Humidification during Invasive and Non-Invasive Ventilation: A Starting Tool Kit for Correct Setting
Abstract
:1. Introduction
2. Physical Aspects
2.1. The Humidity
2.2. Humidification Target Values in Clinical Practice
3. Technical Aspects
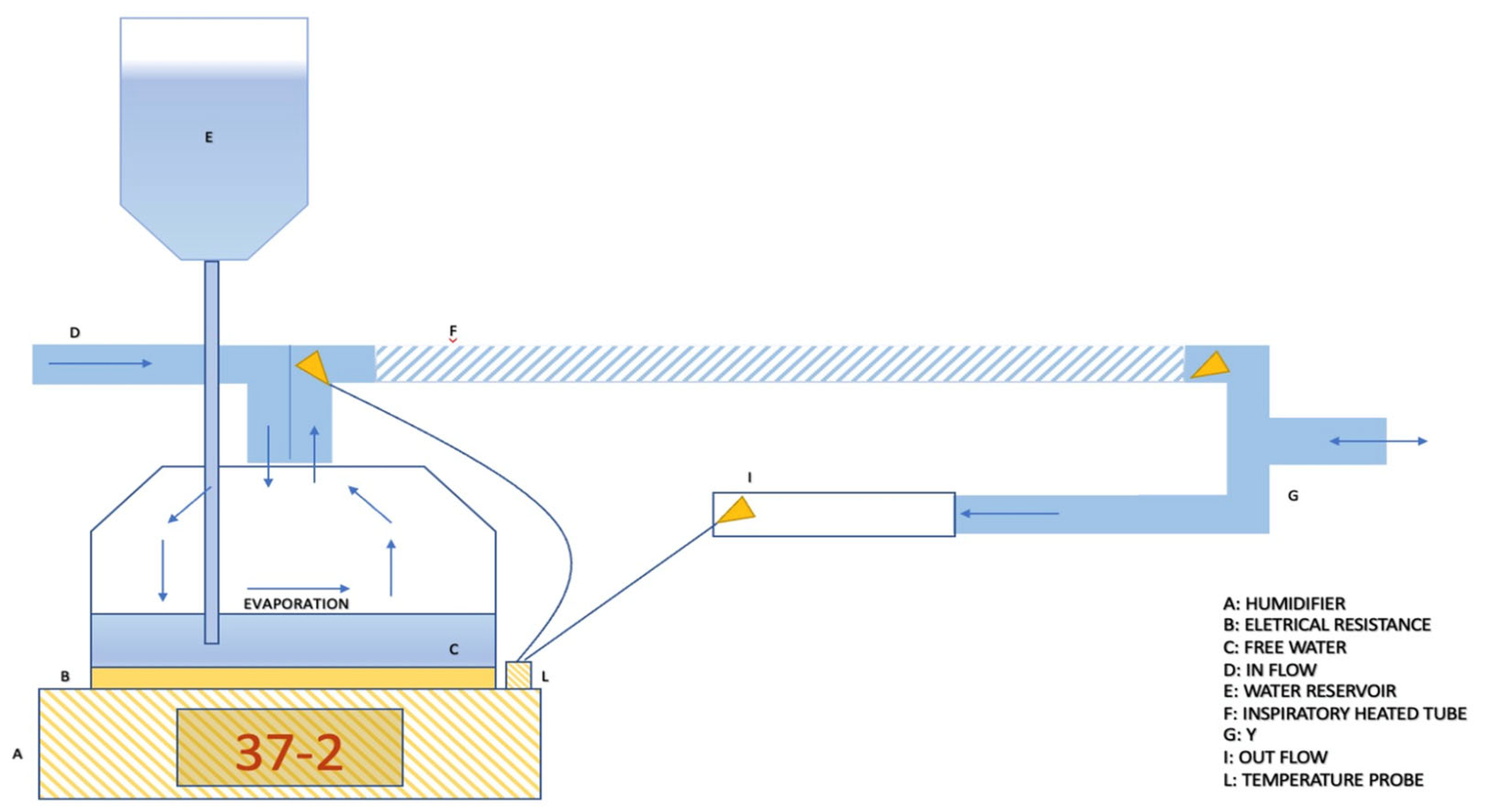
3.1. Passive Humidification
3.2. Active Humidification
- Bubble-through humidifiers: gases pass through a heated water reservoir where they are humidified through bubbling.
