Use of Natural Zeolite Clinoptilolite in the Preparation of Photocatalysts and Its Role in Photocatalytic Activity
Abstract
:1. Introduction
2. Structural Features of Clinoptilolite
2.1. Photocatalytic Activity of Semiconductor Oxides
2.1.1. TiO2
2.1.2. ZnO
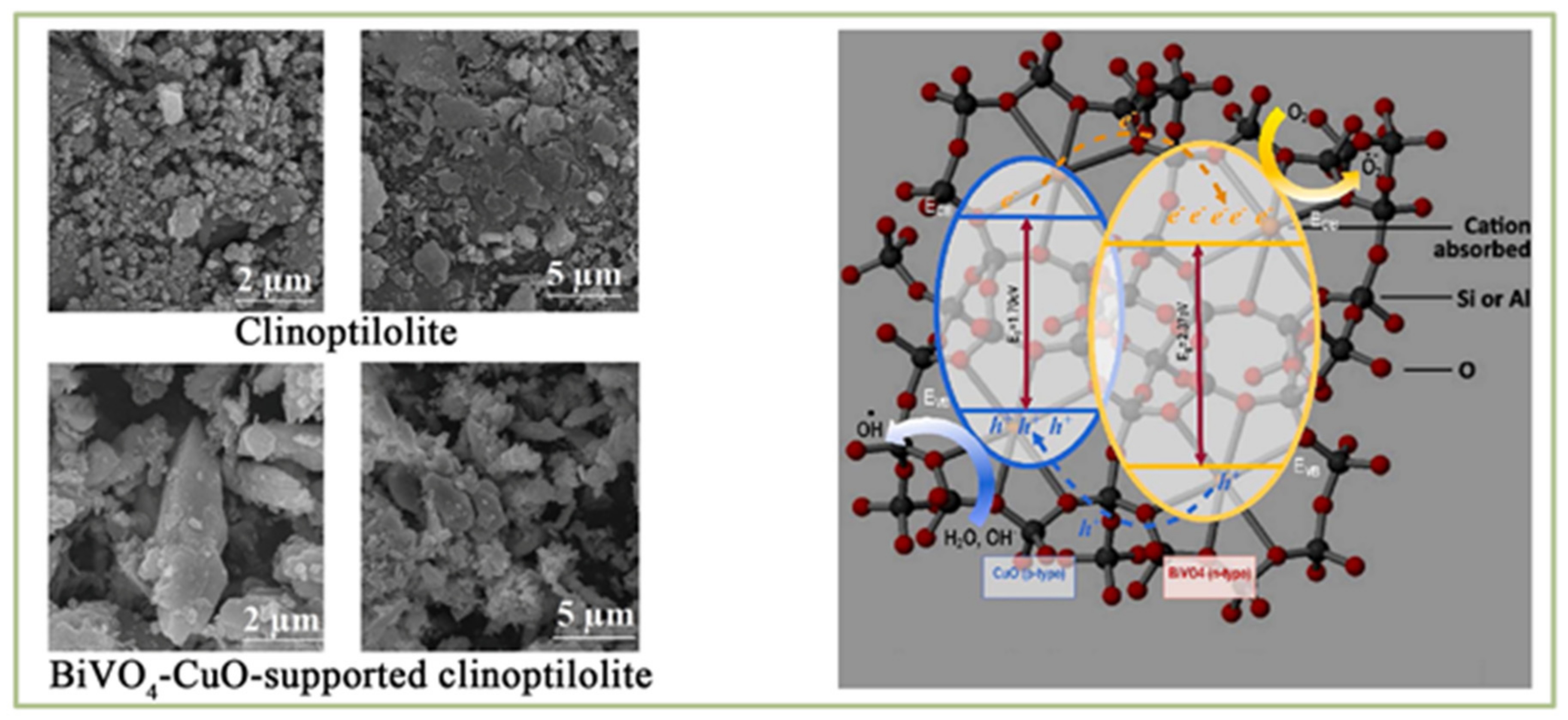
2.1.3. CuO
2.1.4. SnO2
2.1.5. NiO
2.2. Other Semiconductors
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mamulová Kutláková, K.; Tokarský, J.; Kovář, P.; Vojtěšková, S.; Kovářová, A.; Smetana, B.; Kukutschová, J.; Čapková, P.; Matějka, V. Preparation and characterization of photoactive composite kaolinite/TiO2. J. Hazard. Mater. 2011, 188, 212–220. [Google Scholar] [CrossRef]
- Castañeda-Contreras, J.; Marañón-Ruiz, V.F.; Chiu-Zárate, R.; Pérez-Ladrón de Guevara, H.; Rodriguez, R.; Michel-Uribe, C. Photocatalytic activity of erbium-doped TiO2 nanoparticles immobilized in macro-porous silica films. Mater. Res. Bull. 2012, 47, 290–295. [Google Scholar] [CrossRef]
- Wang, C.; Shi, H.; Li, Y. Synthesis and characterization of natural zeolite supported Cr-doped TiO2 photocatalysts. Appl. Surf. Sci. 2012, 258, 4328–4333. [Google Scholar] [CrossRef]
- Guimarães, V.; Teixeira, A.R.; Lucas, M.S.; Silva, A.M.T.; Peres, J.A. Pillared interlayered natural clays as heterogeneous photocatalysts for H2O2-assisted treatment of a winery wastewater. Sep. Purif. Technol. 2019, 228, 115768. [Google Scholar] [CrossRef]
- Mekatel, E.; Trari, M.; Nibou, D.; Sebai, I.; Amorkrane, S. Preparation and characterization of α-Fe2O3 supported clay as a novel photocatalyst for hydrogen evolution. Int. J. Hydrogen Energy 2019, 44, 10309–10315. [Google Scholar] [CrossRef]
- Alakhras, F.; Alhajri, E.; Haounati, R.; Ouachtak, H.; Ait Addi, A.; Saleh, T.A. A comparative study of photocatalytic degradation of Rhodamine B using natural-based zeolite composites. Surf. Interfaces 2020, 20, 100611. [Google Scholar] [CrossRef]
- Hass Caetano Lacerda, E.; Casanova Monteiro, F.; Regina Kloss, J.; Fujiwara, S.T. Bentonite clay modified with Nb2O5: An efficient and reused photocatalyst for the degradation of reactive textile dye. J. Photochem. Photobiol. A Chem. 2020, 388, 112084. [Google Scholar] [CrossRef]
- Khennaouia, B.; Zehani, F.; Malouki, M.; Menacerd, R.; Canle, M. Chemical and physical characterization of a natural clay and its use as photocatalyst for the degradation of the methabenzthiazuron herbicide in water. Optik 2020, 219, 165024. [Google Scholar] [CrossRef]
- Fatimah, I.; Ardianti, S.; Sahroni, I.; Purwiandono, G.; Sagadevan, S.; Doong, R.A. Visible light sensitized porous clay heterostructure photocatalyst of zinc-silica modified montmorillonite by using tris (2,2′-bipyridyl) dichlororuthenium. Appl. Clay Sci. 2021, 204, 106023. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P.; Li, X. A review on heterogeneous photocatalysis for environmental remediation: From semiconductors to modification strategies. Chin. J. Catal. 2022, 43, 178–214. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibánez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, D. Photocatalysis: Basic Principles, Diverse Forms of Implementations and Emerging Scientific Opportunities. Adv. Energy Mater. 2017, 7, 17100841. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D. Photocatalysis: From fundamental principles to materials and applications. ACS Appl. Energy Mater. 2018, 1, 6657–6693. [Google Scholar] [CrossRef]
- Loeb, S.K.; Alvarez, P.J.J.; Brame, J.A.; Cates, E.L.; Choi, W.; Crittenden, J.; Dionysiou, D.D.; Li, Q.; Li-Puma, G.; Quan, X.; et al. The technology horizon for photocatalytic water treatment: Sunrise or Sunset? Environ. Sci. Technol. 2019, 53, 2937–2947. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent Advances of Photocatalytic Application in Water Treatment: A Review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef] [PubMed]
