An Overview Analysis of Current Research Status in Iron Oxides Reduction by Hydrogen
Abstract
:1. Introduction
2. Application of Hydrogen in Metallurgical Technologies
- As a reducing agent for the reduction of iron oxides, primarily used in the blast furnace process and the gas-based direct reduction iron (DRI) process.
- As fuel for heating, such as in the processes of sintering, pelletizing, or heating steel ladles.
- As part of protective atmospheres and prevention of oxidation of the processed material. During heat treatments like annealing and sintering, hydrogen is used to prevent metals from coming into contact with oxygen and other reactive gases in the atmosphere. This helps avoid corrosion or oxidation of metals during processing. During the heat treatment of carbon steels, hydrogen is also employed for their decarburization. For this purpose, a protective atmosphere consisting of a mixture of hydrogen and nitrogen is used [15,16,17,18].
2.1. Overview of Laboratory Research on the Reduction of Fe Materials by Hydrogen
- Fe ores/concentrates.
- Fe pellets.
- Fe synthetic powders.
2.2. Results of Research Studies Focused on the Reduction of Iron Oxides with Hydrogen
2.2.1. Thermodynamics and Kinetics Basics of Reduction in Fe Oxides by Hydrogen
ΔH° = −3.197 kJ/mol
T ˂ 570 °C
ΔH° = 151.079 kJ/mol
T > 570 °C
ΔH° = 74.747 kJ/mol
T > 570 °C
ΔH° = 25.444 kJ/mol
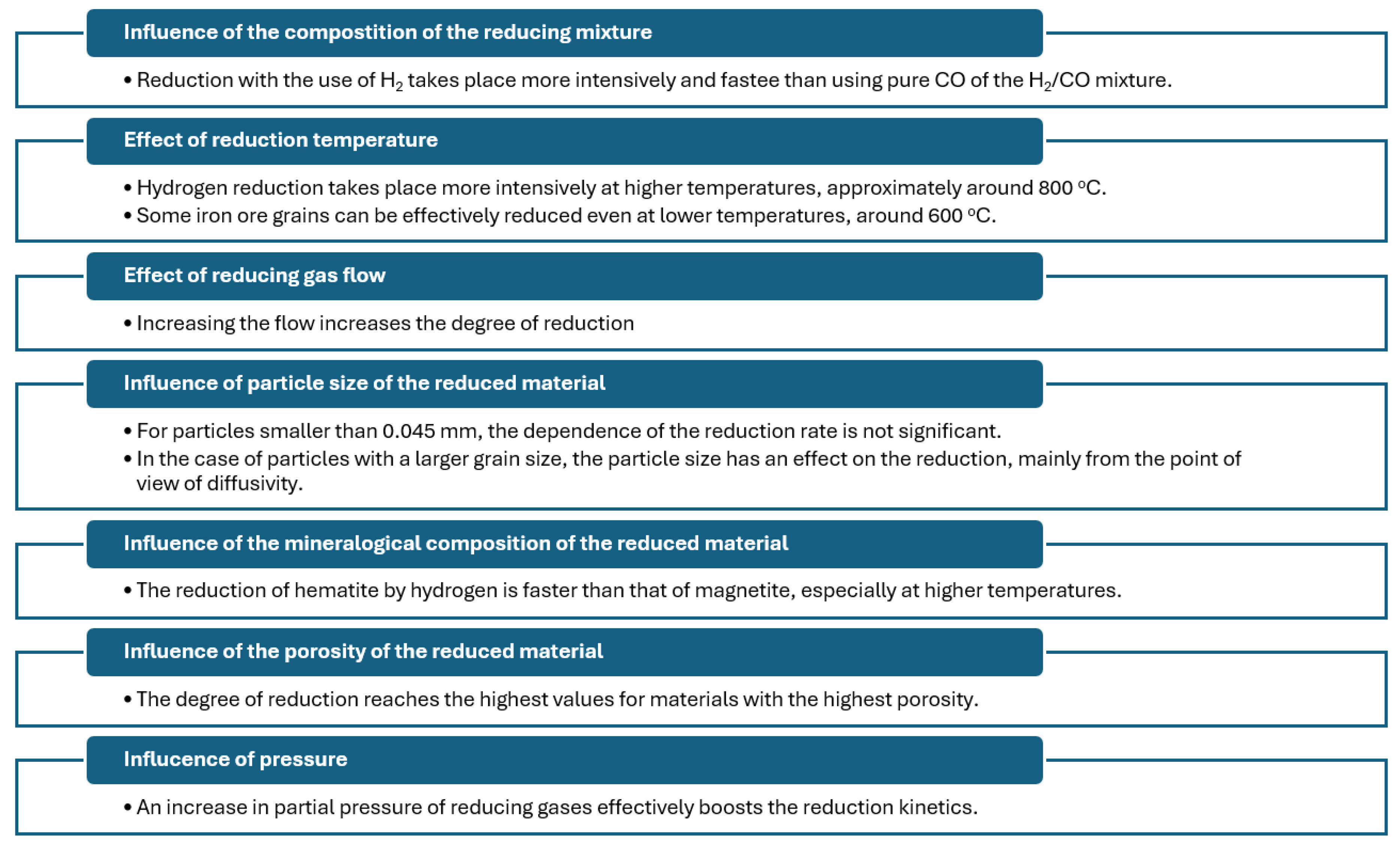
2.2.2. Influence of the Composition of the Reducing Atmosphere, Temperature and Kinetics on the Reduction in Fe Oxides by Hydrogen
2.2.3. Influence of Gas Flow on the Reduction in Fe Oxides by Hydrogen
- Particle diameters, dρ, in size range of 2 to 90 μm, to account for 95 wt.%.
- Densities of the ore and DRI, ρp, between 5000 and 3500 kg/m3.
- Gas densities, ρf, and dynamic viscosities, ηf, for the H2 and H2/H2O mixtures at temperatures of 873 to 1123 K and a pressure of 0.1 bar.
- Superficial gas velocity, u, between 0.15 and 0.30 m/s [83].