- Passover humidifiers: gases are humidified by passing through heated cells equipped with permeable membranes or water-free surfaces.
- Counter flow humidifiers: water is heated outside the system and then flows within the ventilatory circuit, counter to the direction of gases, providing humidification.
- Inline vaporizer humidifiers: gases are humidified through a process of direct water vaporization inside the ventilatory circuit.
Passover Humidifier
- Invasive mode and non-invasive mode. This parameter allows for the adjustment of the working temperature range and gas absolute humidity, depending on whether the upper airways are bypassed or not. As a reference, depending on the humidifier models, the temperature level allowed in the invasive setting typically ranges from 33 °C to 39 °C, while for the non-invasive setting, it ranges between 28 °C and 37 °C [41].
- Patient temperature. This parameter allows for setting the desired gas temperature at the circuit “Y” point, which is the bifurcation between the inspiratory and expiratory branches [42].
- Temperature gradient. With the presence of two temperature sensors, the inspiratory heated branch tube enables the setting of a temperature difference between the patient’s temperature and the evaporation cell temperature. Temperature gradients typically range between −3 and +3 °C. Consequently, this parameter indirectly allows the setting of the temperature of the water in the heating cell, where a higher cell temperature corresponds to increased water evaporation (absolute humidity) (Figure 5) [42].
4. Settings
4.1. Best Setting for Invasive Ventilation
- Set invasive mode.
- Set patient temperature to 37 °C (±2): this setting allows an adequate pre-alveolar physiologic gas temperature to be reached with 44 mg/L of absolute humidity and 100% relative humidity [45].
- Gradient settings:
- A. Zero Gradient: the goal is to achieve a balanced humidity relationship. Although theoretically maintaining a constant humidity, it is necessary to be cautious of suboptimal tube performance and room temperature influence. This setting is recommended when the inspiratory branch has condensation collectors.
- B. Negative gradient (−1 or −2): this setting minimizes condensation risk, albeit with a slightly lower humidification level, aligning with ventilator-associated pneumonia prevention. This setting is recommended when the inspiratory branch lacks condensation collectors [45].
4.2. Best Setting for Non-Invasive Ventilation
- Set non-invasive mode.
- Gradient setting: zero gradient. This setting ensures an optimal balance between provided humidity and the humidity reaching the patient. Given the reduced humidity production at 28 °C, this setting prevents condensation in the tube or in the ventilatory device.
- Special consideration during High Flow Nasal Cannula (HFNC) therapy:
- A.
- During high flow nasal cannula therapy, it is necessary to elevate the humidifier temperature due to the direct high flow in the patient’s upper airways.
- B.
