X-ray Diffraction Study of Metallized Polyethylene for Creating Heat Storage Systems
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mourad, A.; Aissa, A.; Said, Z.; Younis, O.; Iqbal, M.; Alazzam, A. Recent advances on the applications of phase change materials for solar collectors, practical limitations, and challenges: A critical review. J. Energy Storage 2022, 49, 104186. [Google Scholar] [CrossRef]
- Deng, Z.; Nian, Y.; Liu, Q.; Cheng, W.L. Numerical analysis of borehole heat exchanger using a single shape-stabilized phase change material in heating and cooling seasons. J. Energy Storage 2023, 70, 107897. [Google Scholar] [CrossRef]
- Lin, S.; Ling, Z.; Li, S.; Cai, C.; Zhang, Z.; Fang, X. Mitigation of lithium-ion battery thermal runaway and inhibition of thermal runaway propagation using inorganic salt hydrate with integrated latent heat and thermochemical storage. Energy 2023, 266, 126481. [Google Scholar] [CrossRef]
- Duquesne, M.; Godin, A.; Palomo del Barrio, E.; Achchaq, F. Crystal growth kinetics of sugar alcohols as phase change materials for thermal energy storage. Energy Procedia 2017, 139, 315–321. [Google Scholar] [CrossRef]
- Shao, X.F.; Yang, S.; Shi, H.Y.; Fan, L.W.; Yuan, Y.P. A comprehensive evaluation on the cycling stability of sugar alcohols for medium-temperature latent heat storage. J. Energy Storage 2023, 64, 107190. [Google Scholar] [CrossRef]
- Cao, L.; Tang, Y.; Fang, G. Preparation and properties of shape-stabilized phase change materials based on fatty acid eutectics and cellulose composites for thermal energy storage. Energy 2015, 80, 98–103. [Google Scholar] [CrossRef]
- Nosova, N.; Roiter, Y.; Samaryk, V.; Varvarenko, S.; Stetsyshyn, Y.; Minko, S.; Stamm, M.; Voronov, S. Polypropylene surface peroxidation with heterofunctional polyperoxides. Macromol. Symp. 2004, 210, 339–348. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, R. N-octanoic acid-based phase change composites synthesized by absorption polymerization for efficient thermal energy storage. J. Energy Storage 2023, 64, 107169. [Google Scholar] [CrossRef]
- Zauner, C.; Hengstberger, F.; Etzel, M.; Lager, D.; Hofmann, R.; Walter, H. Experimental characterization and simulation of a fin-tube latent heat storage using high density polyethylene as PCM. Appl. Energy 2016, 179, 237–246. [Google Scholar] [CrossRef]
- Malovanyy, M.S.; Synelnikov, S.D.; Nagurskiy, O.A.; Soloviy, K.M.; Tymchuk, I.S. Utilization of sorted secondary PET waste-raw materials in the context of sustainable development of the modern city. In IOP Conference Series: Materials Science and Engineering, Innovative Technology in Architecture and Design (ITAD 2020), Kharkiv, Ukraine, 21–22 May 2020; IOP Publishing: Bristol, UK, 2020; p. 012067. [Google Scholar] [CrossRef]
- Nagurskyy, O.; Krylova, H.; Vasiichuk, V.; Kachan, S.; Dziurakh, Y.; Nahursky, A.; Paraniak, N. Safety Usage of Encapsulated Mineral Fertilizers Based on Polymeric Waste. Ecol. Eng. Environ. Technol. 2022, 23, 156–161. [Google Scholar] [CrossRef]
- Nagurskyy, O.; Krylova, H.; Vasiichuk, V.; Kachan, S.; Nahursky, A.; Paraniak, N.; Sabadash, V.; Malovanyy, M. Utilization of Household Plastic Waste in Technologies with Final Biodegradation. Ecol. Eng. Environ. Technol. 2022, 23, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Moravskyi, V.; Kucherenko, A.; Kuznetsova, M.; Dulebova, L.; Spišák, E.; Majerníková, J. Utilization of Polypropylene in the Production of Metal-Filled Polymer Composites: Development and Characteristics. Materials 2020, 13, 2856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, B.G.; Zheng, X.B.; Singh, P.K.; Ayed, H.; Mouldi, A.; Mohamed, A.; Mehrez, S. Investigation on effect of connection angle of “L” shaped fin on charging and discharging process of PCM in vertical enclosure. Case Stud. Therm. Eng. 2022, 33, 101908. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Z.; Li, P.; Qin, H.; Heng, W. Multi-parameter heat transfer analysis of molten PCM in an inclined enclosure. Appl. Therm. Eng. 2022, 208, 118209. [Google Scholar] [CrossRef]
- Chaichan, M.T.; Kazem, H.A.; Al-Waeli, A.; Sopian, K. Controlling the melting and solidification points temperature of PCMs on the performance and economic return of the water-cooled photovoltaic thermal system. Sol. Energy 2021, 224, 1344–1357. [Google Scholar] [CrossRef]
- Teja, P.; Gugulothu, S.K.; Reddy, P.; Deepanraj, B.; Sundar, L.S. Computational investigation of the influencing parameters on the melting of phase change material in a square enclosure with built in fin and Al2O3 nanoparticles. Appl. Therm. Eng. 2023, 232, 120942. [Google Scholar] [CrossRef]
- Zheng, S.; Li, S.; Li, M.; Dai, R.; Wei, M.; Tian, R. Experimental and numerical investigation of a rectangular finned-tube latent heat storage unit for Carnot battery. J. Energy Storage 2023, 71, 108092. [Google Scholar] [CrossRef]