2.2.4. Influence of the Composition of the Reduced Material on the Reduction in Fe Oxides by Hydrogen
2.2.5. Influence of Pressure on the Reduction in Fe Oxides by Hydrogen
2.3. Results of Research Studies Focused on the Reduction in Iron Pellets with Hydrogen
2.4. Results of Research Studies Focused on the Reduction in Iron Materials with Hydrogen Using Hydrogen Plasma
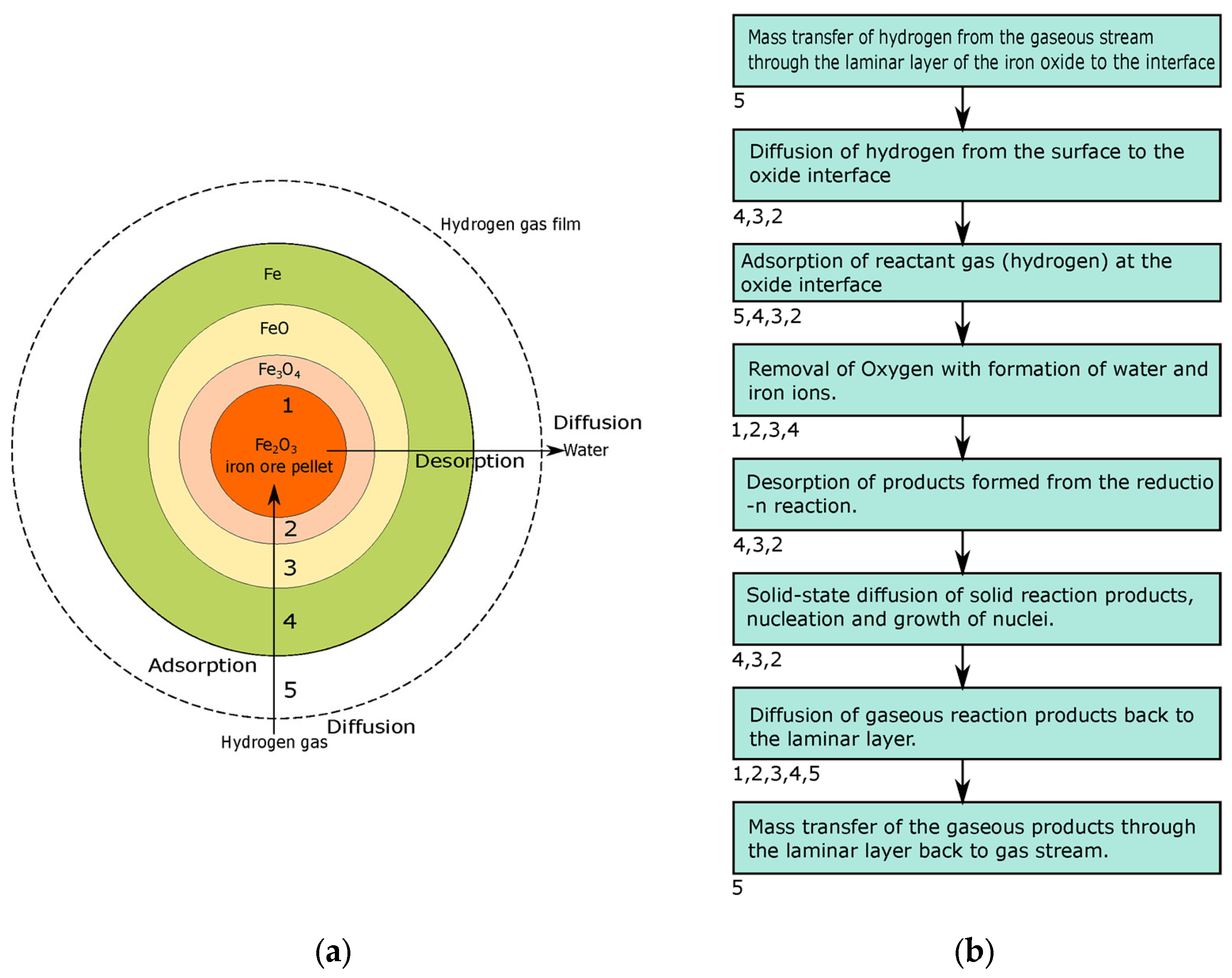
2.5. Critical Analysis of Reduction in Iron Oxides with Hydrogen, Advantages and Disadvantages of Reduction of Iron Oxides with Hydrogen
- mass transfer of the hydrogen from the stream to the surface of the reduced material;
- diffusion of the approaching gas through the tick film surrounding the reduced material;
- diffusion inside the surface pores;
- adsorption of hydrogen at the different oxides interphases;
- consequent oxygen removal through phase boundary reactions;
- formation of water vapor, iron oxides and ferrous iron;
- desorption of all the gases belonging to the reactions;
- solid state diffusion of the reacted products;
- diffusion of gaseous products back toward the surface;
- and mass transfer of the gaseous product toward the stream.
3. Discussion
- Kinetics of reduction: Researchers study the kinetics of hydrogen reduction reactions at the laboratory level. This involves examining the rate at which iron oxides (usually hematite or magnetite) are reduced to metallic iron in the presence of hydrogen.
- Optimum temperature and pressure: Identifying the optimal temperature and pressure that maximize the speed and completeness of the reduction process is a key area of research. Scientists are working to pinpoint these conditions.
- Mechanisms of reduction: Understanding the mechanisms in hydrogen reduction reactions is crucial. Researchers are identifying reaction pathways to better control and enhance the reduction process.
- Gas composition: Researchers experiment with different gas compositions, including variations in hydrogen concentration, to assess their effects on reduction efficiency. This may involve using pure hydrogen or a mixture of gases with a high hydrogen content.
- Material characterization: Before and after reduction, researchers use analytical techniques such as X-ray diffraction (XRD), scanning electron microscopy (SEM), and energy-dispersive X-ray spectroscopy (EDS) to characterize the iron ore and reduced products.
- Effect of impurities: The research explores the impact of impurities in iron ore and hydrogen gas on the reduction process and the quality of the obtained iron.
- Reaction Modeling: Mathematical modeling and simulations are employed to predict and optimize process parameters, providing insights into reaction behavior and guiding experimental design.
- Energy efficiency: The research aims to enhance the energy efficiency of the hydrogen reduction process. This includes assessing the energy required for hydrogen production and iron ore reduction, and exploring ways to minimize energy consumption.
- Product quality: Research focuses on improving the quality of the iron reduced by hydrogen. This includes achieving the desired chemical compositions, metallurgical properties, and purity levels of the reduced Fe.
- Environmental Impact: Studies assess the environmental impact of hydrogen-based reduction processes, including by-product emissions and their potential effects on air quality and greenhouse gas emissions.
- Economic sustainability: Researchers evaluate the economic viability of implementing hydrogen-based reduction processes in the steel industry. This includes analyzing the costs associated with hydrogen production and its impact on the overall economics of the process.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, R.R.; Zhao, Y.Q.; Babich, A.; Senk, D.; Fan, X.Y. Hydrogen Direct Reduction (H-DR) in Steel Industry—An Overview of Challenges and Opportunities. J. Clean. Prod. 2021, 329, 129797. [Google Scholar] [CrossRef]
- Okolie, J.A.; Patra, B.R.; Mukherjee, A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Futuristic Applications of Hydrogen in Energy, Biorefining, Aerospace, Pharmaceuticals and Metallurgy. Int. J. Hydrogen Energy 2021, 46, 8885–8905. [Google Scholar] [CrossRef]
- Hasanbeigi, A.; Arens, M.; Cardenas, J.C.R.; Price, L.; Triolo, R. Comparison of Carbon Dioxide Emissions Intensity of Steel Production in China, Germany, Mexico, and the United States. Resour. Conserv. Recycl. 2016, 113, 127–139. [Google Scholar] [CrossRef]
- Vogl, V.; Åhman, M.; Nilsson, L.J. Assessment of Hydrogen Direct Reduction for Fossil-Free Steelmaking. J. Clean. Prod. 2018, 203, 736–745. [Google Scholar] [CrossRef]
- Morfeldt, J.; Nijs, W.; Silveira, S. The Impact of Climate Targets on Future Steel Production—An Analysis Based on a Global Energy System Model. J. Clean. Prod. 2015, 103, 469–482. [Google Scholar] [CrossRef]
- Ariyama, T.; Takahashi, K.; Kawashiri, Y.; Nouchi, T. Diversification of the Ironmaking Process toward the Long-Term Global Goal for Carbon Dioxide Mitigation. J. Sustain. Metall. 2019, 5, 276–294. [Google Scholar] [CrossRef]