5. Clinical Evidence and Future Directions
5.1. Clinical Evidence
5.2. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al Ashry, H.S.; Modrykamien, A.M. Humidification during mechanical ventilation in the adult patient. BioMed Res. Int. 2014, 2014, 715434. [Google Scholar] [CrossRef]
- Kacmarek, R.M. The mechanical ventilator: Past, present, and future. Respir. Care 2011, 56, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.L.; Park, G.R. Humidification of inspired gases during mechanical ventilation. Minerva Anestesiol. 2012, 78, 496–502. [Google Scholar] [PubMed]
- Doctor, T.N.; Foster, J.P.; Stewart, A.; Tan, K.; Todd, D.A.; McGrory, L. Heated and humidified inspired gas through heated humidifiers in comparison to non-heated and non-humidified gas in hospitalised neonates receiving respiratory support. Cochrane Database Syst. Rev. 2017, 2017, CD012549. [Google Scholar] [CrossRef]
- Vasconcelos Pereira, A.; Simões, A.V.; Rego, L.; Pereira, J.G. New technologies in airway management: A review. Medicine 2022, 101, e32084. [Google Scholar] [CrossRef]
- Branson, R.D. Secretion management in the mechanically ventilated patient. Respir. Care 2007, 52, 1328. [Google Scholar]
- Martin, C.; Papazian, L.; Perrin, G.; Saux, P.; Gouin, F. Preservation of humidity and heat of respiratory gases in patients with a minute ventilation greater than 10 L/min. Crit Care Med. 1994, 22, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.Y.; Acosta, E.; Policova, Z.; Cox, P.N.; Hair, M.L.; Neumann, A.W. Effect of humidity on the stability of lung surfactant films adsorbed at air–water interfaces. Biochim Biophys Acta. 2006, 1758, 1609–1620. [Google Scholar] [CrossRef]
- D’Amato, M.; Molino, A.; Calabrese, G.; Cecchi, L.; Annesi-Maesano, I.; D’Amato, G. The impact of cold on the respiratory tract and its consequences to respiratory health. Clin. Transl. Allergy 2018, 8, 20. [Google Scholar] [CrossRef]
- Stein, S.W.; Thiel, C.G. The History of Therapeutic Aerosols: A Chronological Review. J. Aerosol. Med. Pulm. Drug Deliv. 2017, 30, 20–41. [Google Scholar] [CrossRef]
- Kanda, A.; Kobayashi, Y.; Asako, M.; Tomoda, K.; Kawauchi, H.; Iwai, H. Regulation of Interaction between the Upper and Lower Airways in United Airway Disease. Med. Sci. 2019, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- American Association for Respiratory Care; Restrepo, R.D.; Walsh, B.K. Humidification during invasive and noninvasive mechanical ventilation: 2012. Respir. Care 2012, 57, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Lucchini, A.; Valsecchi, D.; Elli, S.; Doni, V.; Corsaro, P.; Tundo, P.; Re, R.; Foti, G.; Manici, M. The comfort of patients ventilated with the Helmet Bundle. Assist. Inferm. Ric. 2010, 29, 174–183. [Google Scholar] [PubMed]
- Lucchini, A.; Giani, M.; Minotti, D.; Elli, S.; Bambi, S. Helmet CPAP bundle: A narrative review of practical aspects and nursing interventions to improve patient’s comfort. Intensive Crit. Care Nurs. 2023, 74, 103335. [Google Scholar] [CrossRef] [PubMed]
- Ambrosetti, L.; Giani, M.; Rezoagli, E.; Fiorillo, C.; Vitale, D.; Giacchè, D.; Ravasio, G.; Fumagalli, R.; Foti, G.; Lucchini, A. Gas Humidification during Helmet Continuonus Positive Airway Pressure. Dimes Crit. Care Nurs. 2024, 43, 21–27. [Google Scholar] [CrossRef]
- Plotnikow, G.A.; Villalba, D.; Gogniat, E.; Quiroga, C.; Calvo, E.P.; Scapellato, J.L. Performance of Different Active Humidification Systems in High-Flow Oxygen Therapy. Respir. Care 2020, 65, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Antonio Matìas Esquinas, Hospital Morales Meseguer, Murcia Spain. Humidification in the Intensive Care Unit: The Essentials; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- McCullough, L.; Arora, S. Diagnosis and treatment of hypothermia. Am. Fam. Physician 2004, 70, 2325–2332. [Google Scholar] [PubMed]
