Microscopic Mechanism and Reagent Activation of Waste Glass Powder for Solidifying Soil
Abstract
:1. Introduction
2. Experimental Section
2.1. Production of Glass Powder
2.2. Selection of Activators
2.3. Preparation
3. Results and Discussion
3.1. Unconfined Compressive Strength
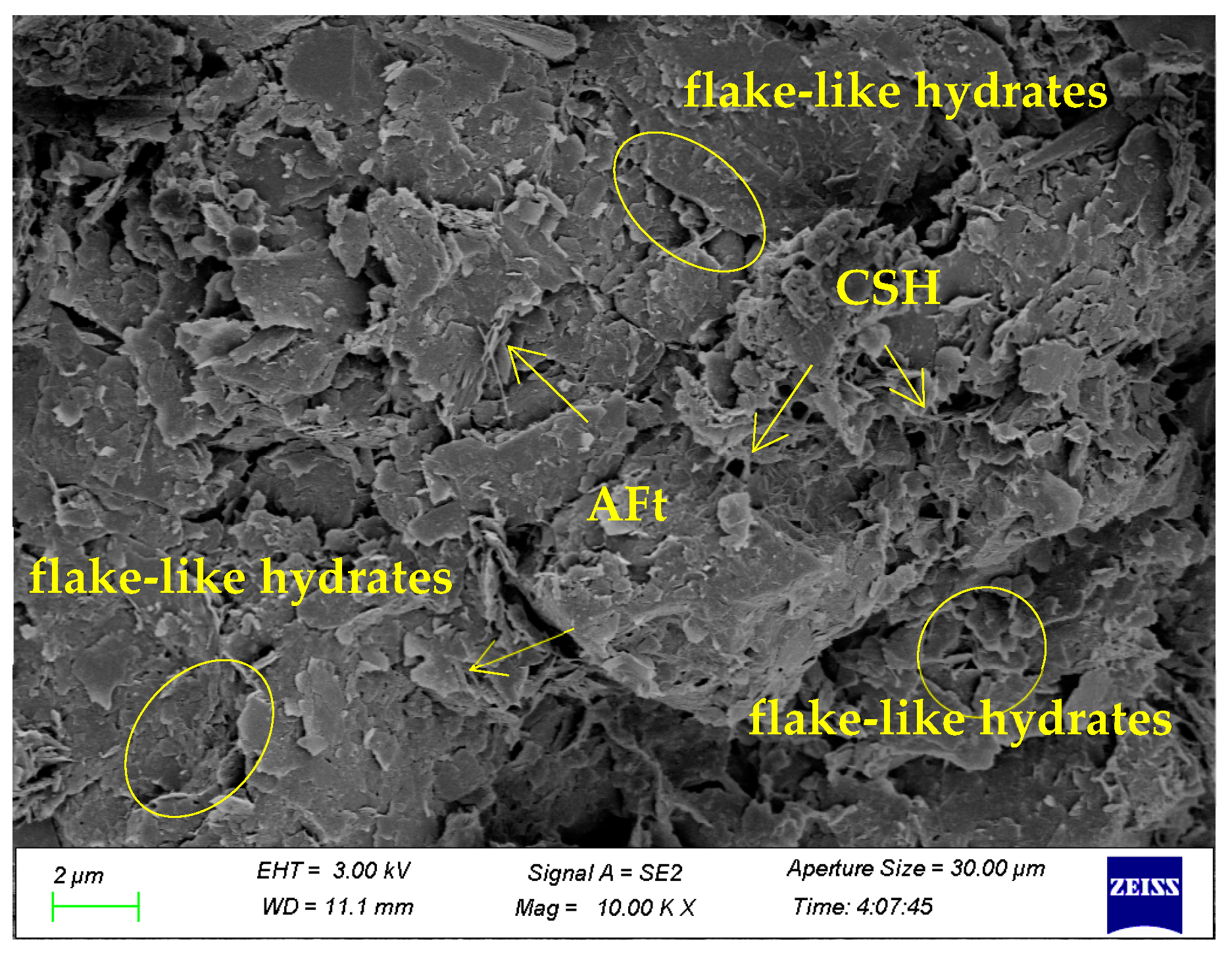
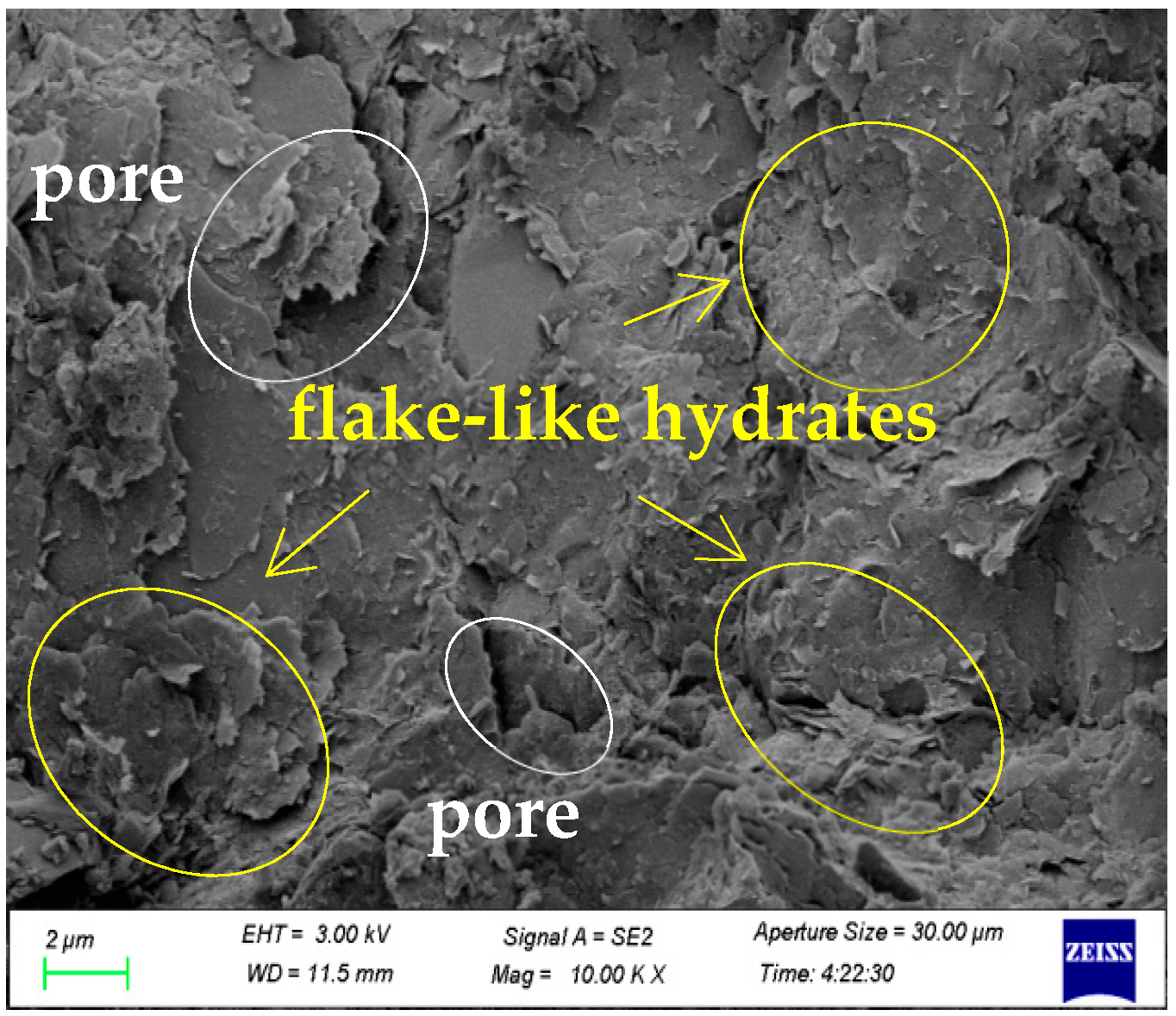
3.2. Scanning Electron Microscopic (SEM) Analysis
3.3. ImageJ Microstructure Analysis
3.4. X-ray Diffractometer (XRD) Analysis
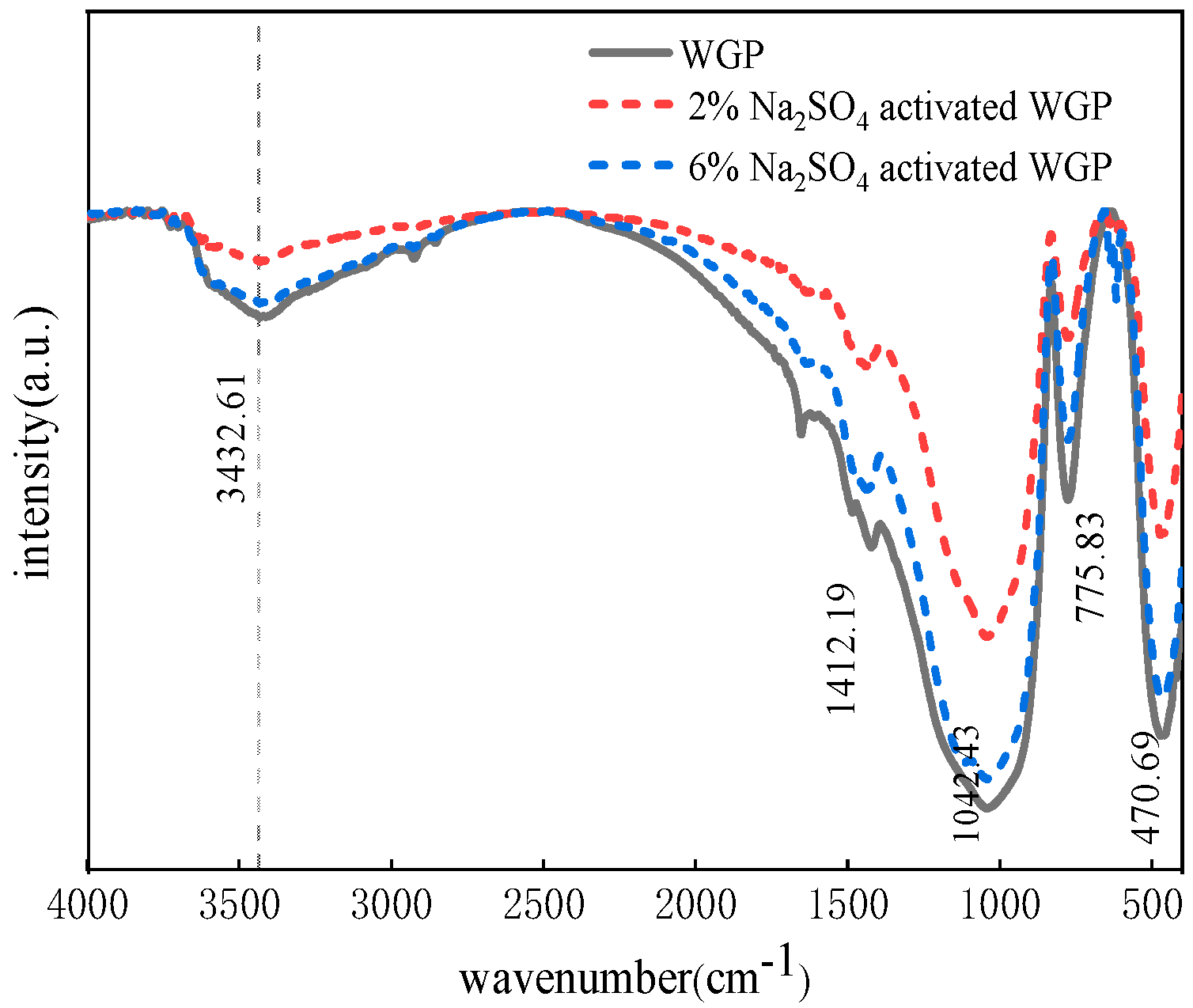
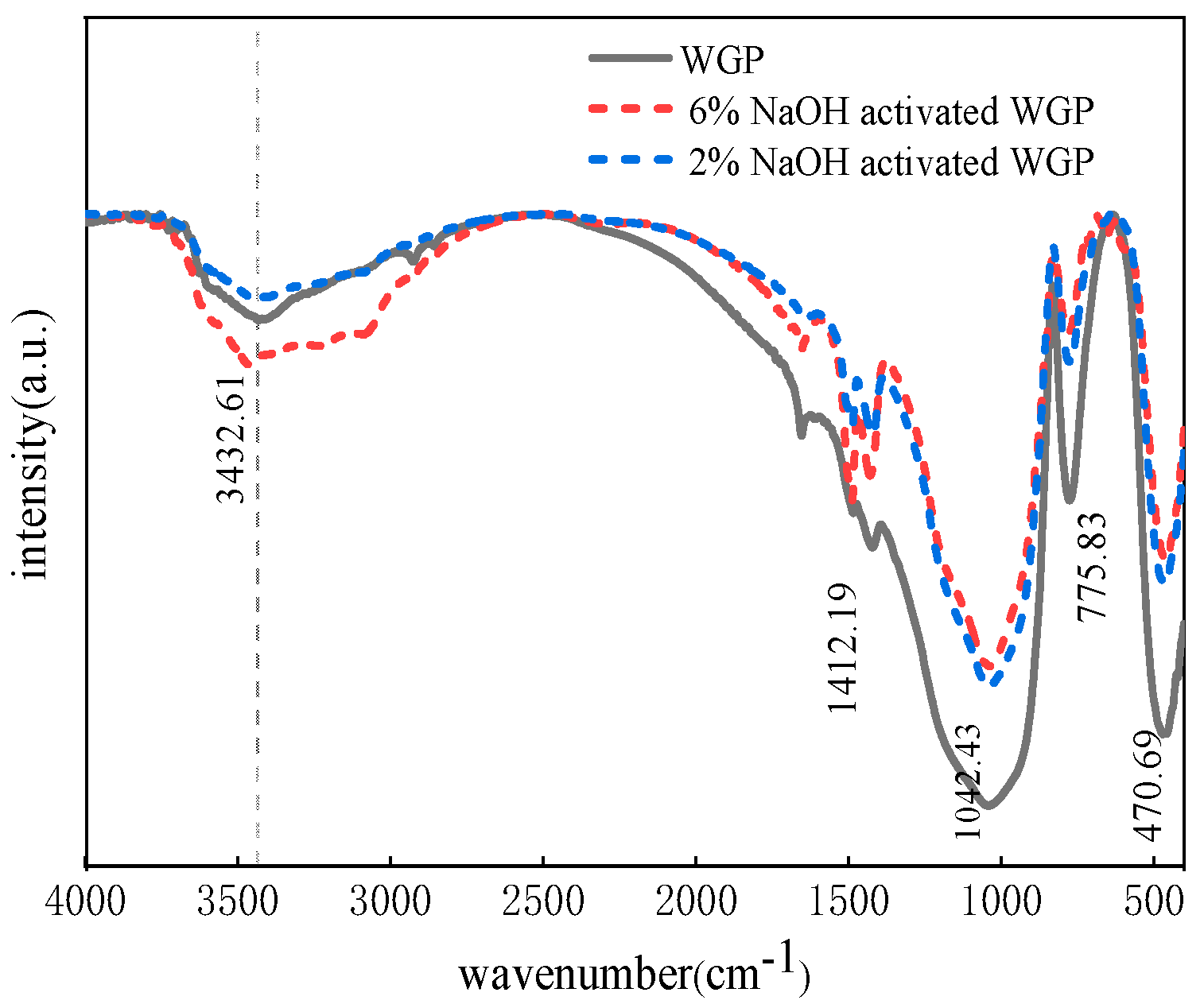
3.5. Fourier-Transform Infrared Spectroscopic (FTIR) Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jain, J.A.; Neithalath, N. Chloride transport in fly ash and glass powder modified concretes—Influence of test methods on microstructure. Cem. Concr. Compos. 2010, 32, 148–156. [Google Scholar] [CrossRef]
- Nassar, R.U.D.; Soroushian, P. Green and durable mortar produced with milled waste glass. Mag. Concr. Res. 2012, 64, 605–615. [Google Scholar] [CrossRef]
- Li, A.; Qiao, H.; Li, Q.; Hakuzweyezu, T.; Chen, B. Study on the performance of pervious concretemixed with waste glass powder. Constr. Build. Mater. 2021, 300, 123997. [Google Scholar] [CrossRef]
- Khmiri, A.; Chaabouni, M.; Samet, B. Chemical behaviour of ground waste glass when usedas partial cement replacement in mortars. Constr. Build. Mater. 2013, 44, 74–80. [Google Scholar] [CrossRef]