- Mohod, A.V.; Imran, M.; Momotzko, M.; Shah, N.S.; Marchel, M.; Imram, M.; Kong, L.; Boczkaj, G. Degradation of Rhodamine dyes by Advanced Oxidation Processes (AOPs)—Focus on cavitation and photocatalysis—A critical review. Water Resour. Ind. 2023, 30, 100220. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Fang, W.; Yi, Q.; Zhang, J. A comprehensive review on reactive oxygen species (ROS) in advanced oxidation processes (AOPs). Chemosphere 2022, 308, 136205. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El-Nemr, A.; Elkatory, M.R.; Ragab, S.; Niculescu, V.C.; El Nemr, A. Principles of photocatalysts and their different applications: A Review. Top Curr. Chem. 2023, 381, 31. [Google Scholar] [CrossRef]
- Mahbub, P.; Duke, M. Scalability of advanced oxidation processes (AOPs) in industrial applications: A review. J. Environ. Manag. 2023, 345, 118861. [Google Scholar] [CrossRef]
- Friedmann, R. A General overview of heterogeneous photocatalysis as a remediation technology for wastewaters containing pharmaceutical compounds. Water 2022, 14, 3588. [Google Scholar] [CrossRef]
- Huang, D.; Chen, S.; Zeng, G.; Gong, X.; Zhou, C.; Cheng, M.; Xue, W.; Yan, X.; Li, J. Artificial Z-scheme photocatalytic system: What have been done and where to go? Coordin. Chem. Rev. 2019, 385, 44–80. [Google Scholar] [CrossRef]
- Tahir, M.; Tasleem, S.; Tahir, B. Recent development in band engineering of binary semiconductor materials for solar driven photocatalytic hydrogen production. Int. J. Hydrogen Energy 2020, 45, 15985–16038. [Google Scholar] [CrossRef]
- Yin, S.; Sun, L.; Zhou, Y.; Li, X.; Li, J.; Song, X.; Huo, P.; Wang, H.; Yan, Y. Enhanced electron–hole separation in SnS2/Au/g-C3N4 embedded structure for efficient CO2 photoreduction. Chem. Eng. J. 2021, 406, 126776. [Google Scholar] [CrossRef]
- Brame, J.; Long, M.; Li, Q.; Alvarez, P. Inhibitory effect of natural organic matter or other background constituents on photocatalytic advanced oxidation processes: Mechanistic model development and validation. Water Res. 2015, 84, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, R.; Mohsemi, M. Impact of natural organic matter on the degradation of 2,4-dichlorophenoxy acetic acid in a fluidized bed photocatalytic reactor. Chem. Eng. J. 2017, 310, 457–463. [Google Scholar] [CrossRef]
- Lado Ribeira, A.R.; Moreiraa, N.F.F.; Li Puma, G.; Silva, A.M.T. Impact of water matrix on the removal of micropollutants by advanced oxidation technologies. Chem. Eng. J. 2019, 363, 155–173. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H. Zeolite-based photocatalysts. Chem. Commun. 2004, 13, 1443–1459. [Google Scholar]
- Sandoval-Díaz, L.-E.; González-Amaya, J.-A.; Carlos-Alexander Trujillo, C.-A. General aspects of zeolite acidity characterization. Micropor. Mesopor. Mater. 2015, 215, 229–243. [Google Scholar] [CrossRef]
- Reeve, P.J.; Fallowfield, H.J. Natural and surfactant modified zeolites: A review of their applications for water remediation with a focus on surfactant desorption and toxicity towards microorganisms. J. Environ. Manag. 2018, 205, 253–261. [Google Scholar] [CrossRef]
- Hu, G.; Yang, J.; Duan, X.; Farnood, R.; Yang, C.; Yang, J.; Liu, W.; Liu, Q. Recent developments and challenges in zeolite-based composite photocatalysts for environmental applications. Chem. Eng. J. 2021, 417, 129209. [Google Scholar] [CrossRef]
- Liaquat, I.; Munir, R.; Fazila Younas, F.; Abbasi, N.A.; Sadia, B.; Muneer, A.; Younas, F.; Sardar, M.F.; Zahid, M.; Noreen, S. Exploring zeolite-based composites in adsorption and photocatalysis for toxic wastewater treatment: Preparation, mechanisms, and future perspectives. Environ. Pollut. 2024, 349, 123922. [Google Scholar] [CrossRef] [PubMed]
- Godelitsas, A.; Armbruster, T. HEU-type zeolites modified by transition elements and lead. Micropor. Mesopor. Mater. 2003, 61, 3–24. [Google Scholar] [CrossRef]
