Bacillus siamensis Improves the Immune Status and Intestinal Health of Weaned Piglets by Improving Their Intestinal Microbiota
Abstract
1. Introduction
2. Material and Methods
2.1. Animals and Diets
2.2. Sample and Data Collection
2.3. Determination of Serum Biochemistry
2.4. Determination of Immunity, Inflammation, Gut Permeability and Growth Factors
2.5. Analysis of SCFAs
2.6. DNA Extraction, PCR and Library Construction and Sequencing
2.7. Analysis of Data
3. Results
3.1. Growth Performance
3.2. Serum Biochemistry
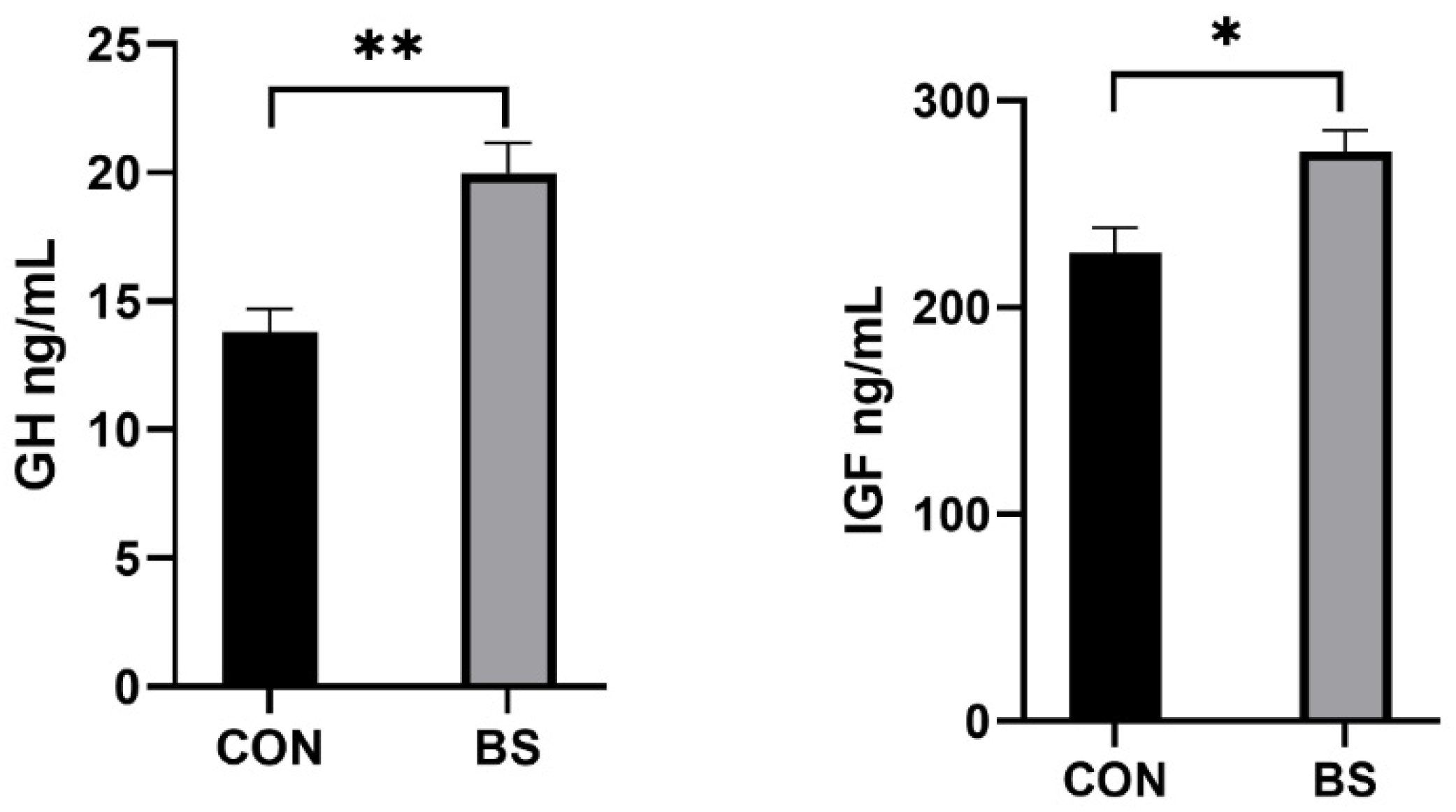
3.3. Serum Growth Factor
3.4. Serum Immunity and Inflammation
3.5. Gut Permeability
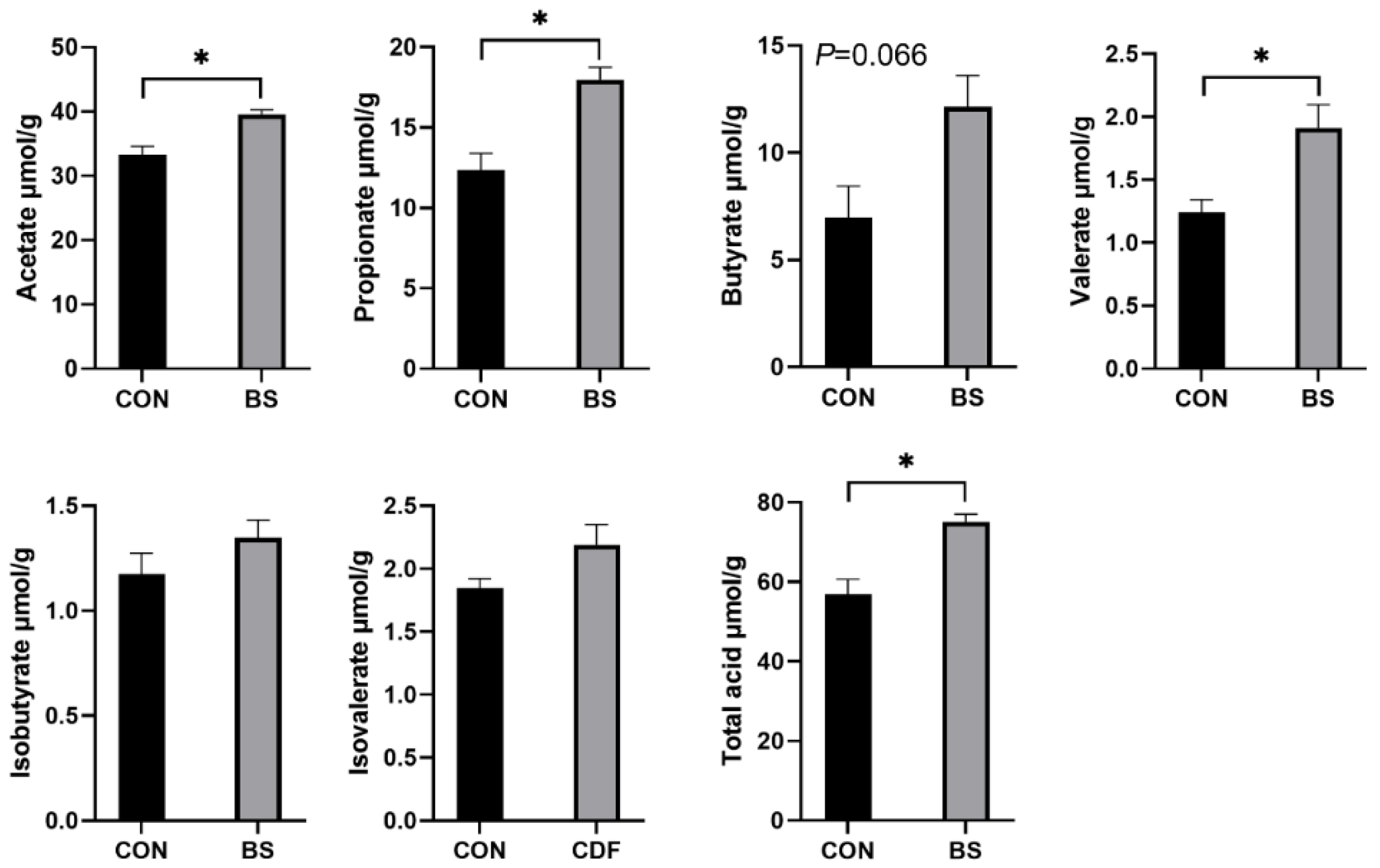
3.6. Fecal SCFA Content
3.7. Fecal Microbiota
3.8. Spearman’s Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J.; Hu, N.Q.; Mao, Y.Q.; Hu, A.M.; Jiang, W.J.; Huang, A.M.; Wang, Y.; Meng, P.Y.; Hu, M.W.; Yang, X.B.; et al. Traditional Chinese medicine prescriptions (XJZ, JSS) ameliorate spleen inflammatory response and antioxidant capacity by synergistically regulating NF-κB and Nrf2 signaling pathways in piglets. Front. Vet. Sci. 2022, 9, 993018. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tan, X.; Wang, H.R.; Wang, Q.Y.; Huang, P.F.; Li, Y.L.; Li, J.Z.; Huang, J.; Yang, H.S.; Yin, Y.L. Effects of varying dietary folic acid during weaning stress of piglets. Anim. Nutr. 2021, 7, 101–110. [Google Scholar] [CrossRef]
- Gilani, S.M.H.; Rashid, Z.; Galani, S.; Ilyas, S.; Sahar, S.; Hassan, Z.U.; Al-Ghanim, K.; Zehra, S.; Azhar, A.; Al-Misned, F.; et al. Growth performance, intestinal histomorphology, gut microflora and ghrelin gene expression analysis of broiler by supplementing natural growth promoters: A nutrigenomics approach. Saudi J. Biol. Sci. 2021, 28, 3438–3447. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wang, A.; Fu, M.; Wang, A.; Chen, K.; Jia, Q.; Huang, Z.Y. Investigation of Incidents and Trends of Antimicrobial Resistance in Foodborne Pathogens in Eight Countries from Historical Sample Data. Int. J. Environ. Res. Public Health 2020, 17, 472. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yin, Y.X.; Wang, F.; Bao, X.T.; Long, L.N.; Tan, B.; Yin, Y.L.; Chen, J.S. Effects of dietary rosemary extract supplementation on growth performance, nutrient digestibility, antioxidant capacity, intestinal morphology, and microbiota of weaning pigs. J. Anim. Sci. 2021, 99, skab237. [Google Scholar] [CrossRef]
- Hu, S.L.; Cao, X.F.; Wu, Y.P.; Mei, X.Q.; Xu, H.; Wang, Y.; Zhang, X.P.; Gong, L.; Li, W.F. Effects of Probiotic Bacillus as an Alternative of Antibiotics on Digestive Enzymes Activity and Intestinal Integrity of Piglets. Front. Microbiol. 2018, 9, 2427. [Google Scholar] [CrossRef] [PubMed]