- Shayan, A.; Xu, A. Value-added utilisation of waste glass in concrete. Cem. Concr. Res. 2004, 34, 81–89. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.; Li, X.; Yuan, Y. Assessing the modification efficiency of waste glass powder in hydraulic construction materials. Constr. Build. Mater. 2020, 263, 120111. [Google Scholar] [CrossRef]
- Song, B. Experimental Study on Activation of Waste Glass Powder. Master’s Thesis, Nanhua University, Chiayi, China, 2013. [Google Scholar]
- Du, H.J.; Tan, K.H. Use of waste glass as sand in mortar: Part II-alkali-silicareaction and mitigation methods. Cem. Concr. Compos. 2013, 35, 118–126. [Google Scholar] [CrossRef]
- Zhao, H.; Ke, G.; Song, B.; Zou, P. Effect of waste glass pow der on hydration products of cement paste and structure of plastic sand. Concrete 2018, 10, 106–109+122. [Google Scholar]
- Ali, E.E.; Al-Tersawy, S.H. Recycled glass as a partial replacement for fine aggregate in self compacting concrete. Constr. Build. Mater. 2012, 35, 785–791. [Google Scholar] [CrossRef]
- Lam, C.S.; Poon, C.S.; Chan, D. Enhancing the performance of pre-cast concrete blocks incorporating waste glass e ASR consideration. Cem. Concr. Compos. 2007, 29, 616–625. [Google Scholar] [CrossRef]
- De Moura, A.A.; Effting, L.; Moisés, M.P.; Carbajal, G.G.A.; Tarley, C.R.T.; Câmara, E.; Gracioli, A.; Bail, A. The influence of calcium-rich environments in siliceous industrial residues on the hydration reaction of cementitious mixtures. J. Clean. Prod. 2019, 225, 152–162. [Google Scholar] [CrossRef]