- Dziedzicka, A.; Sulikowski, B.; Ruggiero-Mikolajczyk, M. Catalytic and physicochemical properties of modified natural clinoptilolite. Catal. Today 2016, 259, 50–58. [Google Scholar] [CrossRef]
- Johnson, M.; O’Connor, D.; Barnes, P.; Bell, R.; Catlow, C.R.A.; Owens, S.L.; Sankar, G.; Teat, S.J.; Stephenson, R. Cation exchange, dehydration and calcination in clinoptilolite: In situ X-ray diffraction and computer modeling. J. Phys. Chem. B 2003, 107, 942–951. [Google Scholar] [CrossRef]
- Snellings, R.A.; Gualtieri, A.F.; Elsen, J. The Rietveld structure refinement of an exceptionally pure sample of clinoptilolite from Ecuador and its Na-, K-, and Ca-exchanged forms. In Eleventh European Powder Diffraction Conference. Z. Kristallogr. Suppl. 2009, 30, 395–400. [Google Scholar] [CrossRef]
- Farías, T.; Ruiz-Salvador, A.R.; Velazco, L.; de Ménorval, L.C.; Rivera, A. Preparation of natural zeolitic supports for potential biomedical applications. Mater. Chem. Phys. 2009, 118, 322–328. [Google Scholar] [CrossRef]
- Garcia-Basabe, Y.; Rodriguez-Iznaga, I.; de Menorval, L.-C.; Llewellyn, P.; Maurin, G.; Lewis, D.W.; Binions, R.; Autie, M.; Ruiz-Salvador, A.R. Step-wise dealumination of natural clinoptilolite: Structural and physicochemical characterization. Micropor. Mesopor. Mater. 2010, 135, 187–196. [Google Scholar] [CrossRef]
- Pavlovic, J.; Popova, M.; Mihalyi, R.M.; Mazaj, M.; Mali, G.; Kovač, J.; Lazarova, H.; Rajic, N. Catalytic activity of SnO2- and SO4/SnO2-containing clinoptilolite in the esterification of levulinic acid. Micropor. Mesopor. Mater. 2019, 279, 10–18. [Google Scholar] [CrossRef]
- Lin, H.; Liu, Q.; Dong, Y.; He, Y.; Wang, L. Physicochemical properties and mechanism study of clinoptilolite modified by NaOH. Micropor. Mesopor. Mater. 2015, 218, 174–179. [Google Scholar] [CrossRef]
- De Souza, V.C.; Villarroel-Rocha, J.; De Araújo, M.J.G.; Sapag, K.; Pergher, S.B.C. Basic treatment in natural clinoptilolite for improvement of physicochemical properties. Minerals 2018, 8, 595. [Google Scholar] [CrossRef]
- Ates, A. Effect of alkali-treatment on the characteristics of natural zeolites with different compositions. J. Colloid Interf. Sci. 2018, 523, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Leng, S.; Guo, H.; Cao, L.; Huang, J. Acid and alkaline treatments for regulation of hydrophilicity/hydrophobicity of natural zeolite. Appl. Surf. Sci. 2019, 478, 319–326. [Google Scholar] [CrossRef]
- Pavlović, J.; Hrenović, J.; Povrenović, D.; Rajić, N. Advances in the applications of clinoptilolite-rich tuffs. Materials 2024, 17, 1306. [Google Scholar] [CrossRef]
- Rajic, N.; Stojakovic, D.; Daneu, N.; Recnik, A. The formation of oxide nanoparticles on the surface of natural clinoptilolite. J. Phys. Chem. Solids 2011, 72, 800–803. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Moazzeni, N. Sunlight photodecolorization of a mixture of Methyl Orange and Bromocresol Green by CuS incorporated in a clinoptilolite zeolite as a heterogeneous catalyst. J. Ind. Eng. Chem. 2013, 19, 1433–1442. [Google Scholar] [CrossRef]
- Jafari, S.; Nezamzadeh-Ejhieh, A. Supporting of coupled silver halides onto clinoptilolite nanoparticles as simple method for increasing their photocatalytic activity in heterogeneous photodegradation of mixture of 4-methoxy aniline and 4-chloro-3-nitro aniline. J. Colloid Interface Sci. 2017, 490, 478–487. [Google Scholar] [CrossRef]
- Gharbalifard, M.; Nezamzadeh-Ejhieh, A. Synergistic photocatalytic activity of PbS/clinoptilolite in ciprofloxacin photodegradation: An experimental design study. J. Photochem. Photobiol. A 2024, 446, 115159. [Google Scholar] [CrossRef]
- Heidari, Z.; Alizadeh, R.; Ebadi, A.; Oturan, N.; Oturan, M.A. Efficient photocatalytic degradation of furosemide by a novel sonoprecipited ZnO over ion exchanged clinoptilolite nanorods. Sep. Purif. Technol. 2020, 242, 116800. [Google Scholar] [CrossRef]