- Faber, W.; Stolwijk-Swuste, J.; van Ginkel, F.; Nachtegaal, J.; Zoetendal, E.; Winkels, R.; Witteman, B. Faecal Microbiota in Patients with Neurogenic Bowel Dysfunction and Spinal Cord Injury or Multiple Sclerosis-A Systematic Review. J. Clin. Med. 2021, 10, 1598. [Google Scholar] [CrossRef] [PubMed]
- Vemuri, R.; Shinde, T.; Gundamaraju, R.; Gondalia, S.V.; Karpe, A.V.; Beale, D.J.; Martoni, C.J.; Eri, R. Lactobacillus acidophilus DDS-1 Modulates the Gut Microbiota and Improves Metabolic Profiles in Aging Mice. Nutrients 2018, 10, 1255. [Google Scholar] [CrossRef] [PubMed]
- Upreti, D.; Ishiguro, S.; Robben, N.; Nakashima, A.; Suzuki, K.; Comer, J.; Tamura, M. Oral Administration of Water Extract from Euglena gracilis Alters the Intestinal Microbiota and Prevents Lung Carcinoma Growth in Mice. Nutrients 2022, 14, 678. [Google Scholar] [CrossRef]
- Tian, Z.L.; Wang, X.D.; Duan, Y.H.; Zhao, Y.; Zhang, W.M.; Azad, M.A.K.; Wang, Z.B.; Blachier, F.; Kong, X.F. Dietary Supplementation With Bacillus subtilis Promotes Growth and Gut Health of Weaned Piglets. Front. Vet. Sci. 2021, 7, 600772. [Google Scholar] [CrossRef] [PubMed]
- Jinno, C.; Wong, B.D.; Klünemann, M.; Htoo, J.; Li, X.D.; Liu, Y.H. Effects of supplementation of Bacillus amyloliquefaciens on performance, systemic immunity, and intestinal microbiota of weaned pigs experimentally infected with a pathogenic enterotoxigenic E. coli F18. Front. Microbiol. 2023, 14, 1101457. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S.X.; Liu, H.; Zhang, D.Y.; Wang, Y.M.; Ji, H.F. Effects of oligosaccharides on the growth and stress tolerance of Lactobacillus plantarum ZLP001 in vitro, and the potential synbiotic effects of L. plantarum ZLP001 and fructo-oligosaccharide in post-weaning piglets. J. Anim. Sci. 2019, 97, 4588–4597. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.; Cao, G.T.; Zhang, H.R.; Li, Q.; Yang, C.M. Effects of Clostridium butyricum and Enterococcus faecalis on growth performance, immune function, intestinal morphology, volatile fatty acids, and intestinal flora in a piglet model. Food Funct. 2019, 10, 7844–7854. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.S.; Gu, W.; Liu, X.Y.; Zou, Y.W.; Wu, Y.J.; Xu, Y.H.; Han, D.D.; Wang, J.J.; Zhao, J.B. Joint Application of Lactobacillus plantarum and Bacillus subtilis Improves Growth Performance, Immune Function and Intestinal Integrity in Weaned Piglets. Vet. Sci. 2022, 9, 668. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Zhao, X.C.; Azad, M.A.; Ma, C.; Gao, Q.K.; He, J.H.; Kong, X.F. Dietary supplementation with Bacillus subtilis and xylo-oligosaccharides improves growth performance and intestinal morphology and alters intestinal microbiota and metabolites in weaned piglets. Food Funct. 2021, 12, 5837–5849. [Google Scholar] [CrossRef] [PubMed]
- Council, N.R. Nutrient Requirements of Swine: Eleventh Revised Edition; The National Academies Press: Washington, DC, USA, 2012; p. 420. [Google Scholar]