- Geng, C.; Wu, X.; Yao, X.; Wang, C.; Mei, Z.; Jiang, T. Reusing waste glass powder to improve the strength stability of cement at HTHP. J. Pet. Sci. Eng. 2022, 213, 110394. [Google Scholar] [CrossRef]
- Varma, D.N.; Singh, S.P. A Review on Waste Glass-based Geopolymer Composites as a Sustainable Binder. Silicon 2023, 15, 7685–7703. [Google Scholar] [CrossRef]
- Cai, Y.; Xuan, D.; Poon, C.S. Effects of nano-SiO2 and glass powder on mitigating alkali-silica reaction of cement glass mortars. Constr. Build. Mater. 2019, 201, 295–302. [Google Scholar] [CrossRef]
- Schwarz, N.; Cam, H.; Neithalath, N. Influence of a fine glass powder on the durability characteristics of concrete and its comparison to fly ash. Cem. Concr. Compos. 2008, 30, 486–496. [Google Scholar] [CrossRef]
- Yang, J. Study on the Auxiliary Cementitious Effect of Waste Glass Micro Powder in Recycled Concrete. Master’s Thesis, Kunming University of Science and Technology, Kunming, China, 2010. [Google Scholar]
- Shi, C.J.; Day, R.L. Comparison of different methods for enhancing reactivity of pozzolans. Cem. Concr. Res. 2001, 31, 813–818. [Google Scholar] [CrossRef]
- Idir, R.; Cyr, M. Pozzolanic properties of fine coarse color-mixed glass cullet. Cem. Concr. Compos. 2011, 33, 19–29. [Google Scholar] [CrossRef]
- Shi, C.; Zheng, K. A review on the use of waste glasses in the production of cement and concrete. Resour. Conserv. Recycl. 2007, 52, 234–247. [Google Scholar] [CrossRef]
- Dyer, T.D.; Dhir, R.K. Chemical Reaction of Glass Cullet used as Cement Component. Mater. Civ. Eng. 2001, 13, 412–417. [Google Scholar] [CrossRef]
