Carbon Mineralization Dynamics of Switchgrass (Panicum virgatum) Biochar in a Northern Florida Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biochar Production and Characterization
2.2. Soil Characterization and the Biochar-Amended Soil Incubation
2.3. CO2 Sample Collection and 13CO2 Analysis
2.4. Experimental Design and Statistics
3. Results
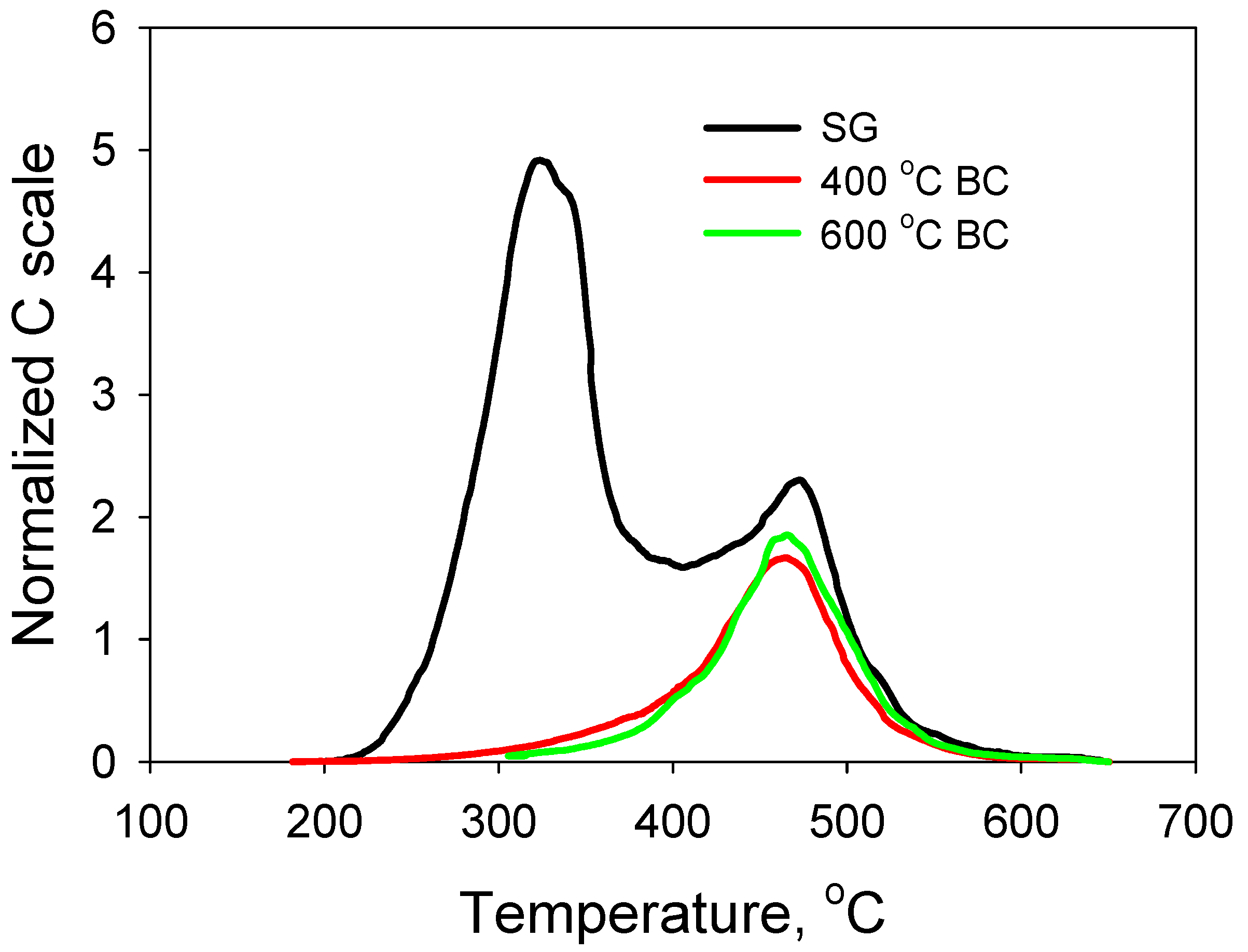
3.1. Characterization of SG Feedstock and Its Biochars by MESTA
3.2. Carbon Mineralization Rates of the 400 °C Biochar in Soil
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laird, D. The Charcoal Vision: A Win–Win–Win scenario for simultaneously producing bioenergy, permanently sequestering carbon, while improving soil and water quality. Agron. J. 2008, 100, 178–181. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Amonette, J.E.; Joseph, S. Characteristics of biochar: Microchemical properties. In Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S., Eds.; Routledge Taylor & Francis Group: New York, NY, USA, 2012; pp. 65–84. [Google Scholar]
- Rajkovich, S.; Enders, A.; Hanley, K.; Hyland, C.; Zimmerman, A.R.; Lehmann, J. Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biol. Fertil. Soils 2011, 48, 271–284. [Google Scholar] [CrossRef]
- Gaskin, J.W.; Steiner, C.; Harris, K.; Das, K.C.; Bibens, B. Effects of low-temperature pyrolysis conditions on biochar for agricultural use. Am. Soc. Agric. Biol. Eng. 2008, 51, 2061–2069. [Google Scholar]
- Hilscher, A.; Heister, K.; Siewert, C.; Knicker, H. Mineralization and structural changes during the initial phase of microbial degradation of pyrogenic plant residues in soil. Org. Geochem. 2009, 40, 332–342. [Google Scholar] [CrossRef]
- Novak, J.M.; Lima, I.; Xing, B.; Gaskin, J.W.; Steiner, C.; Sas, K.C.; Ahmedna, M.; Rehrah, D.; Watts, D.W.; Busscher, W.J.; et al. Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. Ann. Environ. Sci. 2009, 3, 195–206. [Google Scholar]
- Novak, J.M.; Busscher, W.J.; Laird, D.L.; Ahmedna, M.; Watts, D.W.; Niandou, M.A. Impact of biochar amendment of fertility of a southeastern coastal plain soil. Soil Sci. 2009, 174, 105–112. [Google Scholar] [CrossRef]
- Karhu, K.; Mattila, T.; Bergström, I.; Regina, K. Biochar addition to agricultural soil increased CH4 uptake and water holding capacity—Results from a short-term pilot field study. Agric. Ecosyst. Environ. 2011, 140, 309–313. [Google Scholar] [CrossRef]
- Cheng, C.; Lehmann, J.; Thies, J.E.; Burton, S.D.; Engelhard, M.H. Oxidation of black carbon by biotic and abiotic processes. Org. Geochem. 2006, 37, 1477–1488. [Google Scholar] [CrossRef]
- Yuan, J.H.; Xu, R.K. The amelioration effects of low temperature biochar generated from nine crop residues on an acidic Ultisol. Soil Use Manag. 2011, 27, 110–115. [Google Scholar] [CrossRef]