- González-Solé, F.; Solà-Oriol, D.; Ramayo-Caldas, Y.; Rodriguez-Prado, M.; Ortiz, G.G.; Bedford, M.R.; Pérez, J.F. Supplementation of xylo-oligosaccharides to suckling piglets promotes the growth of fiber-degrading gut bacterial populations during the lactation and nursery periods. Sci. Rep. 2022, 12, 11594. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.J.; Yang, Y.Z.; Bao, C.L.; Cao, Y.H. High-level expression of an acidic thermostable xylanase in Pichia pastoris and its application in weaned piglets. J. Anim. Sci. 2020, 98, skz364. [Google Scholar] [CrossRef]
- Chen, X.Y.; Zhao, X.; Wang, H.W.; Yang, Z.L.; Li, J.; Suo, H.Y. Prevent Effects of Lactobacillus Fermentum HY01 on Dextran Sulfate Sodium-Induced Colitis in Mice. Nutrients 2017, 9, 545. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Q.; Xia, Y.; Zhu, S.B.; Yang, J.; Yao, J.; Di, J.Z.; Liang, Y.; Gao, R.Y.; Wu, W.; Yang, Y.Z.; et al. Lactobacillus plantarum LP-Onlly alters the gut flora and attenuates colitis by inducing microbiome alteration in interleukin-10 knockout mice. Mol. Med. Rep. 2017, 16, 5979–5985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Ma, M.M.; Li, Z.Y.; Zhang, H.H.; He, X.; Song, Z.H. Protective Effects of L-Theanine on IPEC-J2 Cells Growth Inhibition Induced by Dextran Sulfate Sodium via p53 Signaling Pathway. Molecules 2021, 26, 7002. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.P.; Su, W.F.; Li, W.T.; Wen, C.Y.; Du, S.; He, H.; Zhang, Y.; Gong, T.; Wang, X.X.; Wang, Y.Z.; et al. Bacillus amyloliquefaciens 40 regulates piglet performance, antioxidant capacity, immune status and gut microbiota. Anim. Nutr. 2023, 12, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Jin, P.T.; Qin, S.Z.; Liu, J.Z.; Yang, Z.J.; Zhao, H.B.; Sheng, Q.K. Effects of dietary supplementation with S. platensis and probiotics on the growth performance, immune response and the fecal Lactobacillus spp. and E. coli contents of weaned piglets. Livest. Sci. 2019, 225, 32–38. [Google Scholar] [CrossRef]
- Cui, K.; Wang, Q.; Wang, S.Q.; Diao, Q.Y.; Zhang, N.F. The Facilitating Effect of Tartary Buckwheat Flavonoids and Lactobacillus plantarum on the Growth Performance, Nutrient Digestibility, Antioxidant Capacity, and Fecal Microbiota of Weaned Piglets. Animals 2019, 9, 986. [Google Scholar] [CrossRef] [PubMed]
- Duddeck, K.A.; Petersen, T.E.; Adkins, H.J.; Smith, A.H.; Hernandez, S.; Wenner, S.J.; Yao, D.; Chen, C.; Li, W.L.; Fregulia, P.; et al. Dose-Dependent Effects of Supplementing a Two-Strain Bacillus subtilis Probiotic on Growth Performance, Blood Parameters, Fecal Metabolites, and Microbiome in Nursery Pigs. Animals 2024, 14, 109. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Xiong, S.T.; Li, Z.; Wu, J.J.; Zhou, L.; Gui, J.F.; Mei, J. A feedback regulatory loop involving p53/miR-200 and growth hormone endocrine axis controls embryo size of zebrafish. Sci. Rep. 2015, 5, 15906. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.K.; Zhang, X.Y.; Wu, Y.J.; Che, D.S.; Ye, H.; Pi, Y.; Tao, S.Y.; Wang, J.J.; Han, D.D. Maternal Amino Acid Mixtures Supplementation during Late Gestation and Lactation Improved Growth Performance of Piglets through Improving Colostrum Composition and Antioxidant Capacity. Antioxidants 2022, 11, 2144. [Google Scholar] [CrossRef] [PubMed]
- Caputo, M.; Pigni, S.; Agosti, E.; Daffara, T.; Ferrero, A.; Filigheddu, N.; Prodam, F. Regulation of GH and GH Signaling by Nutrients. Cells 2021, 10, 1376. [Google Scholar] [CrossRef] [PubMed]
- Lavajoo, F.; Perelló-Amorós, M.; Vélez, E.J.; Sánchez-Moya, A.; Balbuena-Pecino, S.; Riera-Heredia, N.; Fernández-Borràs, J.; Blasco, J.; Navarro, I.; Capilla, E.; et al. Regulatory mechanisms involved in muscle and bone remodeling during refeeding in gilthead sea bream. Sci. Rep. 2020, 10, 184. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, C.; Wang, Z.S.; Cao, G.; Hu, R.; Wang, X.Y.; Zou, H.W.; Kang, K.; Peng, Q.H.; Xue, B.; et al. Active dry yeast supplementation improves the growth performance, rumen fermentation, and immune response of weaned beef calves. Anim. Nutr. 2021, 7, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.F.; Yue, S.Z.; Qiao, Y.H.; Sun, Z.J.; Wang, C.; Li, H.F. Dietary supplementation with selenium-enriched earthworm powder improves antioxidative ability and immunity of laying hens. Poult. Sci. 2020, 99, 5344–5349. [Google Scholar] [CrossRef] [PubMed]
- Huo, W.Y.; Li, M.; Wang, J.P.; Wang, Z.X.; Huang, Y.Q.; Chen, W. On growth performance, nutrient digestibility, blood T lymphocyte subsets, and cardiac antioxidant status of broilers. Anim. Nutr. 2019, 5, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Probiotics, Prebiotics and Synbiotics-A Promising Strategy in Prevention and Treatment of Cardiovascular Diseases? Int. J. Mol. Sci. 2020, 21, 9737. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.Q.; Du, J.; Li, T.T.; Gao, N.; Yang, S.Y.; Zhang, Y.X.; Pan, L.L. Elevated serum immunoglobulin level predicts high risk of 1-year recurrence in patients with Takayasu arteritis. Arthritis Res. Ther. 2023, 25, 36. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Zhang, Y.; He, W.T.; Wei, Y.H.; Han, S.S.; Xia, L.; Tan, B.; Yu, J.; Kang, H.Y.; Ma, M.E.; et al. Effects of Small Peptide Supplementation on Growth Performance, Intestinal Barrier of Laying Hens During the Brooding and Growing Periods. Front. Immunol. 2022, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Wang, T.H.; Lu, Z.Q.; Song, D.G.; Zhao, J.; Wang, Y.Z. Effects of Clostridium butyricum or in combination with Bacillus licheniformis on the growth performance, blood indexes, and intestinal barrier function of weanling piglets. Livest. Sci. 2019, 220, 137–142. [Google Scholar] [CrossRef]