- Shen, Y.; Zhou, P.; Zhao, S.; Li, A.; Chen, Y.; Bai, J.; Han, C.; Wei, D.; Ao, Y. Synthesis of high-efficient TiO2/clinoptilolite photocatalyst for complete degradation of xanthate. Miner. Eng. 2020, 159, 106640. [Google Scholar] [CrossRef]
- Ullah, R.; Liu, C.; Panezai, H.; Gul, A.; Sun, J.; Wu, X. Controlled crystal phase and particle size of loaded-TiO2 using clinoptiloliteas support via hydrothermal method for degradation of crystal violet dye in aqueous solution. Arab. J. Chem. 2020, 13, 4092–4101. [Google Scholar] [CrossRef]
- Ullah, R.; Sun, J.; Gul, A.; Munir, T.; Wu, X. Evaluations of physico-chemical properties of TiO2/clinoptilolite synthesized via three methods on photocatalytic degradation of crystal violet. Chin. J. Chem. Eng. 2021, 33, 181–189. [Google Scholar] [CrossRef]
- Karunakaran, C.; Senthilvelan, S.; Karuthapandian, S. TiO2—Photocatalyzed oxidation of aniline. J. Photochem. Photobiol. A 2005, 172, 207–213. [Google Scholar] [CrossRef]
- Chen, K.; Li, J.; Li, J.; Zhang, Y.; Wang, W. Synthesis and characterization of TiO2–montmorillonites doped with vanadium and/or carbon and their application for the photodegradation of sulphorhodamine B under UV–vis irradiation. Colloid Surf. A 2010, 360, 47–56. [Google Scholar] [CrossRef]
- Kang, X.; Liu, S.; Dai, Z.; He, Y.; Song, X.; Tan, Z. Titanium Dioxide: From Engineering to Applications. Catalysts 2019, 9, 191. [Google Scholar] [CrossRef]
- Liang, Y.; Huang, G.; Xin, X.; Yao, Y.; Li, Y.; Yin, J.; Li, X.; Wu, Y.; Gao, S. Black titanium dioxide nanomaterials for photocatalytic removal of pollutants: A review. J. Mater. Sci. Technol. 2022, 112, 239–262. [Google Scholar] [CrossRef]
- Akbari Sene, R.; Sharifnia, S.; Moradi, G.R. On the impact evaluation of various chemical treatments of support on the photocatalytic properties and hydrogen evolution of sonochemically synthesized TiO2/Clinoptilolite. Int. J. Hydrogen Energy 2018, 43, 695–707. [Google Scholar] [CrossRef]
- Elghniji, K.; Elaloui, E.; Moussaoui, Y. Coating of anatase titania on clinoptilolite by metal organic chemical vapor deposition method: Enhanced mesoporosity and photocatalytic activity. Chem. Pap. 2018, 72, 1159–1168. [Google Scholar] [CrossRef]
- Castañeda-Juárez, M.; Martínez-Miranda, V.; Almazán-Sánchez, P.T.; Linares-Hernández, I.; Santoyo-Tepole, F.; Vázquez-Mejía, G. Synthesis of TiO2 catalysts doped with Cu, Fe, and Fe/Cu supported on clinoptilolite zeolite by an electrochemical-thermal method for the degradation of diclofenac by heterogeneous photocatalysis. J. Photochem. Photobiol. A Chem. 2019, 380, 111834. [Google Scholar] [CrossRef]
- Zabihi-Mobarakeh, H.; Nezamzadeh-Ejhieh, A. Application of supported TiO2 onto Iranian clinoptilolite nanoparticles in the photodegradation of mixture of aniline and 2,4-dinitroaniline aqueous solution. J. Ind. Eng. Chem. 2015, 26, 315–321. [Google Scholar] [CrossRef]
- Ko, S.; Fleming, P.D.; Joyce, M.; Ari-Gur, P. High performance nano-titania photocatalytic paper composite. Part II: Preparation and characterization of natural zeolite-based nano-titania composite sheets and study of their photocatalytic activity. Mater. Sci. Eng. B-Adv. 2009, 164, 135–139. [Google Scholar] [CrossRef]
- Khodadoust, S.; Sheini, A.; Armand, N. Photocatalytic degradation of monoethanolamine in wastewater using nanosized TiO2 loaded on clinoptilolite. Spectrochim. Acta Part A 2012, 92, 91–95. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Lu, S.; Guo, W.; Xi, B. Performance and mechanism into TiO2/Zeolite composites for sulfadiazine adsorption and photodegradation. Chem. Eng. J. 2018, 350, 131–147. [Google Scholar] [CrossRef]
- Sanni, S.O.; Modise, S.J.; Viljoen, E.L.; Ofomaja, A.E. Enhanced degradation of dye mixtures: Physicochemical and electrochemical properties of titania dispersed on clinoptilolite, synergistic influence. SN Appl. Sci. 2020, 2, 1668. [Google Scholar] [CrossRef]