- Shi, C.; Wu, Y.; Riefler, C.; Wang, H. Characteristics and pozzolanic reactivity of glass powders. Cem. Concr. Res. 2005, 35, 987–993. [Google Scholar] [CrossRef]
- Archibald, J.F.; DeGagne, D.O.; Lausch, P.; De Souza, E.M. Ground waste glass as a pozzolanic consolidation agent for mine backfill. CIM Bull. 1995, 88, 80–87. [Google Scholar]
- Fan, L.; Liu, G.; Su, H.; Jin, D.; Lu, R.; Li, L. The influence of glass powder particle size distribution on the properties of cementitious materials. Sci. Technol. Eng. 2016, 16, 271–274. [Google Scholar]
- Yue, L.; Ji, T.; Zhou, Y.; Wang, S.; Jiang, M.; Fu, T.; Zhang, S. Active Excitation of Waste Glass Powder and Its Effect on the Properties of Mortar. Concr. Cem. Prod. 2022, 7, 97–100. [Google Scholar]
- Kong, Q. Research on the Activity of Waste Glass Powder by Chemical Excitation and Hydrothermal Excitation. Master’s Thesis, South China University, Chiayi, China, 2014. [Google Scholar]
- Shi, C.; Day, R.L. Pozzolanic reaction in the presence of chemical activators Part I. Reaction kinetics. Cem. Concr. Res. 2000, 30, 51–58. [Google Scholar] [CrossRef]
- Li, Z. Study on Characterizing the Volcanic ash Activity of Waste Glass Powder Using pH Value and Conductivity. Master’s Thesis, South China University, Chiayi, China, 2014. [Google Scholar]
- Zhang, C.; Wang, J.; Song, W.; Fu, J. Effect of Waste Glass Powder on Pore Structure, Mechanical Properties and Microstructure of Cemented Tailings Backfill; Elsevier Ltd.: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Yu, X. Study on The Influence of Glass Powder on The Microcosmic and Mechanical Properties of Concrete. Master’s Thesis, Guilin University of Technology, Guilin, China, 2020. [Google Scholar]