- Wang, W.H.; Zhang, J.S.; Feng, T.; Deng, J.; Lin, C.C.; Fan, H.; Yu, W.J.; Bao, H.Y.; Jia, W. Structural elucidation of a polysaccharide from &ITFlammulina velutipes &ITand its immunomodulation activities on mouse B lymphocytes. Sci. Rep. 2018, 8, 3120. [Google Scholar] [CrossRef] [PubMed]
- Dreschers, S.; Gille, C.; Haas, M.; Grosse-Ophoff, J.; Schneider, M.; Leiber, A.; Bühring, H.J.; Orlikowsky, T.W. Infection-induced Bystander-Apoptosis of Monocytes Is TNF-alpha-mediated. PLoS ONE 2013, 8, e53589. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.H.; Liu, H.S.; Liu, S.J.; He, T.F.; Piao, X.S. Effects of dietary fiber sources during late gestation and lactation on sow performance, milk quality, and intestinal health in piglets. J. Anim. Sci. 2019, 97, 4922–4933. [Google Scholar] [CrossRef] [PubMed]
- Dirajlal-Fargo, S.; El-Kamari, V.; Weiner, L.; Shan, L.P.; Sattar, A.; Kulkarni, M.; Funderburg, N.; Nazzinda, R.; Karungi, C.; Kityo, C.; et al. Altered Intestinal Permeability and Fungal Translocation in Ugandan Children With Human Immunodeficiency Virus. Clin. Infect. Dis. 2020, 70, 2413–2422. [Google Scholar] [CrossRef]
- de Punder, K.; Pruimboom, L. Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Front. Immunol. 2015, 6, 223. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.Z.; Chen, H.Q.; Liang, Y.; Xia, Y.; Yang, Y.Z.; Yang, J.; Zhang, J.D.; Wang, S.H.; Liu, J.; Qin, H.L. Combined probiotic bacteria promotes intestinal epithelial barrier function in interleukin-10-gene-deficient mice. World J. Gastroenterol. 2014, 20, 4636–4647. [Google Scholar] [CrossRef] [PubMed]
- Emal, D.; Rampanelli, E.; Stroo, I.; Butter, L.M.; Teske, G.J.; Claessen, N.; Stokman, G.; Florquin, S.; Leemans, J.C.; Dessing, M.C. Depletion of Gut Microbiota Protects against Renal Ischemia-Reperfusion Injury. J. Am. Soc. Nephrol. 2017, 28, 1450–1461. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.J.; Yin, B.M.; Li, W.; Chai, T.J.; Liang, W.W.; Huang, Y.; Tan, X.M.; Zheng, P.; Wu, J.; Li, Y.F.; et al. Age-related changes in microbial composition and function in cynomolgus macaques. Aging-Us 2019, 11, 12080–12096. [Google Scholar] [CrossRef]
- Hsu, Y.J.; Huang, W.C.; Lin, J.S.; Chen, Y.M.; Ho, S.T.; Huang, C.C.; Tung, Y.T. Kefir Supplementation Modifies Gut Microbiota Composition, Reduces Physical Fatigue, and Improves Exercise Performance in Mice. Nutrients 2018, 10, 862. [Google Scholar] [CrossRef]
- Pereira, G.V.; Abdel-Hamid, A.M.; Dutta, S.; D’Alessandro-Gabazza, C.N.; Wefers, D.; Farris, J.A.; Bajaj, S.; Wawrzak, Z.; Atomi, H.; Mackie, R.I.; et al. Degradation of complex arabinoxylans by human colonic Bacteroidetes. Nat. Commun. 2021, 12, 459. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, Y.; Xiao, K.P.; Jiang, F.; Wang, H.C.; Tang, D.Z.; Liu, D.; Liu, B.; Liu, Y.S.; He, X.; et al. The chicken gut metagenome and the modulatory effects of plant-derived benzylisoquinoline alkaloids. Microbiome 2018, 6, 211. [Google Scholar] [CrossRef] [PubMed]