- Plotnikow, G.A.; Accoce, M.; Navarro, E.; Tiribelli, N. Humidification and heating of inhaled gas in patients with artificial airway. A narrative review. Rev. Bras. Ter. Intensiva 2018, 30, 86–97. [Google Scholar] [CrossRef]
- Schulze, A. Respiratory gas conditioning and humidification. Clin. Perinatol. 2007, 34, 19–33. [Google Scholar] [CrossRef]
- E Holland, A.; Denehy, L.; Buchan, C.A.; Wilson, J.W. Efficacy of a heated passover humidifier during noninvasive ventilation: A bench study. Respir. Care 2007, 52, 38–44. [Google Scholar]
- Randerath, W.; Meier, J.; Genger, H.; Domanski, U.; Ruhle, K.-H. Efficiency of cold passover and heated humidification under continuous positive airway pressure. Eur. Respir. J. 2002, 20, 183–186. [Google Scholar] [CrossRef]
- Popat, B.; Jones, A.T. Invasive and non-invasive mechanical ventilation. Medicine 2012, 40, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Hardavella, G.; Karampinis, I.; Frille, A.; Sreter, K.; Rousalova, I. Oxygen devices and delivery systems. Breathe 2019, 15, E108–E116. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.; Gillies, D.; Todd, D.A.; Lockwood, C. Heated humidification versus heat and moisture exchangers for ventilated adults and children. Anesth Analg. 2010, 111, 1072. [Google Scholar] [CrossRef] [PubMed]
- Gillies, D.; Todd, D.A.; Foster, J.P.; Batuwitage, B.T. Heat and moisture exchangers versus heated humidifiers for mechanically ventilated adults and children. Cochrane Database Syst Rev. 2017, 2018, CD004711. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.; Chiumello, D.; Sutherasan, Y.; Ball, L.; Esquinas, A.M.; Pelosi, P.; Servillo, G. Heat and moisture exchangers (HMEs) and heated humidifiers (HHs) in adult critically ill patients: A systematic review, meta-analysis and meta-regression of randomized controlled trials. Crit. Care 2017, 21, 123. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yuan, W.; Gao, F.; Guo, B. A review of membrane-based air dehumidification. Indoor Built Environ. 2015, 24, 11–26. [Google Scholar] [CrossRef]
- Schumann, B.W.; Gattinoni, H.S.; Koller, T.; Wisliceny, B. In vitro evaluation of humidifiers with respect to their hygienic properties. Anaesthesia 2000, 55, 358–362. [Google Scholar]
- Jones, P.G.; Kamona, S.; Doran, O.; Sawtell, F. Randomized controlled trial of humidified high-flow nasal oxygen for acute respiratory distress in the emergency department: The HOT-ER study. Respir. Care 2011, 56, 1018–1024. [Google Scholar] [CrossRef]
- Fraser, J.F.; Spooner, A.J. High-flow nasal cannula oxygen therapy in adults: Physiological benefits, indication, clinical benefits, and adverse effects. Can. J. Anesth. 2016, 63, 1359–1366. [Google Scholar]
- Nishimura, M. High-flow nasal cannula oxygen therapy in adults. J. Bras. Pneumol. 2016, 42, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Zha, S.S.; He, Z.F.; Guan, L.L.; Niu, J.Y.; Huang, Q.Y.; Chen, R.C. Clinical research progress in non-invasive positive pressure ventilation in 2023. Zhonghua Jie He He Hu Xi Za Zhi 2024, 47, 146–151. (In Chinese) [Google Scholar] [CrossRef]
- Chatburn, R.L. Fundamentals of mechanical ventilation: A short course on the theory and application of mechanical ventilators. Respir. Care 2013, 58, 133–140. [Google Scholar]
- Hess, D.R. Humidification during mechanical ventilation: Updated review. Respir. Care 2014, 59, 782–788. [Google Scholar]