- Bataille, C.; Åhman, M.; Neuhoff, K.; Nilsson, L.J.; Fischedick, M.; Lechtenböhmer, S.; Solano-Rodriquez, B.; Denis-Ryan, A.; Stiebert, S.; Waisman, H.; et al. A Review of Technology and Policy Deep Decarbonization Pathway Options for Making Energy-Intensive Industry Production Consistent with the Paris Agreement. J. Clean. Prod. 2018, 187, 960–973. [Google Scholar] [CrossRef]
- Li, Z.; Davis, C. Ironmaking and Steelmaking. Metals 2019, 9, 525. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A Comparative Overview of Hydrogen Production Processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Ma, K.; Deng, J.; Wang, G.; Zhou, Q.; Xu, J. Utilization and Impacts of Hydrogen in the Ironmaking Processes: A Review from Lab-Scale Basics to Industrial Practices. Int. J. Hydrogen Energy 2021, 46, 26646–26664. [Google Scholar] [CrossRef]
- Mazloomi, K.; Gomes, C. Hydrogen as an Energy Carrier: Prospects and Challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Wietschel, M.; Hasenauer, U.; de Groot, A. Development of European Hydrogen Infrastructure Scenarios—CO2 Reduction Potential and Infrastructure Investment. Energy Policy 2006, 34, 1284–1298. [Google Scholar] [CrossRef]
- Sun, M.; Pang, K.; Barati, M.; Meng, X. Hydrogen-Based Reduction Technologies in Low-Carbon Sustainable Ironmaking and Steelmaking: A Review. J. Sustain. Metall. 2024, 10, 10–25. [Google Scholar] [CrossRef]
- Fan, Z.; Friedmann, S.J. Low-Carbon Production of Iron and Steel: Technology Options, Economic Assessment, and Policy. Joule 2021, 5, 829–862. [Google Scholar] [CrossRef]
- Liu, W.; Zuo, H.; Wang, J.; Xue, Q.; Ren, B.; Yang, F. The Production and Application of Hydrogen in Steel Industry. Int. J. Hydrogen Energy 2021, 46, 10548–10569. [Google Scholar] [CrossRef]
- Chen, Y.; Zuo, H. Review of Hydrogen-Rich Ironmaking Technology in Blast Furnace. Ironmak. Steelmak. 2021, 48, 749–768. [Google Scholar] [CrossRef]
- Yilmaz, C.; Wendelstorf, J.; Turek, T. Modeling and Simulation of Hydrogen Injection into a Blast Furnace to Reduce Carbon Dioxide Emissions. J. Clean. Prod. 2017, 154, 488–501. [Google Scholar] [CrossRef]
- Mittemeijer, E.J. Steel Heat Treating Fundamentals and Processes. In ASM Handbook; Dossett, J., Totten, G.E., Eds.; Introduction to Surface Hardening of Steels; ASM International: Detroit, MI, USA, 2013; Volume 4A, p. 784. [Google Scholar]
- Quader, M.A.; Ahmed, S.; Ghazilla, R.A.R.; Ahmed, S.; Dahari, M. A Comprehensive Review on Energy Efficient CO2 Breakthrough Technologies for Sustainable Green Iron and Steel Manufacturing. Renew. Sustain. Energy Rev. 2015, 50, 594–614. [Google Scholar] [CrossRef]
- Cavaliere, P. Direct Reduced Iron: Most Efficient Technologies for Greenhouse Emissions Abatement. In Clean Ironmaking and Steelmaking Processes; Springer: Cham, Switzerland, 2019; pp. 419–484. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, L.; Shen, F.M. Shaft Furnace Direct Reduction Technology-Midrex and Energiron. Adv. Mat. Res. 2013, 805–806, 654–659. [Google Scholar] [CrossRef]
- Tang, J.; Chu, M.S.; Li, F.; Feng, C.; Liu, Z.G.; Zhou, Y.S. Development and Progress on Hydrogen Metallurgy. Int. J. Miner. Metall. Mater. 2020, 27, 713–723. [Google Scholar] [CrossRef]
- Zhang, X.; Jiao, K.; Zhang, J.; Guo, Z. A Review on Low Carbon Emissions Projects of Steel Industry in the World. J. Clean. Prod. 2021, 306, 127259. [Google Scholar] [CrossRef]
- Pei, M.; Petäjäniemi, M.; Regnell, A.; Wijk, O. Toward a Fossil Free Future with HYBRIT: Development of Iron and Steelmaking Technology in Sweden and Finland. Metals 2020, 10, 972. [Google Scholar] [CrossRef]
- The Adoption of Hydrogen Metallurgy in the Climate-Neutral Production of Steel|HELIOS|Project|Fact Sheet|HORIZON|CORDIS|European Commission. Available online: https://cordis.europa.eu/project/id/101120068 (accessed on 25 November 2023).
- Green H2 and Circular Bio-Coal from Biowaste for Cost-Competitive Sustainable Steel|H2STEEL|Project|Fact Sheet|HORIZON|CORDIS|European Commission. Available online: https://cordis.europa.eu/project/id/101070741 (accessed on 25 November 2023).
- Hydrogen Technologies for Decarbonization of Industrial Heating Processes|HyInHeat|Project|Fact Sheet|HORIZON|CORDIS|European Commission. Available online: https://cordis.europa.eu/project/id/101091456 (accessed on 25 November 2023).
- Hybrid Hydrogen-Based Reduction of Iron Ores|Max-Planck-Institut Für Eisenforschung GmbH. Available online: https://www.mpie.de/4728546/hybrid_hydrogen-based_reduction (accessed on 25 November 2023).
- Hydrogen Plasma-Based Reduction of Iron Ores|Max-Planck-Institut Für Eisenforschung GmbH. Available online: https://www.mpie.de/4727993/hydrogen_plasma-based_reduction (accessed on 25 November 2023).
- Hydrogen As the Reducing Agent in the REcovery of Metals and Minerals from Metallurgical Waste|HARARE|Project|Fact Sheet|H2020|CORDIS|European Commission. Available online: https://cordis.europa.eu/project/id/958307 (accessed on 28 January 2024).
- Decarbonising Steel Production in China: H2-DRI-EAF Technology. Available online: https://transitionasia.org/wp-content/uploads/2024/02/TA_DecarbonisingSteelProductionInChina_Feb2024N.pdf (accessed on 14 May 2024).
