0.489 | 0.189 | 0.237 |
| 2 | −0.078 | −0.236 | −0.276 | −0.013 | 0.690 | 0.621 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, T.; Guo, J.; Yang, Z.; Yao, Z.; Liu, X.; Wang, Z. Effects of Different Grazing Treatments on the Root System of Stipa krylovii Steppe. Sustainability 2024, 16, 3975. https://doi.org/10.3390/su16103975
Tian T, Guo J, Yang Z, Yao Z, Liu X, Wang Z. Effects of Different Grazing Treatments on the Root System of Stipa krylovii Steppe. Sustainability. 2024; 16(10):3975. https://doi.org/10.3390/su16103975
Chicago/Turabian StyleTian, Tian, Jianying Guo, Zhenqi Yang, Zhenyu Yao, Xinyu Liu, and Ziwei Wang. 2024. "Effects of Different Grazing Treatments on the Root System of Stipa krylovii Steppe" Sustainability 16, no. 10: 3975. https://doi.org/10.3390/su16103975