- Yener, H.B.; Yılmaz, M.; Deliismail, Ö.; Özkan, S.F.; Helvacı, Ş.Ş. Clinoptilolite supported rutile TiO2 composites: Synthesis, characterization, and photocatalytic activity on the degradation of terephthalic acid. Sep. Purif. Technol. 2017, 173, 17–26. [Google Scholar] [CrossRef]
- Tan, Y.; Li, C.; Sun, Z.; Liang, C.; Zheng, S. Ternary structural assembly of BiOCl/TiO2/clinoptilolite composite: Study of coupled mechanism and photocatalytic performance. J. Colloid Interface Sci. 2020, 564, 143–154. [Google Scholar] [CrossRef]
- Zhou, P.; Shen, Y.; Zhao, S.; Chen, Y.; Gao, S.; Liu, W.; Wei, D. Hydrothermal synthesis of novel ternary hierarchical MoS2/TiO2/clinoptilolite nanocomposites with remarkably enhanced visible light response towards xanthates. Appl. Surf. Sci. 2021, 542, 148578. [Google Scholar] [CrossRef]
- Zhou, P.; Shen, Y.; Zhao, S.; Bai, J.; Han, C.; Liu, W.; Wei, D. Facile synthesis of clinoptilolite-supported Ag/TiO2 nanocomposites for visible-light degradation of xanthates. J. Taiwan Inst. Chem. Eng. 2021, 122, 231–240. [Google Scholar] [CrossRef]
- Amiri, S.; Anbia, M. Insights into the effect of parameters and pathway of visible-light photodegradation of glyphosate and diazinon by C-TiO2/clinoptilolite nanocomposite. J. Photochem. Photobiol. A 2024, 446, 115146. [Google Scholar] [CrossRef]
- Ali Johar, M.; Arslan Afzal, R.; Ali Alazba, A.; Manzoor, U. Photocatalysis and bandgap engineering using ZnO nanocomposites. Adv. Mater. Sci. Eng. 2015, 2015, 934587. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Z.; Liu, D.; Gao, Z. Preparation of ZnO Photocatalyst for the efficient and rapid photocatalytic degradation of azo dyes. Nanoscale Res. Lett. 2017, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Razavi-Khosroshahi, H.; Edalati, K.; Wu, J.; Nakashima, Y.; Arita, M.; Ikoma, Y.; Sadakiyo, M.; Inagaki, Y.; Staykov, A.; Yamauchi, Y.; et al. High-pressure zinc oxide phase as visible-light-active photocatalyst with narrow band gap. J. Mater. Chem. A 2017, 5, 20298–20303. [Google Scholar] [CrossRef]
- Lee, S.J.; Jung, H.J.; Koutavarapu, R.; Lee, S.H.; Arumugam, M.; Kim, J.H.; Choi, M.Y. ZnO supported Au/Pd bimetallic nanocomposites for plasmon improved photocatalytic activity for methylene blue degradation under visible light irradiation. Appl. Surf. Sci. 2019, 496, 143665. [Google Scholar] [CrossRef]
- Taha, A.; Da’na, E.; Hassanin, H.A. Modified activated carbon loaded with bio-synthesized Ag/ZnO nanocomposite and its application for the removal of Cr (VI) ions from aqueous solution. Surf. Interfaces 2021, 23, 100928. [Google Scholar] [CrossRef]
- Jagannatha, R.B.; Rani, R.S.; Padaki, M. ZnO zeolite nanocomposite for photocatalytic elimination of benzophenone and caffeine. ChemistrySelect 2019, 4, 1989–1993. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Khorsandi, S. Photocatalytic degradation of 4-nitrophenol with ZnO supported nano-clinoptilolite zeolite. J. Ind. Eng. Chem. 2014, 20, 937–946. [Google Scholar] [CrossRef]
- Iazdani, F.; Nezamzadeh-Ejhieh, A. The photocatalytic rate of ZnO supported onto natural zeolite nanoparticles in the photodegradation of an aromatic amine. Environ. Sci. Pollut. Res. 2021, 28, 53314–53327. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Khodabakhshi-Chermahini, F. Incorporated ZnO onto nano clinoptilolite particles as the active centers in the photodegradation of phenylhydrazine. J. Ind. Eng. Chem. 2014, 20, 695–704. [Google Scholar] [CrossRef]
- Bahrami, M.; Nezamzadeh-Ejhieh, A. Effect of the supported ZnO on clinoptilolite nano-particles in the photodecolorization of semi-real sample bromothymol blue aqueous solution. Mater. Sci. Semicond. Process. 2015, 30, 275–284. [Google Scholar] [CrossRef]
- De Dios, F.S.; Morales, E.R.; Cortaza, M.D.A.; Hernández, G.P.; Mandujano, E.V.M.; Alejandro, E.M.L.; Blanco, L.R. Improvement of photocatalysis using ZnO/zeolite nanocomposites for contaminant removal in aqueous media. Desalin. Water Treat. 2023, 312, 79–88. [Google Scholar] [CrossRef]