- Jones, D.L.; Rousk, J.; Edwards-Jones, G.; DeLuca, T.H.; Murphy, D.V. Biochar-mediated changes in soil quality and plant growth in a three year field trial. Soil Biol. Biochem. 2012, 45, 113–124. [Google Scholar] [CrossRef]
- Gaskin, J.W.; Speir, R.A.; Harris, K.; Das, K.C.; Lee, R.D.; Morris, A.; Fisher, D.S. Effects of peanut hull and pine chip biochar on soil nutrients, corn nutrient status, and yield. Agron. J. 2010, 102, 623–633. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.Y.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- Thies, J.E.; Rillig, M.C. Characteristics of biochar: Biological properties. In Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S., Eds.; Routledge Taylor & Francis Group: New York, NY, USA, 2012; pp. 117–138. [Google Scholar]
- Singh, B.P.; Fang, Y.; Boersma, M.; Collins, D.; Van Zwieten, L.; Macdonald, L.M. In situ persistence and migration of biochar carbon and its impact on native carbon emission in contrasting soils under managed temperate pastures. PLoS ONE 2015, 10, e0141560. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiong, Z.; Kuzyakov, Y. Biochar stability in soil: Meta-analysis of decomposition and priming effects. GCB Bioenergy 2016, 8, 512–523. [Google Scholar] [CrossRef]
- Rasse, D.P.; Budai, A.; O’Toole, A.; Ma, X.; Rumpel, C.; Abiven, S. Persistence in soil of Miscanthus biochar in laboratory and field conditions. PLoS ONE 2017, 12, e0184383. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Xu, X.; Wei, L.; Fan, L.; Huang, H.; Li, J.; Lu, Q.; Li, J.; Zhou, W. Biochar stability assessment by incubation and modelling: Methods, drawbacks and recommendations. Sci. Total Environ. 2019, 664, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Jindo, K.; Sonoki, T. Comparative assessment of biochar stability using multiple indicators. Agronomy 2019, 9, 254. [Google Scholar] [CrossRef]
- Spokas, K.A. Review of the stability of biochar in soils: Predictability of O:C molar ratios. Carbon Manag. 2010, 1, 289–303. [Google Scholar] [CrossRef]
- Luo, Y.; Durenkamp, M.; De Nobili, M.; Lin, Q.; Brookes, P.C. Short-term soil priming effects and the mineralization of biochar following its incorporation to soils of different pH. Soil Biol. Biochem. 2011, 43, 2304–2314. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Gao, B.; Ahn, M.Y. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Cross, A.; Sohi, S.P. The priming potential of biochar products in relation to labile carbon contents and soil organic matter status. Soil Biol. Biochem. 2011, 43, 2127–2134. [Google Scholar] [CrossRef]