- Öhman, A.; Karakaya, E.; Urban, F. Enabling the Transition to a Fossil-Free Steel Sector: The Conditions for Technology Transfer for Hydrogen-Based Steelmaking in Europe. Energy Res. Soc. Sci. 2022, 84, 102384. [Google Scholar] [CrossRef]
- Oh, J.; Noh, D. The Reduction Kinetics of Hematite Particles in H2 and CO Atmospheres. Fuel 2017, 196, 144–153. [Google Scholar] [CrossRef]
- Hessling, O.; Tottie, M.; Sichen, D. Experimental Study on Hydrogen Reduction of Industrial Fines in Fluidized Bed. Ironmak. Steelmak. 2021, 48, 936–943. [Google Scholar] [CrossRef]
- Bai, M.; Long, H.; Li, L.; Liu, D.; Ren, S.B.; Zhao, C.F.; Cheng, J. Kinetics of Iron Ore Pellets Reduced by H2–N2 under Non-Isothermal Condition. Int. J. Hydrogen Energy 2018, 43, 15586–15592. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, J.; Qin, B.; Dong, Y.; Lu, Y.; Li, Y.; Hao, W.; Zhang, Y. Reduction Kinetics of Hematite Ore Fines with H2 in a Rotary Drum Reactor. Powder Technol. 2018, 332, 18–26. [Google Scholar] [CrossRef]
- Nyankson, E.; Kolbeinsen, L. Kinetics of Direct Iron Ore Reduction with CO-H2 Gas Mixtures. Int. J. Eng. Res. Technol. 2015, 4, 934–940. [Google Scholar] [CrossRef]
- Chen, Z.; Dang, J.; Hu, X.; Yan, H. Reduction Kinetics of Hematite Powder in Hydrogen Atmosphere at Moderate Temperatures. Metals 2018, 8, 751. [Google Scholar] [CrossRef]
- Spreitzer, D.; Schenk, J. Iron Ore Reduction by Hydrogen Using a Laboratory Scale Fluidized Bed Reactor: Kinetic Investigation-Experimental Setup and Method for Determination. Metall. Mater. Trans. B 2019, 50, 2471–2484. [Google Scholar] [CrossRef]
- Wang, D.; Sichen, D. Effect of Density on the Reduction of Fe2O3 Pellets by H2-CO Mixtures. Master’s Thesis, School of Industrial Engineering and Management, Royal Institute of Technology, Stockholm, Sweden, 2023. [Google Scholar]
- Zhang, J.; Li, Y.; Liu, Z.; Wang, T.; Wang, Y.; Li, K.; Wang, G.; Xu, T.; Zhang, Y. Isothermal Kinetic Analysis on Reduction of Solid/Liquid Wustite by Hydrogen. Int. J. Miner. Metall. Mater. 2022, 29, 1830–1838. [Google Scholar] [CrossRef]
- Du, W.; Yang, S.; Pan, F.; Shangguan, J.; Lu, J.; Liu, S.; Fan, H. Hydrogen Reduction of Hematite Ore Fines to Magnetite Ore Fines at Low Temperatures. J. Chem. 2017, 2017, 1919720. [Google Scholar] [CrossRef]
- Wagner, D.; Devisme, O.; Patisson, F.; Ablitzer, D. A Laboratory Study of The Reduction of Iron Oxides by Hydrogen. In Proceedings of the Sohn International Symposium, San Diego, CA, USA, 27–31 August 2006. [Google Scholar]
- Kuila, S.K.; Chatterjee, R.; Ghosh, D. Kinetics of Hydrogen Reduction of Magnetite Ore Fines. Int. J. Hydrogen Energy 2016, 41, 9256–9266. [Google Scholar] [CrossRef]
- Spreitzer, D.; Schenk, J. Fluidization Behavior and Reducibility of Iron Ore Fines during Hydrogen-Induced Fluidized Bed Reduction. Particuology 2020, 52, 36–46. [Google Scholar] [CrossRef]
- Qu, Y.; Xing, L.; Shao, L.; Luo, Y.; Zou, Z. Microstructural Characterization and Gas-Solid Reduction Kinetics of Iron Ore Fines at High Temperature. Powder Technol. 2019, 355, 26–36. [Google Scholar] [CrossRef]
- Zhang, T.; Lei, C.; Zhu, Q. Reduction of Fine Iron Ore via a Two-Step Fluidized Bed Direct Reduction Process. Powder Technol. 2014, 254, 1–11. [Google Scholar] [CrossRef]
- Guo, D.; Hu, M.; Pu, C.; Xiao, B.; Hu, Z.; Liu, S.; Wang, X.; Zhu, X. Kinetics and Mechanisms of Direct Reduction of Iron Ore-Biomass Composite Pellets with Hydrogen Gas. Int. J. Hydrogen Energy 2015, 40, 4733–4740. [Google Scholar] [CrossRef]
- Kazemi, M.; Pour, M.S.; Sichen, D. Experimental and Modeling Study on Reduction of Hematite Pellets by Hydrogen Gas. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2017, 48, 1114–1122. [Google Scholar] [CrossRef]
- Jozwiak, W.K.; Kaczmarek, E.; Maniecki, T.P.; Ignaczak, W.; Maniukiewicz, W. Reduction Behavior of Iron Oxides in Hydrogen and Carbon Monoxide Atmospheres. Appl. Catal. A Gen. 2007, 326, 17–27. [Google Scholar] [CrossRef]
- Zuo, H.B.; Wang, C.; Dong, J.J.; Jiao, K.X.; Xu, R.S. Reduction Kinetics of Iron Oxide Pellets with H2 and CO Mixtures. Int. J. Miner. Metall. Mater. 2015, 22, 688–696. [Google Scholar] [CrossRef]
- Kim, W.H.; Lee, S.; Kim, S.M.; Min, D.J. The Retardation Kinetics of Magnetite Reduction Using H2 and H2–H2O Mixtures. Int. J. Hydrogen Energy 2013, 38, 4194–4200. [Google Scholar] [CrossRef]
- Ngoy, D.; Sukhomlinov, D.; Tangstad, M. Pre-Reduction Behaviour of Manganese Ores in H2 and CO Containing Gases. ISIJ Int. 2020, 60, 2325–2331. [Google Scholar] [CrossRef]
- Li, P.; Li, Y.; Yu, J.; Gao, P.; Han, Y. Kinetics and Microstructural Changes during Fluidized Reduction of Magnetite with Hydrogen at Low Temperatures. Int. J. Hydrogen Energy 2022, 47, 31140–31151. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Nie, J.; Dewil, R.; Baeyens, J.; Deng, Y. The Direct Reduction of Iron Ore with Hydrogen. Sustainability 2021, 13, 8866. [Google Scholar] [CrossRef]
- Zheng, H.; Daghagheleh, O.; Wolfinger, T.; Taferner, B.; Schenk, J.; Xu, R. Fluidization Behavior and Reduction Kinetics of Pre-Oxidized Magnetite-Based Iron Ore in a Hydrogen-Induced Fluidized Bed. Int. J. Miner. Metall. Mater. 2022, 29, 1873–1881. [Google Scholar] [CrossRef]
- Dhawan, N.; Manzoor, U.; Agrawal, S.; Dhawan, N.; Manzoor, U.; Agrawal, S. Hydrogen Reduction of Low-Grade Banded Iron Ore. MiEng 2022, 187, 107794. [Google Scholar] [CrossRef]
- Scharm, C.; Küster, F.; Laabs, M.; Huang, Q.; Volkova, O.; Reinmöller, M.; Guhl, S.; Meyer, B. Direct Reduction of Iron Ore Pellets by H2 and CO: In-Situ Investigation of the Structural Transformation and Reduction Progression Caused by Atmosphere and Temperature. Miner. Eng. 2022, 180, 107459. [Google Scholar] [CrossRef]
- Lyu, Q.; Qie, Y.; Liu, X.; Lan, C.; Li, J.; Liu, S. Effect of Hydrogen Addition on Reduction Behavior of Iron Oxides in Gas-Injection Blast Furnace. Thermochim. Acta 2017, 648, 79–90. [Google Scholar] [CrossRef]
- Abu Tahari, M.N.; Salleh, F.; Tengku Saharuddin, T.S.; Samsuri, A.; Samidin, S.; Yarmo, M.A. Influence of Hydrogen and Carbon Monoxide on Reduction Behavior of Iron Oxide at High Temperature: Effect on Reduction Gas Concentrations. Int. J. Hydrogen Energy 2021, 46, 24791–24805. [Google Scholar] [CrossRef]
- Hessels, C.J.M.; Homan, T.A.M.; Deen, N.G.; Tang, Y. Reduction Kinetics of Combusted Iron Powder Using Hydrogen. Powder Technol. 2022, 407, 117540. [Google Scholar] [CrossRef]
- El-Geassy, A.A.; Nasr, M.I. Influence of the Original Structure on the Kinetics of Hydrogen Reduction of Hematite Compacts. Trans. Iron Steel Inst. Jpn. 1988, 28, 650–658. [Google Scholar] [CrossRef]