- Da Silvaa, P.L.; Nippes, R.P.; Macruz, P.D.; Hegetob, F.L.; Scaliante, M.H.N.O. Photocatalytic degradation of hydroxychloroquine using ZnO supported on clinoptilolite zeolite. Water Sci. Technol. 2021, 84, 764. [Google Scholar] [CrossRef] [PubMed]
- Wahyuni, T.H.; Diantariani, N.P.; Kartini, I.; Kuncaka, A. Enhancement of the photostability and visible photoactivity of ZnO photocatalyst used for reduction of Cr(VI) ions. Results Eng. 2022, 13, 100351. [Google Scholar] [CrossRef]
- Bahrami, M.; Nezamzadeh-Ejhieh, A. Effect of supporting and hybridizing of FeO and ZnO semiconductors onto an Iranian clinoptilolite nano-particles and the effect of ZnO/FeO ratio in the solar photodegradation of fish ponds waste water. Mat. Sci. Semicon. Proc. 2014, 27, 833–840. [Google Scholar] [CrossRef]
- Verma, N.; Kumar, N. Synthesis and Biomedical Applications of Copper Oxide Nanoparticles: An Expanding Horizon. ACS Biomater. Sci. Eng. 2019, 5, 1170–1188. [Google Scholar] [CrossRef] [PubMed]
- Katal, R.; Masudy-Panah, S.; Kong, E.Y.-J.; Khiavi, N.D.; DavoodAbadi Farahani, M.H.; Gong, X. Nanocrystal-engineered thin CuO film photocatalyst for visible-light-driven photocatalytic degradation of organic pollutant in aqueous solution. Catal. Today 2020, 40, 236–244. [Google Scholar] [CrossRef]
- Soori, F.; Nezamzadeh-Ejhieh, A. Synergistic effects of copper oxide-zeolite nanoparticles composite on photocatalytic degradation of 2,6-dimethylphenol aqueous solution. J. Mol. Liq. 2018, 255, 250–256. [Google Scholar] [CrossRef]
- Saberian, M.; Nezamzadeh-Ejhieh, A. Synergistic photocatalytic degraded tetracycline upon supported CuO clinoptilolite nanoparticles. Solid State Sci. 2024, 147, 107381. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Zabihi-Mobarakeh, H. Heterogeneous photodecolorization of mixture of methylene blue and bromophenol blue using CuO-nano-clinoptilolite. J. Ind. Eng. Chem. 2014, 20, 1421–1431. [Google Scholar] [CrossRef]
- Iazdani, F.; Nezamzadeh-Ejhieh, A. Supported cuprous oxide-clinoptilolite nanoparticles: Brief identification and the catalytic kinetics in the photodegradation of dichloroaniline. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 250, 119348. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Amiri, M. CuO supported Clinoptilolite towards solar photocatalytic degradation of p-aminophenol. Powder Technol. 2013, 235, 279–288. [Google Scholar] [CrossRef]
- Amiri, M.; Nezamzadeh-Ejhieh, A. Improvement of the photocatalytic activity of cupric oxide by deposition onto a natural clinoptilolite substrate. Mat. Sci. Semicon. Proc. 2015, 31, 501–508. [Google Scholar] [CrossRef]
- Shirzadi, A.; Nezamzadeh-Ejhieh, A. Enhanced photocatalytic activity of supported CuO–ZnO semiconductors towards the photodegradation of mefenamic acid aqueous solution as a semi real sample. J. Mol. Catal. A Chem. 2016, 411, 222–229. [Google Scholar] [CrossRef]
- Dashtpeyma, G.; Shabanian, S.R. Efficient photocatalytic oxidative desulfurization of liquid petroleum fuels under visible-light irradiation using a novel ternary heterogeneous BiVO4-CuO/modified natural clinoptilolite zeolite. J. Photochem. Photobiol. A 2023, 445, 115024. [Google Scholar] [CrossRef]
- Zhang, M.; An, T.; Hu, X.; Wang, C.; Sheng, G.; Fu, J. Preparation and photocatalytic properties of a nanometer ZnO–SnO2 coupled oxide. Appl. Catal. A Gen. 2004, 260, 215–222. [Google Scholar] [CrossRef]
- Kim, S.P.; Choi, M.Y.; Choi, H.C. Photocatalytic activity of SnO2 nanoparticles in methylene blue degradation. Mater. Res. Bull. 2016, 74, 85–89. [Google Scholar] [CrossRef]
- Šuligoj, A.; Pavlović, J.; Arčon, I.; Rajić, N.; Novak Tušar, N. SnO2-containing clinoptilolite as a composite photocatalyst for dyes removal from wastewater under solar light. Catalysts 2020, 10, 253. [Google Scholar] [CrossRef]