- Martin, C.; Perrin, G.; Gevaudan, M.-J.; Saux, P.; Gouin, F. Heat and moisture exchangers and vaporizing humidifiers in the intensive care unit. Chest 1990, 97, 144–149. [Google Scholar] [CrossRef]
- Christiansen, S.; Renzing, K.; Hirche, H.; Reidemeister, J.C. Measurement of the humidity of inspired air in ventilated patients with various humidifer systems. Anasthesiol. Intensivmed. Notfallmed. Schmerzther. 1998, 33, 300–305. [Google Scholar] [CrossRef]
- Branson, R.D. Humidification of respired gases during mechanical ventilation: Mechanical considerations. Respir. Care Clin. N. Am. 2006, 12, 253–261. [Google Scholar] [PubMed]
- Chatburn, R.L.; Primiano, F.P. A rational basis for humidity therapy. Respir. Care 2002, 47, 1276–1293. [Google Scholar]
- Hess, D.R. Humidification of inspired gases in the intensive care unit. Respir. Care 2000, 45, 652–660. [Google Scholar]
- Hess, D.R. Humidification of Respiratory Gases in Mechanical Ventilation. Respir. Care 2017, 62, 890–897. [Google Scholar]
- Branson, R.D. Humidification in the intensive care unit. Respir. Care 2000, 45, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, A.R. Heat and moisture exchangers and breathing system filters: Their use in anaesthesia and intensive care. Part 2—Practical use, including problems, and their use with paediatric patients. Anaesthesia 2011, 66, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Chikata, Y.; Imanaka, H.; Onishi, Y.; Nishimura, M. Effects of flow pattern and humidity on water condensation in breathing circuits for ICU ventilators. J. Anesth. 2008, 22, 39–45. [Google Scholar]
- Rello, J.; Lorente, C.; Diaz, E.; Bodi, M.; Boque, C.; Sandiumenge, A.; Santamaria, J.M. Incidence, etiology, and outcome of nosocomial pneumonia in ICU patients requiring percutaneous tracheotomy for mechanical ventilation. Chest 2003, 124, 2239–2243. [Google Scholar] [CrossRef] [PubMed]
- Chiumello, D.; Gattinoni, L.; Pelosi, P. Conditioning of inspired gases in mechanically ventilated patients. In Yearbook of Intensive Care and Emergency 2002; Vincent, J.L., Ed.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 275–286. [Google Scholar]
- Badiger, S.; John, M.; Fearnley, R.A.; Ahmad, I. Optimizing the humidification and heating of inspired gases during invasive mechanical ventilation. Curr. Opin. Anaesthesiol. 2019, 32, 173–179. [Google Scholar]
- Hess, D.R. Humidification options in mechanical ventilation. Respir. Care 2017, 62, 764–777. [Google Scholar]
- Mauri, T.; Alban, L.; Turrini, C.; Cambiaghi, B.; Carlesso, E.; Taccone, P.; Bottino, N.; Lissoni, A.; Spadaro, S.; Volta, C.A.; et al. Optimum support by high-flow nasal cannula in acute hypoxemic respiratory failure: Effects of increasing flow rates. Intensive Care Med. 2017, 43, 1453–1463. [Google Scholar] [CrossRef]
- Parke, R.L.; Eccleston, M.L.; McGuinness, S.P. The effects of flow on airway pressure during nasal high-flow oxygen therapy. Respir. Care 2011, 56, 1151–1155. [Google Scholar] [CrossRef]
- Mauri, T.; Galazzi, A.; Binda, F.; Masciopinto, L.; Corcione, N.; Carlesso, E.; Lazzeri, M.; Spinelli, E.; Tubiolo, D.; Volta, C.A.; et al. Impact of flow and temperature on patient comfort during respiratory support by high-flow nasal cannula. Crit. Care 2018, 22, 120. [Google Scholar] [CrossRef]
- Cook, D.; de Jonghe, B.; Brochard, L.; Brun-Buisson, C. Influence of airway management on ventilator-associated pneumonia: Evidence from randomized trials. J. Am. Med. Assoc. 1998, 279, 781–787. [Google Scholar] [CrossRef]