- Mach, N.; Fuster-Botella, D. Endurance exercise and gut microbiota: A review. J. Sport Health Sci. 2017, 6, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Vilchez-Vargas, R.; Skieceviciene, J.; Lehr, K.; Varkalaite, G.; Thon, C.; Urba, M.; Morkunas, E.; Kucinskas, L.; Bauraite, K.; Schanze, D.; et al. Gut microbial similarity in twins is driven by shared environment and aging. Ebiomedicine 2022, 79, 104011. [Google Scholar] [CrossRef] [PubMed]
- Carrothers, J.M.; York, M.A.; Brooker, S.L.; Lackey, K.A.; Williams, J.E.; Shafii, B.; Price, W.J.; Settles, M.L.; McGuire, M.A.; McGuire, M.K. Fecal Microbial Community Structure Is Stable over Time and Related to Variation in Macronutrient and Micronutrient Intakes in Lactating Women. J. Nutr. 2015, 145, 2379–2388. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.S.; Geng, S.J.; Li, Y.; Cheng, S.S.; Fu, X.F.; Yue, X.J.; Han, X.Y. Exogenous Fecal Microbiota Transplantation from Local Adult Pigs to Crossbred Newborn Piglets. Front. Microbiol. 2018, 8, 2663. [Google Scholar] [CrossRef] [PubMed]
- Kalkan, H.; Pagano, E.; Paris, D.; Panza, E.; Cuozzo, M.; Moriello, C.; Piscitelli, F.; Abolghasemi, A.; Gazzerro, E.; Silvestri, C.; et al. Targeting gut dysbiosis against inflammation and impaired autophagy in Duchenne muscular dystrophy. Embo Mol. Med. 2023, 15, e16225. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.G.; Fusieger, A.; Miliao, G.L.; Martins, E.; Drider, D.; Nero, L.A.; de Carvalho, A.F. Weissella: An Emerging Bacterium with Promising Health Benefits. Probiotics Antimicrob. Proteins 2021, 13, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.C.; Zhang, Y.; Ling, T.; Zhao, C.J.; Li, Y.R.; Geng, M.; Gai, S.L.; Qi, W.; Luo, X.G.; Chen, L.H.; et al. Chitosan Oligosaccharides Alleviate Colitis by Regulating Intestinal Microbiota and PPARγ/SIRT1-Mediated NF-κB Pathway. Mar. Drugs 2022, 20, 96. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.T.; Wang, Z.X.; Cao, J.; Dong, Y.L.; Chen, Y.X. Gut microbiota-derived metabolites mediate the neuroprotective effect of melatonin in cognitive impairment induced by sleep deprivation. Microbiome 2023, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.H.; Yang, W.L.; Su, C.Y.; Lu, X.H.; He, L.; Zhang, A.H. Relationship between gut microbiota and vascular calcification in hemodialysis patients. Ren. Fail. 2023, 45, 2148538. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Usyk, M.; Sollecito, C.C.; Qiu, Y.P.; Williams-Nguyen, J.; Hua, S.M.; Gradissimo, A.; Wang, T.; Xue, X.N.; Kurland, I.J.; et al. Altered Gut Microbiota and Host Metabolite Profiles in Women With Human Immunodeficiency Virus. Clin. Infect. Dis. 2020, 71, 2345–2353. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Fujita, Y.; Ren, Q.; Ma, M.; Dong, C.; Hashimoto, K. Bifidobacterium in the gut microbiota confer resilience to chronic social defeat stress in mice. Sci. Rep. 2017, 7, srep45942. [Google Scholar] [CrossRef] [PubMed]