- Zhao, Y.; Yin, H. Glass Technology; Chemical Industry Press: Beijing, China, 2006; Volume 412. [Google Scholar]
- Wang, Z.; Zheng, H.; Qian, J. Comparison of sulfate activation on fly ash activity. Compr. Util. Fly Ash 1999, 12, 15–18. [Google Scholar]
- Li, F.; Yang, J.; Li, L. Effect and Mechanism of Waste Glass Powder as Supplementary Cementitious Material on Mortar Properties. Silic. Bull. 2022, 41, 3208–3218. [Google Scholar]
- Lei, F. The Research on Hydration Activity and Mechanism of Cement Paste with Glass Powder. Master’s Thesis, Guilin University of Technology, Guilin, China, 2017. [Google Scholar]
- Niyomukiza, J.B. Use of waste glass powder in improving the properties of expansive clay soils. Glob. NEST Int. J. Glob. NEST J. 2023, 25, 139–145. [Google Scholar]
- Zhang, W.; Li, S.; Song, L.; Sheng, Y.; Xiao, J.; Zhang, T. Studying the Effects of Varied Dosages and Grinding Times on the Mechanical Properties of Mortar. Sustainability 2023, 15, 5936. [Google Scholar] [CrossRef]
- Xu, Y.; He, T. Effect of Mitigating Strength Retrogradation of Alkali Accelerator by the Synergism of Sodium Sulfate and Waste Glass Powder. J. Renew. Mater. 2021, 9, 1991–1999. [Google Scholar] [CrossRef]
- Dan, J.; Wang, P. Research on the variation law of ion concentration in early cement hydration liquid phase. J. Shihezi Univ. (Nat. Sci. Ed.) 2007, 25, 494–499. [Google Scholar]