- He, J.; Li, K.; Zhang, J.; Conejo, A.N. Reduction Kinetics of Compact Hematite with Hydrogen from 600 to 1050 °C. Metals 2023, 13, 464. [Google Scholar] [CrossRef]
- Bahgat, M.; Khedr, M.H. Reduction Kinetics, Magnetic Behavior and Morphological Changes during Reduction of Magnetite Single Crystal. Mater. Sci. Eng. B 2007, 138, 251–258. [Google Scholar] [CrossRef]
- Zieliński, J.; Zglinicka, I.; Znak, L.; Kaszkur, Z. Reduction of Fe2O3 with Hydrogen. Appl. Catal. A Gen. 2010, 381, 191–196. [Google Scholar] [CrossRef]
- Barde, A.A.; Klausner, J.F.; Mei, R. Solid State Reaction Kinetics of Iron Oxide Reduction Using Hydrogen as a Reducing Agent. Int. J. Hydrogen Energy 2016, 41, 10103–10119. [Google Scholar] [CrossRef]
- Heidari, A.; Niknahad, N.; Iljana, M.; Fabritius, T. A Review on the Kinetics of Iron Ore Reduction by Hydrogen. Materials 2021, 14, 7540. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, T.; Shishin, D.; Jak, E.; Decterov, S.A. Thermodynamic Reevaluation of the Fe–O System. Calphad 2015, 48, 131–144. [Google Scholar] [CrossRef]
- Kawasaki, E.; Sanscrainte, J.; Walsh, T.J. Kinetics of Reduction of Iron Oxide with Carbon Monoxide and Hydrogen. AIChE J. 1962, 8, 48–52. [Google Scholar] [CrossRef]
- Ma, Y.; Souza Filho, I.R.; Bai, Y.; Schenk, J.; Patisson, F.; Beck, A.; van Bokhoven, J.A.; Willinger, M.G.; Li, K.; Xie, D.; et al. Hierarchical Nature of Hydrogen-Based Direct Reduction of Iron Oxides. Scr. Mater. 2022, 213, 114571. [Google Scholar] [CrossRef]
- Bhaskar, A.; Assadi, M.; Somehsaraei, H.N. Decarbonization of the Iron and Steel Industry with Direct Reduction of Iron Ore with Green Hydrogen. Energies 2020, 13, 758. [Google Scholar] [CrossRef]
- Hammam, A.; Nasr, M.I.; Elsadek, M.H.; Khan, I.U.; Omran, M.; Wei, H.; Qiu, D.; Yu, Y. Studies on the Reduction Behavior of Iron Oxide Pellet Fines with Hydrogen Gas: Mechanism and Kinetic Analysis. J. Sustain. Metall. 2023, 9, 1289–1302. [Google Scholar] [CrossRef]
- He, K.; Zheng, Z.; Chen, Z.; Chen, H.; Hao, W. Kinetics of Hydrogen Reduction of Brazilian Hematite in a Micro-Fluidized Bed. Int. J. Hydrogen Energy 2021, 46, 4592–4605. [Google Scholar] [CrossRef]
- Mao, X.; Garg, P.; Hu, X.; Li, Y.; Nag, S.; Kundu, S.; Zhang, J. Kinetic Analysis of Iron Ore Powder Reaction with Hydrogen—Carbon Monoxide. Int. J. Miner. Metall. Mater. 2022, 29, 1882–1890. [Google Scholar] [CrossRef]
- Fogelström, J.B.; Martinsson, J.; Kojola, N. The Influence of Nitrogen on Hydrogen Reduction of Iron Ore Pellets. Steel Res. Int. 2024, 95, 2300655. [Google Scholar] [CrossRef]
- Lyu, B.; Wang, G.; Yang, F.; Zuo, H.; Xue, Q.; Wang, J. Kinetic Analysis of Isothermal and Non-Isothermal Reduction of Iron Ore Fines in Hydrogen Atmosphere. Metals 2022, 12, 1754. [Google Scholar] [CrossRef]
- Teplov, O.A. Kinetics of the Low-Temperature Hydrogen Reduction of Magnetite Concentrates. Russ. Metall. Met. 2012, 2012, 8–21. [Google Scholar] [CrossRef]
- Abdelrahim, A.; Iljana, M.; Omran, M.; Vuolio, T.; Bartusch, H.; Fabritius, T. Influence of H2–H2O Content on the Reduction of Acid Iron Ore Pellets in a CO–CO2–N2 Reducing Atmosphere. ISIJ Int. 2020, 60, 2206–2217. [Google Scholar] [CrossRef]
- He, K.; Zheng, Z.; Chen, H.; Hao, W. Reduction Behaviors of Hematite to Metallic Iron by Hydrogen at Low Temperatures. In Minerals, Metals and Materials Series; Springer: Cham, Switzerland, 2021; pp. 111–122. [Google Scholar] [CrossRef]
- Yi, L.; Huang, Z.; Peng, H.; Jiang, T. Action Rules of H2 and CO in Gas-Based Direct Reduction of Iron Ore Pellets. J. Cent. South Univ. 2012, 19, 2291–2296. [Google Scholar] [CrossRef]
- Kang, H.; Xu, Q.; Cao, Z.; Lu, X.; Shi, J.; Chen, B.; Guo, L. Influence of Hydrogen Flow Rate on Multistep Kinetics of Hematite Reduction. Int. J. Hydrogen Energy 2024, 49, 1255–1268. [Google Scholar] [CrossRef]
- Mirzajani, A.; Ale Ebrahim, H.; Nouri, S.M.M. Simulation of a Direct Reduction Moving Bed Reactor Using a Three Interface Model. Braz. J. Chem. Eng. 2018, 35, 1019–1028. [Google Scholar] [CrossRef]
- Wolfinger, T.; Spreitzer, D.; Schenk, J. Using Iron Ore Ultra-Fines for Hydrogen-Based Fluidized Bed Direct Reduction—A Mathematical Evaluation. Materials 2022, 15, 3943. [Google Scholar] [CrossRef] [PubMed]
- Olivares, B.; René, I. Reduction of Iron Ore in a Batch Fluidized Bed. Master’s Thesis, University of New South Wales, Sydney, Australia, 1990. [Google Scholar] [CrossRef]
- Heikkilä, A.; Iljana, M.; Bartusch, H.; Fabritius, T. Reduction of Iron Ore Pellets, Sinter, and Lump Ore under Simulated Blast Furnace Conditions. Steel Res. Int. 2020, 91, 2000047. [Google Scholar] [CrossRef]
- Hou, B.; Zhang, H.; Li, H.; Zhu, Q. Study on Kinetics of Iron Oxide Reduction by Hydrogen. Chin. J. Chem. Eng. 2012, 20, 10–17. [Google Scholar] [CrossRef]
- Sundberg, R. Reduction of Iron Oxides with Hydrogen. Master’s Thesis, Abo Akademi University, Turku, Finland, 2021. [Google Scholar]
- Souza Filho, I.R.; Ma, Y.; Raabe, D.; Springer, H. Fundamentals of Green Steel Production: On the Role of Gas Pressure During Hydrogen Reduction of Iron Ores. JOM 2023, 75, 2274–2286. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Ueda, Y.; Nishikawa, Y.; Goto, T. Effect of Pressure on Reduction Rate of Iron Ore with High Pressure Fluidized Bed. Trans. Iron Steel Inst. Jpn. 1986, 26, 697–703. [Google Scholar] [CrossRef]
- Habermann, A.; Winter, F.; Hofbauer, H.; Zirngast, J.; Schenk, J.L. An Experimental Study on the Kinetics of Fluidized Bed Iron Ore Reduction. ISIJ Int. 2000, 40, 935–942. [Google Scholar] [CrossRef]
- Sato, K.; Nishikawa, Y.; Tamura, I. Pressure Increase and Temperature Fall within a Hematite Sphere during Reduction by Hydrogen. Tetsu–Hagane 1983, 69, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Spreitzer, D.; Schenk, J. Reduction of Iron Oxides with Hydrogen—A Review. Steel Res. Int. 2019, 90, 1900108. [Google Scholar] [CrossRef]
- Zare Ghadi, A.; Valipour, M.S.; Vahedi, S.M.; Sohn, H.Y. A Review on the Modeling of Gaseous Reduction of Iron Oxide Pellets. Steel Res. Int. 2020, 91, 1900270. [Google Scholar] [CrossRef]
- Biswas, A.K. Principles of Blast Furnace Ironmaking: Theory and Practice; Cootha: Cootha, Australia, 1981; ISBN 0949917087/9780949917089. [Google Scholar]
- Geerdes, M.; Chaigneau, R.; Kurunov, I.; Lingiardi, O.; Ricketts, J. Modern Blast Furnace Ironmaking: An Introduction, 3rd ed.; Delft University Press: Delft, The Netherlands, 2015; ISBN 978-1-61499-498-5 (Print), 978-1-61499-499-2 (Online). [Google Scholar]