- Susanto, M.; Dunning, J.; Chew, R. Pantoea abscess mimicking sarcoma in a HTLV-1-infected Indigenous Australian man: Case report and literature review. Clin. Case Rep. 2023, 11, e7351. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; McDowell, A.; Kim, E.K.; Seo, H.; Lee, W.H.; Moon, C.M.; Kym, S.M.; Lee, D.H.; Park, Y.S.; Jee, Y.K.; et al. Development of a colorectal cancer diagnostic model and dietary risk assessment through gut microbiome analysis. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yin, J.; Xu, K.; Han, H.; Liu, Z.M.; Wang, C.Y.; Li, T.J.; Yin, Y.L. Protein Level and Infantile Diarrhea in a Postweaning Piglet Model. Mediat. Inflamm. 2020, 2020, 1937387. [Google Scholar] [CrossRef] [PubMed]
- Maraki, S.; Pleyritaki, A.; Kofteridis, D.; Scoulica, E.; Eskitzis, A.; Gikas, A.; Panagiotakis, S.H. Bicuspid aortic valve endocarditis caused by Gemella sanguinis: Case report and literature review. J. Infect. Public Health 2019, 12, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Carmody, L.A.; Zhao, J.; Schloss, P.D.; Petrosino, J.F.; Murray, S.; Young, V.B.; Li, J.Z.; LiPuma, J.J. Changes in cystic fibrosis airway microbiota at pulmonary exacerbation. Ann. Am. Thorac. Soc. 2013, 10, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Vincent, C.; Miller, M.A.; Edens, T.J.; Mehrotra, S.; Dewar, K.; Manges, A.R. Bloom and bust: Intestinal microbiota dynamics in response to hospital exposures and Clostridium difficile colonization or infection. Microbiome 2016, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.; Rud, T.; Karch, A.; Pieper, D.H.; Vital, M. Pathogenic functions of host microbiota. Microbiome 2018, 6, 174. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Xu, T.Q.; Sun, J.Y.; Shi, J.R.; Li, F.L.; Yin, Y.Q.; Wang, Z.Q.; Liu, Y. Nicotinamide Mononucleotide Ameliorates Sleep Deprivation-Induced Gut Microbiota Dysbiosis and Restores Colonization Resistance against Intestinal Infections. Adv. Sci. 2023, 10, e2207170. [Google Scholar] [CrossRef] [PubMed]
- Jaye, K.; Li, C.G.; Chang, D.; Bhuyan, D.J. The role of key gut microbial metabolites in the development and treatment of cancer. Gut Microbes 2022, 14, 2038865. [Google Scholar] [CrossRef] [PubMed]




| Items | Content |
|---|---|
| Ingredient amount (%) | |
| Extruded corn | 49.05 |
| Soybean meal | 3.50 |
| Extruded soybean | 12.50 |
| Yeast culture bio-yeasture | 2.00 |
| Fish meal | 2.00 |
| Mung bean pulp protein powder | 1.25 |
| Flour | 20.00 |