- Haha, M.B.; Le Saout, G.; Winnefeld, F.; Lothenbach, B. Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast-furnace slags. Cem. Concr. Res. 2011, 41, 301–310. [Google Scholar] [CrossRef]
- Omran, A.F.; Etienne, D.; Harbec, D.; Tagnit-Hamou, A. Long-term performance of glass-powder concrete in large-scale field applications. Constr. Build. Mater. 2017, 135, 43–58. [Google Scholar] [CrossRef]
- Ke, G.; Zou, P.; Gan, Y.; Song, B.; Zeng, D. Strength Activity Characteristic and Mechanism of Waste Glass Powders. Chin. Powder Technol. 2015, 21, 87–92. [Google Scholar]
- Zheng, K.R.; Chen, L.; Zhou, J. Pozzolanic Reaction of Soda-lime Glass and Its influences on Composition of Calcium Silicate Hydrate. J. Ceram. 2016, 44, 202–210. [Google Scholar]
- Tang, Q. Engineering Properties and Mechanism of Soft Soil Solidified by Phosphogypsum—Mineral Powder—Carbide Slag Composite Solid Waste Gelling Agent l. Master’s Thesis, Donghua University, Shanghai, China, 2023. [Google Scholar]
- Wang, W. Study on the Effect of Stone Powder on the Properties of Alkali-Activated Slag System. Master’s Thesis, Guangzhou University, Guangzhou, China, 2023. [Google Scholar]
- Li, J. Research on Carbonization of Waste Concrete Powder and Its Application in Brickmaking. Master’s Thesis, Guangzhou University, Guangzhou, China, 2023. [Google Scholar]