- Cavaliere, P.; Dijon, L.; Laska, A.; Koszelow, D. Hydrogen Direct Reduction and Reoxidation Behaviour of High-Grade Pellets. Int. J. Hydrogen Energy 2024, 49, 1235–1254. [Google Scholar] [CrossRef]
- Cavaliere, P.; Perrone, A.; Dijon, L.; Laska, A.; Koszelow, D. Direct Reduction of Pellets through Hydrogen: Experimental and Model Behaviour. Int. J. Hydrogen Energy 2024, 49, 1444–1460. [Google Scholar] [CrossRef]
- Korobeinikov, Y.; Meshram, A.; Harris, C.; Kovtun, O.; Govro, J.; O’Malley, R.J.; Volkova, O.; Sridhar, S. Reduction of Iron-Ore Pellets Using Different Gas Mixtures and Temperatures. Steel Res. Int. 2023, 94, 2300066. [Google Scholar] [CrossRef]
- Zhang, A.; Monaghan, B.J.; Longbottom, R.J.; Nusheh, M.; Bumby, C.W. Reduction Kinetics of Oxidized New Zealand Ironsand Pellets in H2 at Temperatures up to 1443 K. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2020, 51, 492–504. [Google Scholar] [CrossRef]
- Metolina, P.; Ribeiro, T.R.; Guardani, R. Hydrogen-Based Direct Reduction of Industrial Iron Ore Pellets: Statistically Designed Experiments and Computational Simulation. Int. J. Miner. Metall. Mater. 2022, 29, 1908–1921. [Google Scholar] [CrossRef]
- Ma, Y.; Souza Filho, I.R.; Zhang, X.; Nandy, S.; Barriobero-Vila, P.; Requena, G.; Vogel, D.; Rohwerder, M.; Ponge, D.; Springer, H.; et al. Hydrogen-Based Direct Reduction of Iron Oxide at 700 °C: Heterogeneity at Pellet and Microstructure Scales. Int. J. Miner. Metall. Mater. 2022, 29, 1901–1907. [Google Scholar] [CrossRef]
- Bai, M.H.; Long, H.; Ren, S.B.; Liu, D.; Zhao, C.F. Reduction Behavior and Kinetics of Iron Ore Pellets under H2–N2 Atmosphere. ISIJ Int. 2018, 58, 1034–1041. [Google Scholar] [CrossRef]
- Kovtun, O.; Levchenko, M.; Ilatovskaia, M.O.; Aneziris, C.G.; Volkova, O. Results of Hydrogen Reduction of Iron Ore Pellets at Different Temperatures. Steel Res. Int. 2024, 10–11, 2300707. [Google Scholar] [CrossRef]
- Patisson, F.; Mirgaux, O. Hydrogen Ironmaking: How It Works. Metals 2020, 10, 922. [Google Scholar] [CrossRef]
- Zhao, Z.; Tang, J.; Chu, M.; Wang, X.; Zheng, A.; Wang, X.; Li, Y. Direct Reduction Swelling Behavior of Pellets in Hydrogen-Based Shaft Furnaces under Typical Atmospheres. Int. J. Miner. Metall. Mater. 2022, 29, 1891–1900. [Google Scholar] [CrossRef]
- Kovtun, O.; Levchenko, M.; Oldinski, E.; Gräbner, M.; Volkova, O. Swelling Behavior of Iron Ore Pellets during Reduction in H2 and N2/H2 Atmospheres at Different Temperatures. Steel Res. Int. 2023, 94, 2300140. [Google Scholar] [CrossRef]
- Yi, L.; Huang, Z.; Jiang, T.; Wang, L.; Qi, T. Swelling Behavior of Iron Ore Pellet Reduced by H2–CO Mixtures. Powder Technol. 2015, 269, 290–295. [Google Scholar] [CrossRef]
- Sharma, T.; Gupta, R.C.; Prakash, B. Effect of Gangue Content on the Swelling Behaviour of Iron Ore Pellets. Miner. Eng. 1990, 3, 509–516. [Google Scholar] [CrossRef]
- Behera, P.R.; Bhoi, B.; Paramguru, R.K.; Mukherjee, P.S.; Mishra, B.K. Hydrogen Plasma Smelting Reduction of Fe2O3. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2019, 50, 262–270. [Google Scholar] [CrossRef]
- Sabat, K.C.; Murphy, A.B. Hydrogen Plasma Processing of Iron Ore. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2017, 48, 1561–1594. [Google Scholar] [CrossRef]
- Souza Filho, I.R.; Springer, H.; Ma, Y.; Mahajan, A.; da Silva, C.C.; Kulse, M.; Raabe, D. Green Steel at Its Crossroads: Hybrid Hydrogen-Based Reduction of Iron Ores. J. Clean. Prod. 2022, 340, 130805. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Brooks, G.; Rhamdhani, M.A. Decarbonisation and Hydrogen Integration of Steel Industries: Recent Development, Challenges and Technoeconomic Analysis. J. Clean. Prod. 2023, 395, 136391. [Google Scholar] [CrossRef]
- Boretti, A. The Perspective of Hydrogen Direct Reduction of Iron. J. Clean. Prod. 2023, 429, 139585. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Pearson Education Limited: Harlow, UK, 2015; ISBN 978-1-292-04054-7. [Google Scholar]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.M.; Scott, J.H.J.; Joy, D.C. Scanning Electron Microscopy and X-ray Microanalysis; Springer: New York, NY, USA, 2017; 550p. [Google Scholar] [CrossRef]
- Hessels, C. Reduction of Combusted Iron Using Hydrogen. Ph.D. Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 2023. [Google Scholar]
- Ali, A.; Zhang, N.; Santos, R.M. Mineral Characterization Using Scanning Electron Microscopy (SEM): A Review of the Fundamentals, Advancements, and Research Directions. Appl. Sci. 2023, 13, 12600. [Google Scholar] [CrossRef]













| Project | Country | Specification |
|---|---|---|
| H2 FUTURE | Austria, Gemany, Netherlands | Initiated by Voestalpine, VERBUND, and Siemens, this project aims to produce green hydrogen using renewable electricity. This is accomplished through the operation of a 6 MW PEM electrolyzer located at the Voestalpine Linz steelworks [1]. |
| H2 Green Steel | Sweden | The goal of this initiative is to establish a facility capable of producing green hydrogen, intended to replace natural gas in pig iron manufacturing and to electrify the entire process. The only emissions from this operation will be water vapor. Production is scheduled to commence in 2024 [1]. |
| Carbon2Chem | Germany | This project focuses on converting by-product gases from the steel industry into chemicals such as ammonia, methanol, and polymers, while also recycling CO2 emitted during steel production [23]. |
| SALCOS | Germany | The project aims to produce green hydrogen by using wind energy to electrolyze water, generating hydrogen and oxygen for use in iron production [23]. |
| GrInHy 2.0 | Germany | The project focuses on producing green hydrogen via high-temperature water electrolysis, utilizing electricity solely from renewable sources. It also harnesses waste heat from the steel industry to generate water vapor [1]. |
| HYBRIT | Sweden | HYBRIT technology aims to replace coking coal in iron and steel production with emission-free electrical energy and hydrogen, striving to develop the world’s first fossil fuel-free steel production technology [24]. |
| ULCOWIN, ULCOLYSIS | EU’s ULCOS project, which involved many European steelmakers | The ULCOS projects aims to electrolyze iron ore with zero carbon emissions [1]. |
| HELIOS | Netherlands, Finland, Austria, Belgium | The project aims to train 10 doctoral candidates in green steel production using hydrogen. It combines research, internships, and mentorship from leading companies, universities such as KU Leuven and TU Delft, and research centers like K1-MET [25]. |