- Li, S.; Chen, G. Thermogravimetric, thermochemical, and infrared spectral characterization of feedstocks and biochar derived at different pyrolysis temperatures. Waste Manag. 2018, 78, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Ngatia, L.W.; Hsieh, Y.P.; Nemours, D.; Fu, R.; Taylor, R.W. Potential phosphorus eutrophication mitigation strategy: Biochar carbon composition, thermal stability, and pH influence phosphorus sorption. Chemosphere 2017, 180, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Bakar, M.S.A.; Hamdani, R.; Park, Y.-K.; Lam, S.S.; Sukri, R.S.; Hussain, M.; Majeed, K.; Phusunti, N.; Jamil, F. Valorization of underutilized waste biomass from invasive species to produce biochar for energy and other value-added applications. Environ. Res. 2020, 186, 109596. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Duan, X.; Wang, S.; Ren, N.-Q.; Ho, S.-H. Production, properties, and catalytic applications of sludge derived biochar for environmental remediation. Water Res. 2020, 187, 116390. [Google Scholar]
- Dai, Z.; Xiong, X.; Zhu, H.; Xu, H.; Leng, P.; Li, J.; Tang, C.; Xu, J. Association of biochar properties with changes in soil bacterial, fungal and fauna communities and nutrient cycling processes. Biochar 2021, 3, 239–254. [Google Scholar] [CrossRef]
- Han, L.; Ro, K.S.; Wang, Y.; Sun, K.; Sun, H.; Libra, J.A.; Xing, B. Oxidation resistance of biochars as a function of feedstock and pyrolysis condition. Sci. Total Environ. 2018, 616, 335–344. [Google Scholar] [CrossRef]
- Xu, Z.; He, M.; Xu, X.; Cao, X.; Tsang, D.C. Impacts of different activation processes on the carbon stability of biochar for oxidation resistance. Bioresour. Technol. 2021, 338, 125555. [Google Scholar] [CrossRef]
- Li, S.; Tasnady, D. Biochar for soil carbon sequestration: Current knowledge, mechanisms, and future perspectives. C 2023, 9, 67. [Google Scholar] [CrossRef]
- Hsieh, Y.P. A novel multi-elemental scanning thermal analysis (MESTA) for the identification and characterization of solid substances. J. AOAC Int. 2007, 90, 54–59. [Google Scholar] [PubMed]
- Natural Resources Conservation Service (NRCS). Soil Survey of Gadsden County; Natural Resources Conservation Service (NRCS): Washington, DC, USA, 2009. Available online: http://soils.usda.gov/survey/printed_surveys/ (accessed on 1 October 2009).
- Merritt, D.A.; Hayes, J.M.; Des Marais, D.J. Carbon isotopic analysis of atmospheric methane by isotope-ratio-monitoring gas-chromatography mass-spectrometry. J. Geophys. Res. 1995, 100, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.E.; Chanton, J.P.; Tfaily, M.M.; Ogram, A. CO2 and CH4 isotope compositions and production pathways in a tropical peatland. Glob. Biogeochem. Cycles 2015, 29, 1–18. [Google Scholar] [CrossRef]
- Minitab 16 Statistical Software; Minitab, Inc.: State College, PA, USA, 2010. Available online: www.minitab.com (accessed on 15 May 2010).