| Soybean phospholipid powder | 1.25 |
| Beer yeast powder | 0.50 |
| Whey powder | 1.25 |
| Limestone | 0.40 |
| Glucose | 1.25 |
| Salt | 0.35 |
| Choline chloride | 0.40 |
| Mildew preventive | 0.15 |
| Vitamin 1 mix | 3.00 |
| Mineral 2 mix | 1.15 |
| Total | 100 |
| Nutrition level (calculated value 2) | |
| Digestible energy (Mcal/kg) | 3.49 |
| Crude protein (%) | 16.85 |
| Calcium (%) | 0.58 |
| Total phosphorus (%) | 0.47 |
| L-lysine (%) | 0.96 |
| L-methionine (%) | 0.39 |
| L-methionine + L-cysteine, (%) | 0.61 |
| L-threonine (%) | 0.65 |
| L-tryptophane (%) | 0.19 |
| Items | CON 1 | BS 1 | SEM 2 | p-Value |
|---|---|---|---|---|
| Initial weight (kg) | 8.63 | 8.66 | 0.10 | 0.817 |
| Final weight (kg) | 17.51 | 17.94 | 0.69 | 0.565 |
| ADG 1 (g/d) | 316.99 | 331.48 | 23.68 | 0.574 |
| ADFI 1 (g/d) | 473.50 | 467.70 | 18.05 | 0.764 |
| FCR 1 | 1.50 | 1.42 | 0.07 | 0.287 |
| Items | CON 1 | BS 1 | SEM 2 | p-Value |
|---|---|---|---|---|
| UN 1, mmol/L | 4.71 | 3.35 | 0.85 | 0.167 |
| CREA 1, mmol/L | 77.62 | 69.57 | 8.58 | 0.380 |
| GLU 1, mmol/L | 6.36 | 5.55 | 0.58 | 0.202 |
| AST 1, IU/L | 88.98 | 103.30 | 10.85 | 0.263 |
| ALT 1, IU/L | 100.07 | 108.50 | 12.91 | 0.528 |
| ALP 1, IU/L | 382.40 | 300.80 | 56.61 | 0.180 |
| TP 1, g/L | 48.84 | 46.40 | 1.79 | 0.205 |
| ALB 1, g/L | 25.88 | 24.57 | 1.65 | 0.444 |
| GLB 1, g/L | 22.96 | 21.84 | 0.75 | 0.169 |
| TG 1, mmol/L | 0.82 | 0.62 | 0.09 | 0.114 |
| TCHO 1, mmol/L | 2.72 a | 2.10 b | 0.62 | <0.001 |
| HDL 1; mmol/L | 1.11 a | 0.77 b | 0.34 | 0.001 |
| LDL 1; mmol/L | 1.27 | 1.20 | 0.12 | 0.563 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Liu, X.; Liu, H.; Tang, J.; He, W.; Xu, T.; Cheng, B.; Shi, B.; Han, J. Bacillus siamensis Improves the Immune Status and Intestinal Health of Weaned Piglets by Improving Their Intestinal Microbiota. Microorganisms 2024, 12, 1012. https://doi.org/10.3390/microorganisms12051012
Liu H, Liu X, Liu H, Tang J, He W, Xu T, Cheng B, Shi B, Han J. Bacillus siamensis Improves the Immune Status and Intestinal Health of Weaned Piglets by Improving Their Intestinal Microbiota. Microorganisms. 2024; 12(5):1012. https://doi.org/10.3390/microorganisms12051012
Chicago/Turabian StyleLiu, Huawei, Xinyu Liu, Haiyang Liu, Jiaqi Tang, Wei He, Tianqi Xu, Baojing Cheng, Baoming Shi, and Jianchun Han. 2024. "Bacillus siamensis Improves the Immune Status and Intestinal Health of Weaned Piglets by Improving Their Intestinal Microbiota" Microorganisms 12, no. 5: 1012. https://doi.org/10.3390/microorganisms12051012
APA StyleLiu, H., Liu, X., Liu, H., Tang, J., He, W., Xu, T., Cheng, B., Shi, B., & Han, J. (2024). Bacillus siamensis Improves the Immune Status and Intestinal Health of Weaned Piglets by Improving Their Intestinal Microbiota. Microorganisms, 12(5), 1012. https://doi.org/10.3390/microorganisms12051012