| H2STEEL | Italy, Netherlands, Spain, France | The EU-funded H2STEEL project aims to transform organic waste and biomethane into green hydrogen, carbon, and essential materials using a novel catalytic pyrolysis technique in a new reactor. This approach facilitates cost-effective production [26]. |
| HyInHeat | Spain, Italy, Finland, Belgium, Norway, Sweden, Germany, Greece, Austria, Netherlands | The EU-funded HyInHeat project aims to utilize hydrogen for high-temperature heating in the aluminum and steel industries. It focuses on redesigning processes and employing simulations to enhance energy efficiency and minimize hydrogen consumption [27]. |
| Hybrid hydrogen-based reduction of Fe ores | Germany | The project combines hydrogen direct reduction (H-DR) and hydrogen plasma reduction (HPR) techniques to efficiently reduce and transform Fe ore, aiming to enhance energy and hydrogen utilization [28]. |
| Hydrogen plasma-based reduction of Fe ores | Germany | The project focuses on researching the utilization of hydrogen plasma (HPR) [29]. |
| HARARE | Norway, Greece, Germany, Belgium, Sweden | The HARARE project is dedicated to using hydrogen to recover metals and minerals from metallurgical waste, demonstrating sustainable, carbon-free methods for metal production and material valorization [30]. |
| DRI expansion Project | China | Baosteel Zhan-jiang iron and steel project focused on the production of H-DRI at a volume of 1 Mt/year [31]. |
| Researched Material | Fe Ores/Concentrates | Fe Pellets | Fe Synthetic Powders | |||
|---|---|---|---|---|---|---|
| Used Device | Fluidization Device | Laboratory Device | TGA Device | Laboratory Device | TGA Device | Laboratory Device |
| Grain size | 10–500 µm | 44 µm–33 mm | 8–15 mm | 8–16 mm | 1–5 μm | 5 μm–5 mm |
| Sample weight | 5–500 g | 1–1000 g | - | - | 10–20 mg | 50 mg |
| Reaction time | 4–60 min | 30–150 min | 120–150 min | 45–120 min | - | - |
| Reaction temperatures | 400–800 °C | 400–900 °C | 800–1100 °C | 700–1050 °C | 550–1580 °C | 25–900 °C |
| Reducing gas | various ratios of H2:N2:CO; pure gases H2, CO | various ratios of H2:N2:CO; pure gas H2 | various ratios of H2:N2:CO; pure gases H2, CO | various ratios of H2:CO:CO2:N2; pure gas H2 | various ratios of H2:Ar:CO:H2O; pure gas H2 | various ratios of H2/N2, CO/N2; pure gas H2 |
| Fe Ores/Concentrates | |||
|---|---|---|---|
| Material | Device/Reduction Parameters | Results | Reference |
| Iron ore–96.8% Fe2O3 samle weight: 8 g grain size: 106–150 μm | Fluidization device gas flow: 1.5 L/min reaction time: 4–60 min reaction temperatures: 600–800 °C reducing gas: H2, CO, N2 in various mixtures | The reduction of fine-grained iron ore takes place more intensively with pure hydrogen than with pure CO or the H2-CO mixture. | Tao Zhang et al. [47] |
| Powder magnetite ore sample weight: 5 g | Fluidization device Inner diameter: 25 mm gas flow: 1000 mL/min reaction temperatures: 495–570 °C reducing gas: 80% H2: 20% N2 | The degree of reduction increased with the increase in reduction temperatures. The reaction rate increased as the reduction temperature increased. | Peiyu Li et al. [54] |
| Iron ore sample weight: 400 g grain size: 250–500 µm | Fluidization device Inner diameter: 68 mm gas flow: 25.9 NL/min reaction temperatures: 600–800 °C reducing gas: 65% H2: 35% N2 | Higher temperature increases the rate of reduction, especially in the intermediate and final stages of reduction. | Spreitzer D. et al. [45] |
| Fine-grained concentrate based on Fe2O3 sample weight: 170 g | Fluidization device Inner diameter: 26 mm Height: 160 mm gas flow rate: 0.2 m/s reaction temperatures: 500–600 °C reducing gas: H2 | The reduction yield for reduction in a fluidized bed using vibration ranged from 90 to 98% after approx. 50 min of reduction, while the increased frequency (47 Hz) supports the yield. | Shuo Li et al. [55] |
| Low-grade magnetite-based iron ore particle size ranges: 125–250 μm, 250–500 μm, and full size (mixed) | Fluidization device Inner diamter: 68 mm gas flow: 15.9 L/min reaction temperature: 700 °C reducing gas: H2 | Materials with higher temperature pre-oxidation treatment showed better fluidization behaviors. Lower pre-oxidation temperature is more beneficial for the reduction rate. | Zheng H. et al. [56] |
| Hematite ore grain size: 2 mm sample weight: 1 kg | Fixed bed reactor gas flow: 120 L/h reaction time: 30–150 min reaction temperatures: 400–500 °C | At a reduction temperature of 500 °C, the amount of reduced Fe2O3 increased rapidly at the beginning and then increased slowly. The reaction rate increased with increasing temperature. | Wenguang Du et al. [42] |
| Limonite Fe ore grain size: 44–89 μm sample weight: 5 g | Horizontal tube electric furnace Inner diameter: 16 mm Lenght: 950 mm gas flow: 200 mL/min reaction time: 30 min reaction temperatures 700–900 °C reducing gas: H2, N2 | SEM analyses of the partially and fully reduced particles showed that the iron oxide in the ore was reduced to metallic iron. As the reduction time increased, the reduced Fe formed a shell surrounding the unreacted oxides. | Zheng Wei et al. [36] |
| Hematite fine-grained ore grain size: 53–63 μm, 75–90 μm, 100–110 μm | Tube furnace Inner diameter: 100 mm Lenght: 1300 mm reaction time: 5–25 min reaction temperatures: 1450–1550 °C reducing gas: 40% H2: 60% N2 | The effect of particle size 53–110 μm on the degree of reduction is negligible. | Yingxia Qu et al. [46] |
| Low grade iron ore Grain size: 0.5–33 mm | Laboratory device gas flow: 0.5–1.5 L/min reaction time: 30–90 min reaction temperatures: 300–600 °C reducing gas: 100% H2 | Quantitative XRD analysis indicates that magnetite conversion is ~60% at 300 °C, 100% at 400 °C with ~10% ferrite formation, and ferrite formation increased to ~90% at 600 °C. | Nikhil Dhawan et al. [57] |
| Fe Pellets | |||
| Material | Device/Reduction Parameters | Results | Reference |
| KRPS pellets CVRD pellets sample weight: 250 g | TGA device gas flow: 5 L/min. reaction time: 120 min reaction temperatures: 812/822 °C reducing gas: CO/H2 in various ratios | Pellets reduced in H2 swell easily due to the rapid phase transformation (hematite-magnetite-wüstite-iron) which is associated with the higher degree of reduction observed in H2. | Nyankson E. et al. [37] |
| Iron ore pellets from Outotec diameter: 10–13 mm sample weight: 3–3.7 g | TGA device gas flow: 1 L/min. reaction time: 150 min reaction temperatures: 800–1100 °C reducing gas: H2, CO | Higher reduction kinetics and thus higher raw material conversion are achieved in an H2 atmosphere than in a CO atmosphere. In the presence of H2, the porosity is greater. The pellets are almost completely reduced to metallic Fe by H2 at 800 °C. | Scharm Ch. et al. [58] |