- Microsoft Office Software—Excel; Microsoft: Redmond, WA, USA, 2010.
- Hsieh, Y.P.; Bugna, G.C. Analysis of black carbon in sediments and soils using multi-element scanning thermal analysis (MESTA). Org. Geochem. 2008, 39, 1562–1571. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Al-Omran, A.; El-Naggar, A.H.; Nadeem, M.; Usman, A.R.A. Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresour. Technol. 2013, 131, 374–379. [Google Scholar] [CrossRef]
- Takaya, C.A.; Fletcher, L.A.; Singh, S.; Anyikude, K.U.; Ross, A.B. Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes. Chemosphere 2016, 145, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, A.A.; Ahmad, M.; Rafique, M.I.; Al-Wabel, M.I. Variations in composition and stability of biochars derived from different feedstock types at varying pyrolysis temperature. J. Saudi Soc. Agric. Sci. 2023, 22, 25–34. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. Biochar application to soil for climate change mitigation by soil organic carbon sequestration. J. Plant Nutr. Soil Sci. 2014, 177, 651–670. [Google Scholar] [CrossRef]
- Jatav, H.S.; Rajput, V.D.; Minkina, T.; Singh, S.K.; Chejara, S.; Gorovtsov, A.; Barakhov, A.; Bauer, T.; Sushkova, S.; Mandzhieva, S. Sustainable approach and safe use of biochar and its possible consequences. Sustainability 2021, 13, 10362. [Google Scholar] [CrossRef]
- Hsieh, Y.P. Dynamics of soil organic matter formation in croplands—Conceptual analysis. Sci. Total Environ. 1989, 81/82, 381–390. [Google Scholar] [CrossRef]


| C, % | N, % | H, % | LOI, % | O, % | H/C | O/C | |
|---|---|---|---|---|---|---|---|
| SG | 58.8 ± 0.9 | 0.94 ± 0.10 | 6.52 ± 1.15 | 93.5 ± 0.1 | 27.7 ± 1.2 | 1.27 | 0.35 |
| 400 °C char | 68.2 ± 0.7 | 0.98 ± 0.01 | 1.50 ± 0.14 | 78.7 ± 0.2 | 8.2 ± 0.8 | 0.26 | 0.08 |
| 600 °C char | 70.3 ± 0.5 | 0.92 ± 0.02 | 1.25 ± 0.17 | 78.3 ± 0.2 | 4.9 ± 0.6 | 0.21 | 0.06 |
| Treatment | C mineralization Rate | Estimated MRT (Years) | n |
|---|---|---|---|
| 1% biochar-amended soil | 6.3 ± 2.6 µg C/g BC/d | 435 ± 184 | 4 |
| 3% biochar-amended soil | 9.5 ± 3.0 µg C/g BC/d | 288 ± 96 | 10 |
| Combined biochar-amended soil | 7.9 ± 2.7 µg C/g BC/d * | 347 ± 131 | 14 |
| Control (native SOC) | 42.8 ± 16.4 µg C/g SOC/d | 64 ± 10 | 36 |
| Switchgrass-amended soil | 663.8 ± 138 µg C/g SG-C/d | 4 ± 1 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, Y.-P.; Hatakka, K. Carbon Mineralization Dynamics of Switchgrass (Panicum virgatum) Biochar in a Northern Florida Soil. Sustainability 2024, 16, 4060. https://doi.org/10.3390/su16104060
Hsieh Y-P, Hatakka K. Carbon Mineralization Dynamics of Switchgrass (Panicum virgatum) Biochar in a Northern Florida Soil. Sustainability. 2024; 16(10):4060. https://doi.org/10.3390/su16104060
Chicago/Turabian StyleHsieh, Yuch-Ping, and Kristina Hatakka. 2024. "Carbon Mineralization Dynamics of Switchgrass (Panicum virgatum) Biochar in a Northern Florida Soil" Sustainability 16, no. 10: 4060. https://doi.org/10.3390/su16104060