- Khodami, Z.; Nezamzadeh-Ejhieh, A. Investigation of photocatalytic e_ect of ZnO–SnO2/nano clinoptilolite system in the photodegradation of aqueous mixture of 4-methylbenzoic acid/2-chloro-5-nitrobenzoic acid. J. Mol. Catal. A Chem. 2015, 409, 59–68. [Google Scholar] [CrossRef]
- Derikvandi, H.; Nezamzadeh-Ejhieh, A. A comprehensive study on electrochemical and photocatalytic activity of SnO2-ZnO/clinoptilolite nanoparticles. J. Mol. Catal. A Chem. 2017, 426, 158–169. [Google Scholar] [CrossRef]
- Derikvandi, H.; Nezamzadeh-Ejhieh, A. Synergistic effect of p-n heterojunction, supporting and zeolite nanoparticles in enhanced photocatalytic activity of NiO and SnO2. J. Colloid Interf. Sci. 2017, 490, 314–327. [Google Scholar] [CrossRef]
- Khoshdel, K.; Honarmand, M.; Hassani, H. SnO2 and CuO anchored on zeolite as an efficient heterojunction photocatalyst for sunlight-assisted degradation of cefixime. Environ. Sci. Pollut. Res. 2023, 30, 36883–36903. [Google Scholar] [CrossRef]
- Sabouri, Z.; Fereydouni, N.; Akbari, A.; Hosseini, H.A.; Hashemzadeh, A.; Amiri, M.S.; Darroudi, M.; Kazemi Oskuee, R. Plant-based synthesis of NiO nanoparticles using salvia macrosiphon Boiss extract and examination of their water treatment. Rare Met. 2020, 39, 1134–1144. [Google Scholar] [CrossRef]
- Aejitha, S.; Dhanraj, G.; Govindaraj, T.; Senthil Kumar, N.; Maiz, F.; Shkir, M.; Kim, W.K.; Reddy Minnam Reddy, V.; Kim, D.H. Effect of La-doping on NiO photocatalyst for enhancing photocatalytic degradation performance under visible light irradiation: DFT calculations and degradation mechanism. Inorg. Chem. Commun. 2023, 156, 111172. [Google Scholar] [CrossRef]
- Makhado, K.P.; Mphahlele-Makgwane, M.M.; Kumar, N.; Baker, P.G.; Makgwane, P.R. Current updates on p-type nickel oxide (NiO) based photocatalysts towards decontamination of organic pollutants from wastewater. Mater. Today Sustain. 2024, 25, 100664. [Google Scholar] [CrossRef]
- Ajoudanian, N.; Nezamzadeh-Ejhieh, A. Enhanced photocatalytic activity of nickel oxide supported on clinoptilolite nanoparticles for the photodegradation of aqueous cephalexin. Mater. Sci. Semicond. Process. 2015, 36, 162–169. [Google Scholar] [CrossRef]
- Pourtaheri, A.; Nezamzadeh-Ejhieh, A. Enhancement in photocatalytic activity of NiO by supporting onto an Iranian clinoptilolite nano-particles of aqueous solution of cefuroxime pharmaceutical capsule. Spectrochim. Acta Part A 2015, 137, 338–344. [Google Scholar] [CrossRef]
- Pourtaheri, A.; Nezamzadeh-Ejhieh, A. Photocatalytic properties of incorporated NiO onto clinoptilolite nano-particles in the photodegradation process of aqueous solution of cefixime pharmaceutical capsule. Chem. Eng. Res. Des. 2015, 104, 835–843. [Google Scholar] [CrossRef]
- Arabpour, N.; Nezamzadeh-Ejhieh, A. Photodegradation of cotrimaxazole by clinoptilolite-supported nickel oxide. Process Saf. Environ. Prot. 2016, 102, 431–440. [Google Scholar] [CrossRef]
- Iazdani, F.; Nezamzadeh-Ejhieh, A. Photocatalytic kinetics of 2,4-dichloroaniline degradation by NiO-clinoptilolite nanoparticles. Spectrochim. Acta A 2021, 250, 119228. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xue, D. Solvothermal synthesis of CuS semiconductor hollow spheres based on a bubble template route. J. Cryst. Growth 2009, 311, 500–503. [Google Scholar] [CrossRef]
- Mageshwari, K.; Mali, S.S.; Hemalatha, T.; Sathyamoorthy, R.; Patil, P.S. Low temperature growth of CuS nanoparticles by reflux condensation method. Prog. Solid State Chem. 2011, 39, 108–113. [Google Scholar] [CrossRef]
- Isik, M.; Terlemezoglu, M.; Gasanly, N.; Parlak, M. Structural, morphological and temperature-tuned bandgap characteristics of CuS nano-flake thin films. Phys. E 2022, 144, 115407. [Google Scholar] [CrossRef]
- Surendran, S.; Vijaya Sankar, K.; Berchmans, J.L.; Kalai Selvan, R. Polyol synthesis of α-NiS particles and its physico-chemical properties. Mat. Sci. Semicon. Proc. 2015, 33, 16–23. [Google Scholar] [CrossRef]