| Iron pellets with biomass and bentonite pellet size: 10 × 15 mm | Laboratory device gas flow: 7 cm3/s reaction time: 30 min reaction temperatures: 850–1050 °C reducing gas: H2, N2 | The time required to reach 99% reduction range is shorter when biomass is added to the pellets at the same reduction temperature, reflecting the accelerated rate of the reduction reaction. | Dabin Guo et al. [48] |
| Sintered pellets made of hematite powder–99.5% hematite | Laboratory device Inner diameter: 24 mm Height: 105 mm gas flow: 0.75 L/min reaction temperatures: 700–850 °C reducing gas: H2/CO: 1.5 | The reduction rate is higher for pellets with high porosity. Pellets with a lower density have a higher reduction range and reduction rate. | Dongchen Wang et al. [40] |
| Pellets diameter: 12 mm | Laboratory device gas flow: 5 L/min reaction temperatures: 800–1000 °C reducing gas: H2/CO in various ratios | The rate of reduction was highest using H2, the lowest rate was obtained using CO, and the rate of reduction using a gaseous mixture of H2 and CO was intermediate. | Hai-bin Zuo et al. [51] |
| Pellets from industrial production diameter: 10–12.5 mm sample weight: 500 g | Laboratory device gas flow: 15 L/min reaction temperatures: 700–1000 °C reducing gas: CO:H2:N2 in various ratios | During the initial stages of reduction, the rate of reduction proceeds rapidly and the degree of reduction increases rapidly, then becomes less pronounced in the later stages due to the resistance of the product layer. | Qing Lyu et al. [59] |
| Fe pellets from Shouqin metal diameter: 10–12.5 mm sample weight: 500 g | Laboratory device Diameter: 75 mm Height: 800 mm gas flow: 12 L/min. reaction time: 45 min reaction temperatures: 760–1000 °C reducing gas: 75% H2:25% N2 | The highest degrees of reduction were recorded in the isothermal experiment at 900 °C. | Ming-Hua Bai et al. [35] |
| Fe Synthetic Powders | |||
| Material | Device/Reduction Parameters | Reduction Parameters | Reference |
| Synthetically prepared samples of pure hematite | Electric resistance furnace gas flow: 70 cm3/min reaction time: 6–18 min reaction temperatures: 550–900 °C reducing gas: H2 | The reduction in hematite to magnetite and the reduction in magnetite to wüstite are very fast. The slowest step in reduction reactions is the transformation of wüstite into iron. | Damien Wagner et al. [43] |
| Synthetic samples of a-Fe2O3, a-FeOOH, Fe5HO8.4H2O, Fe3O4, FeO | TGA device gas flow–50 cm3/min reaction temperatures: 0–700 °C reducing gas: 5% H2–95% Ar, 5% CO–95% Ar | Complete reduction in hematite to the metallic iron phase can be achieved even at a low temperature of up to 380 °C in pure hydrogen. | W.K. Jozwiak et al. [50] |
| Hematite powder sample weight: 0.013–0.019 g mean grain diameter: 0.74 µm | TGA device gas flow: 0.1 L/min reaction temperatures: 550–1300 °C reducing gas: H2:CO in various ratios | The reduction rate in hematite particles increased with temperature, increasing H2 molecular fraction and atmospheric temperature. The reduction efficiency from Fe2O3 to Fe was more than 99.9% above the temperature of 800 °C. | Jeongseog Oh et al. [33] |
| Laboratory prepared pure FeO sample weight: 20 mg | TGA device Inner diameter: 25 mm gas flow: 20 mL/min reaction temperatures: 900–1580 °C reducing gas: H2 gas flow: 20 mL/min | The reduction conversion tends to increase with increasing reduction temperature during the same reduction time, regardless of the phase state of the reactants and products. | Jianliang Zhang et al. [41] |
| Iron oxide Fe2O3: purity 97% sample weight: 50 mg | Analyzer Micromeritic Autochem 2920 gas flow: 20 mL/min reaction temperatures: 150–900 °C reducing gas: H2:N2, CO:N2 in various ratios | H2 is a better reducing agent than CO in the complete reduction of Fe2O3 to Fe. CO is a more effective reducing agent to initiate the reduction. | Maratun Najiha Abu Tahari et al. [60] |
| Hematite powders: purity 99.5% sample weight: 50 mg | Laboratory device gas flow: 50 mL/min reaction temperatures: 25–600 °C reducing gas: 100% H2 | During reduction at a heating rate of 20 °C/min, a phase transformation from a mixture of iron and magnetite to wüstite occurred already at a temperature above 510 °C. | Zhiyuan Chen et al. [38] |
| Iron oxide powder 40 wt% hematite (Fe2O3), 58 wt% magnetite (Fe3O4) | TGA device hydrogen partial pressure: 0.25–1 atm reaction temperatures: 400–900 °C reducing gas: H2 in various ratios | Full conversion to metallic iron was reached faster at 500 °C than at higher temperatures. Fewer, but larger pores were observed for reduction at higher temperature. | C.J.M. Hessels et al. [61] |
| Green compacts of hematite | TGA device gas flow: 1000 mL/min reaction temperatures: 500–1000 °C reducing gas: H2 | The reduction of porous and dense samples reveals the presence of reduction rate minimum at 650 °C which was attributed to sintering and densification of the freshly reduced iron around the oxide grains. | El-Geassy et al. [62] |
| Hematite powder particle size: 74 µm | Tubular reduction furnace reaction temperatures: 600–1050 °C gas flow: 3.0 NL/min reducing gas: H2 | The reduction rate of pure hematite with hydrogen linearly increased with temperatures from 600 to 1000 °C, without a rate minimum in this temperature range. Above 1000 °C, the reduction rate decreased due to sintering phenomena. | Junguo He et al. [63] |
| Single magnetite crystals | Tube furnace reaction temperatures: 900–1100 °C reaction time: 420 min reducing gas: H2 | The reduction rate increases with reaction temperature. The reduction rate was found to be controlled by the solid-state diffusion mechanism. | Bahgat. M. et al. [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miškovičová, Z.; Legemza, J.; Demeter, P.; Buľko, B.; Hubatka, S.; Hrubovčáková, M.; Futáš, P.; Findorák, R. An Overview Analysis of Current Research Status in Iron Oxides Reduction by Hydrogen. Metals 2024, 14, 589. https://doi.org/10.3390/met14050589
Miškovičová Z, Legemza J, Demeter P, Buľko B, Hubatka S, Hrubovčáková M, Futáš P, Findorák R. An Overview Analysis of Current Research Status in Iron Oxides Reduction by Hydrogen. Metals. 2024; 14(5):589. https://doi.org/10.3390/met14050589
Chicago/Turabian StyleMiškovičová, Zuzana, Jaroslav Legemza, Peter Demeter, Branislav Buľko, Slavomír Hubatka, Martina Hrubovčáková, Peter Futáš, and Róbert Findorák. 2024. "An Overview Analysis of Current Research Status in Iron Oxides Reduction by Hydrogen" Metals 14, no. 5: 589. https://doi.org/10.3390/met14050589