- Liu, G.; Florea, M.V.A.; Brouwers, H.J.H. Waste glass as binder in alkali activated slag–fly ash mortars. Mater. Struct. 2019, 52, 101. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.; Rose, V.; De Gutierrez, R.M. Evolution of binder structure in sodium silicate-activated slag-metakaolin blends. Cem. Concr. Compos. 2011, 33, 46–54. [Google Scholar] [CrossRef]















| 0 h | Particle-size composition | ||||
| Particle size (mm) | 0.5–1 | 0.25–0.5 | 0.075–0.25 | 0–0.075 | |
| Content (%) | 0.9 | 5.8 | 57.97 | 35.33 | |
| 6 h | Particle-size composition | ||||
| Particle size (mm) | 0.075–0.25 | 0–0.075 | |||
| Content (%) | 20.6 | 79.4 | |||
| 12 h | Particle-size composition | ||||
| Particle size (μm) | 0–5.23 | 5.23–14.78 | 14.78–41.8 | ||
| Content (%) | 29.16 | 28.37 | 42.47 | ||
| SiO2 (%) | Al2O3 (%) | CaO (%) | MgO (%) | Na2O (%) | Fe2O3 (%) |
|---|---|---|---|---|---|
| 72 | 2.5 | 8.25 | 2 | 14.6 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Y.; Xu, X.; Zhang, C.; Cheng, Z.; Yang, G. Microscopic Mechanism and Reagent Activation of Waste Glass Powder for Solidifying Soil. Buildings 2024, 14, 1443. https://doi.org/10.3390/buildings14051443
Hong Y, Xu X, Zhang C, Cheng Z, Yang G. Microscopic Mechanism and Reagent Activation of Waste Glass Powder for Solidifying Soil. Buildings. 2024; 14(5):1443. https://doi.org/10.3390/buildings14051443
Chicago/Turabian StyleHong, Yuze, Xinyi Xu, Chaojie Zhang, Zehai Cheng, and Guanshe Yang. 2024. "Microscopic Mechanism and Reagent Activation of Waste Glass Powder for Solidifying Soil" Buildings 14, no. 5: 1443. https://doi.org/10.3390/buildings14051443