- Fazli, Y.; Pourmortazavi, S.M.; Kohsari, I.; Karimi, M.S.; Tajdari, M. Synthesis, characterization and photocatalytic property of nickel sulfide nanoparticles. J. Mater. Sci. Mater. Electron. 2016, 27, 7192–7199. [Google Scholar] [CrossRef]
- Hafdi, H.; Mouldar, J.; Joudi, M.; Hatimi, B.; Nasrellah, H.; Mhammedi, M.A.E.; Bakasse, M. Nickel sulfide impregnated on natural phosphate: Characterization and applications in photocatalytic degradation of indigocarmine dye. Opt. Quant. Electron. 2021, 53, 183. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, Z.K.; Sharma, K.; Dwivedi, D.K. Investigation on physical properties of polycrystalline nickel sulphide films grown by simple & economical screen-printing method. Optik 2018, 156, 43–48. [Google Scholar] [CrossRef]
- Muniyappa, M.; Kalegowda, S.N.; Shetty, M.; Sriramoju, I.B.; Shastri, M.; Rao, S.V.N.; De, D.; Shankar, M.V.; Rangappa, D. Cocatalyst free nickel sulphide nanostructure for enhanced photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2022, 47, 5307–5318. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Moeinirad, S. Heterogeneous photocatalytic degradation of furfural using NiS-clinoptilolite zeolite. Desalination 2011, 273, 248–257. [Google Scholar] [CrossRef]
- Günes, S.; Fritz, K.P.; Neugebauer, H.; Sariciftci, N.S.; Kumar, S.; Scholes, G.D. Hybrid solar cells using PbS nanoparticles. Sol. Energy Mat. Sol. C 2007, 91, 420–423. [Google Scholar] [CrossRef]
- Mamiyev, Z.; Balayeva, N.O. PbS nanostructures: A review of recent advances. Mater. Today Sustain. 2023, 21, 100305. [Google Scholar] [CrossRef]
- Hassan, A.; Zafar, H.K.; Nafady, A.; Sohail, M. Synthesis and characterization of novel ferrocene/PbS nanocomposite material by low-temperature co-precipitation method. Inorg. Chem. Commun. 2024, 162, 112194. [Google Scholar] [CrossRef]
- Mehrabanpour, N.; Nezamzadeh-Ejhieh, A.; Ghattavi, S. A comparative photocatalytic activity between PbS NPs and PbS-clinoptilolite towards Cefotaxime. Solid State Sci. 2022, 131, 106953. [Google Scholar] [CrossRef]
- Almanqur, L.; Vitorica-Yrezabal, I.; Whitehead, G.; Lewis, D.; O’Brien, P. Synthesis of nanostructured powders and thin films of iron sulfide from molecular precursors. RSC Adv. 2018, 8, 29096. [Google Scholar] [CrossRef] [PubMed]
- Rahmani-Aliabadi, A.; Nezamzadeh-Ejhieh, A. A visible light FeS/Fe2S3/zeolite photocatalyst towards photodegradation of ciprofloxacin. J. Photochem. Photobiol. A 2018, 357, 1–10. [Google Scholar] [CrossRef]
- Azimi, S.; Nezamzadeh-Ejhieh, A. Enhanced activity of clinoptilolite-supported hybridized PbS–CdS semiconductors for the photocatalytic degradation of a mixture of tetracycline and cephalexin aqueous solution. J. Mol. Catal. A Chem. 2015, 408, 152–160. [Google Scholar] [CrossRef]
- Mehrabanpour, N.; Nezamzadeh-Ejhieh, A.; Ghattavi, S.; Ershadi, A. A magnetically separable clinoptilolite supported CdS-PbS photocatalyst: Characterization and photocatalytic activity toward cefotaxime. Appl. Surf. Sci. 2023, 614, 156252. [Google Scholar] [CrossRef]
- Mohammadyari, P.; Nezamzadeh-Ejhieh, A. Supporting of mixed ZnS–NiS semiconductors onto clinoptilolite nano-particles to improve its activity in photodegradation of 2-nitrotoluene. RSC Adv. 2015, 5, 75300–75310. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlović, J.; Rajić, N. Use of Natural Zeolite Clinoptilolite in the Preparation of Photocatalysts and Its Role in Photocatalytic Activity. Minerals 2024, 14, 508. https://doi.org/10.3390/min14050508
Pavlović J, Rajić N. Use of Natural Zeolite Clinoptilolite in the Preparation of Photocatalysts and Its Role in Photocatalytic Activity. Minerals. 2024; 14(5):508. https://doi.org/10.3390/min14050508
Chicago/Turabian StylePavlović, Jelena, and Nevenka Rajić. 2024. "Use of Natural Zeolite Clinoptilolite in the Preparation of Photocatalysts and Its Role in Photocatalytic Activity" Minerals 14, no. 5: 508. https://doi.org/10.3390/min14050508