- Chatburn, R.L. Mucus production and clearance in the intubated patient. Respir. Care 2007, 52, 418–429. [Google Scholar]
- Branson, R.D. Humidification for patients with artificial airways. Respir. Care 1999, 44, 630–641. [Google Scholar]
- Kallet, R.H. Adjunct therapies during mechanical ventilation: Airway humidification, monitoring, aerosol therapy, and heliox therapy. Respir. Care 2013, 58, 1053–1073. [Google Scholar] [CrossRef] [PubMed]
- Lellouche, F.; Taillé, S.; Maggiore, S.M.; Qader, S.; L’Her, E.; Deye, N.; Brochard, L. Influence of ambient and ventilator output temperatures on performance of heated-wire humidifiers. Am. J. Respir. Crit. Care Med. 2004, 170, 1073–1079. [Google Scholar] [CrossRef]
- Nava, S.; Bruschi, C.; Rubini, F.; Palo, A.; Iotti, G.; Braschi, A. Respiratory response and inspiratory effort during pressure support ventilation in COPD patients. Intensive Care Med. 1995, 21, 871–879. [Google Scholar] [CrossRef]
- Marini, J.J.; Jaber, S. Dynamic predictors of VILI risk: Beyond the driving pressure. Intensive Care Med. 2016, 42, 1597–1600. [Google Scholar] [CrossRef]
- Iotti, G.A.; Olivei, M.C.; Palo, A.; Galbusera, C.; Veronesi, R.; Comelli, A.; Brunner, J.X.; Braschi, A. Unfavorable mechanical effects of heat and moisture exchangers in ventilated patients. Intensive Care Med. 1997, 23, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.R.; Kallstrom, T.J.; Mottram, C.D.; Myers, T.R.; Sorenson, H.M.; Vines, D.L.; American Association for Respiratory Care. Care of the ventilator circuit and its relation to ventilator-associated pneumonia. Respir. Care 2003, 48, 869–879. [Google Scholar]
- Cerpa, F.; Cáceres, D.; Romero-Dapueto, C.; Giugliano-Jaramillo, C.; Pérez, R.; Budini, H.; Hidalgo, V.; Gutiérrez, T.; Molina, J.; Keymer, J. Humidification on Ventilated Patients: Heated Humidifications or Heat and Moisture Exchangers? Open Respir. Med. J. 2015, 9, 104–111. [Google Scholar] [CrossRef]
- Kneyber, M.C.; Markhorst, D.G. Humidification during mechanical ventilation in the adult patient. J. Intensive Care Med. 2015, 30, 187–199. [Google Scholar]
- Kallet, R.H. Humidification during mechanical ventilation: Impact on airway management. Respir. Care 2015, 60, 1475–1490. [Google Scholar]
- Martin, C.; Papazian, L.; Perrin, G.; Saux, P.; Gouin, F. NCPAP and high-flow nasal oxygen therapy: Current practices in the management of acute hypoxemic respiratory failure. Réanimation 2011, 20, 300–308. [Google Scholar]
- Muñoz, A.C.; Siles, A.G.; Hernández, S.G.; Siles, G. Role of heated humidifiers and heat and moisture exchangers in the prevention of ventilator-associated pneumonia. Enfermería Intensiva 2012, 23, 107–115. [Google Scholar]
- Ricard, J.-D.; Markowicz, P.; Djedaini, K.; Mier, L.; Coste, F.; Dreyfuss, D. Bedside evaluation of efficient airway humidification during mechanical ventilation of the critically ill. Chest 1999, 115, 1646–1652. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Re, R.; Lassola, S.; De Rosa, S.; Bellani, G. Humidification during Invasive and Non-Invasive Ventilation: A Starting Tool Kit for Correct Setting. Med. Sci. 2024, 12, 26. https://doi.org/10.3390/medsci12020026
Re R, Lassola S, De Rosa S, Bellani G. Humidification during Invasive and Non-Invasive Ventilation: A Starting Tool Kit for Correct Setting. Medical Sciences. 2024; 12(2):26. https://doi.org/10.3390/medsci12020026
Chicago/Turabian StyleRe, Riccardo, Sergio Lassola, Silvia De Rosa, and Giacomo Bellani. 2024. "Humidification during Invasive and Non-Invasive Ventilation: A Starting Tool Kit for Correct Setting" Medical Sciences 12, no. 2: 26. https://doi.org/10.3390/medsci12020026