- Wang, Z.; Diao, Y.; Zhao, Y.; Chen, C.; Wang, T.; Liang, L. Experimental and numerical studies of thermal transport in a latent heat storage unit with a plate fin and a flat heat pipe. Energy 2023, 275, 127464. [Google Scholar] [CrossRef]
- Sharma, A.; Pitchumani, R.; Chauhan, R. Melting and solidification performance investigation of latent heat storage unit designs for low-temperature solar thermal applications. J. Energy Storage 2023, 66, 107323. [Google Scholar] [CrossRef]
- Diao, Y.; Wang, Z.; Zhao, Y.; Wang, Z.; Chen, C.; Zhang, D. Heat transfer enhancement of a multichannel flat tube-copper foam latent heat storage unit. Appl. Therm. Eng. 2023, 229, 120559. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, S.; Mazhar, A.R.; Wang, J.; Li, Y. Phase change materials embedded with tuned porous media to alleviate overcharging problem of cascaded latent heat storage system for building heating. Energy Build. 2023, 281, 112746. [Google Scholar] [CrossRef]
- Zhu, R.; Jing, D. Numerical study on thermal and melting performances of a horizontal latent heat storage unit with branched tree-like convergent fins. J. Energy Storage 2023, 62, 106889. [Google Scholar] [CrossRef]
- Sharma, A.; Ding, C.; Kim, S.C.; Chauhan, R. Investigation and optimization of solidification performance of concentration tube type latent heat storage unit with herringbone wavy fin designs. Appl. Therm. Eng. 2023, 222, 119924. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, Z.; Qu, Z.; Xu, H.; Yang, Q. Numerical energy and exergy evaluation for a multiple-layer latent heat storage unit enhanced with nanoparticles under different seasons. J. Clean. Prod. 2023, 417, 138098. [Google Scholar] [CrossRef]
- Lauermannová, A.M.; Lojka, M.; Záleská, M.; Pavlíková, M.; Pivák, A.; Pavlík, Z.; Růžička, K.; Jankovský, O. Magnesium oxychloride cement-based composites for latent heat storage: The effect of the introduction of multi-walled carbon nanotubes. J. Build. Eng. 2023, 72, 106604. [Google Scholar] [CrossRef]
- Yu, D.; Qiu, Y.; Zhang, X. Role of nano-copper in discharging performance of latent heat storage unit. Int. Commun. Heat Mass Transf. 2023, 144, 106748. [Google Scholar] [CrossRef]
- Moravskyi, V.; Kucherenko, A.; Kuznetsova, M.; Dulebova, L.; Spišák, E. Obtainment and characterization of metal-coated polyethylene granules as a basis for the development of heat storage systems. Polymers 2022, 14, 218. [Google Scholar] [CrossRef] [PubMed]
- White, J.L.; Choi, D.D. Polyolefins: Processing, Structure Development, and Properties; Carl Hanser Publishers: Munich, Germany, 2005; p. 271. [Google Scholar]
- Rabiej, M. Application of the particle swarm optimization method for the analysis of wide-angle X-ray diffraction curves of semicrystalline polymers. J. Appl. Crystallogr. 2017, 50, 221–230. [Google Scholar] [CrossRef]
- Rabiej, M. Application of a multicriterial optimization to the resolution of X-ray difraction curves of semicrystalline polymers. Polimery 2017, 62, 821–833. [Google Scholar] [CrossRef]
- Kucherenko, A.; Nikitchuk, O.; Dulebova, L.; Moravskyi, V. Activation of polyethylene granules by finely dispersed zinc. Chem. Technol. Appl. Subst. 2021, 4, 191–197. [Google Scholar] [CrossRef]
- Kucherenko, A.; Nikitchuk, O.; Baran, N.; Dulebova, L.; Kuznetsova, M.; Moravskyi, V. Characteristics of metallized polymeric raw materials. In Proceedings of the 11 International Conference on “Nanomaterials: Applications & Properties” (NAP-2021), Odesa, Ukraine, 5–11 September 2021. TM10. [Google Scholar] [CrossRef]
- Moravskyi, V.; Kucherenko, A.; Kuznetsova, M.; Dulebova, L.; Garbacz, T. Formation of copper coating on polymer granules by chemical method. In Proceedings of the 12 International Conference on “Nanomaterials: Applications & Properties” (NAP-2022), Krakow, Poland, 11–16 September 2022. MTFC13. [Google Scholar] [CrossRef]
- Moravskyi, V.; Kucherenko, A.; Kuznetsova, M.; Dziaman, I.; Grytsenko, O.; Dulebova, L. Studying the effect of concentration factors on the process of chemical metallization of powdered polyvinylchloride. East. -Eur. J. Enterp. Technol. 2018, 3, 40–47. [Google Scholar] [CrossRef]
- Tadayyon, G.; Zebarjad, S.M.; Sajjadi, S.A. Effect of both nano-size alumina particles and severe deformation on polyethylene crystallinity index. J. Thermoplast. Compos. Mater. 2011, 25, 479–490. [Google Scholar] [CrossRef]
- WAXSFIT—Analysis of X-RAY Diffraction Curves; Version 1.0; Informer Technologies, Inc.: Los Angeles, CA, USA, 2020.
- Rabiej, M.; Rabiej, S. Application of the artificial neural network for identification of polymers based on their X-ray diffraction curves. Comput. Mater. Sci. 2021, 186, 110042. [Google Scholar] [CrossRef]

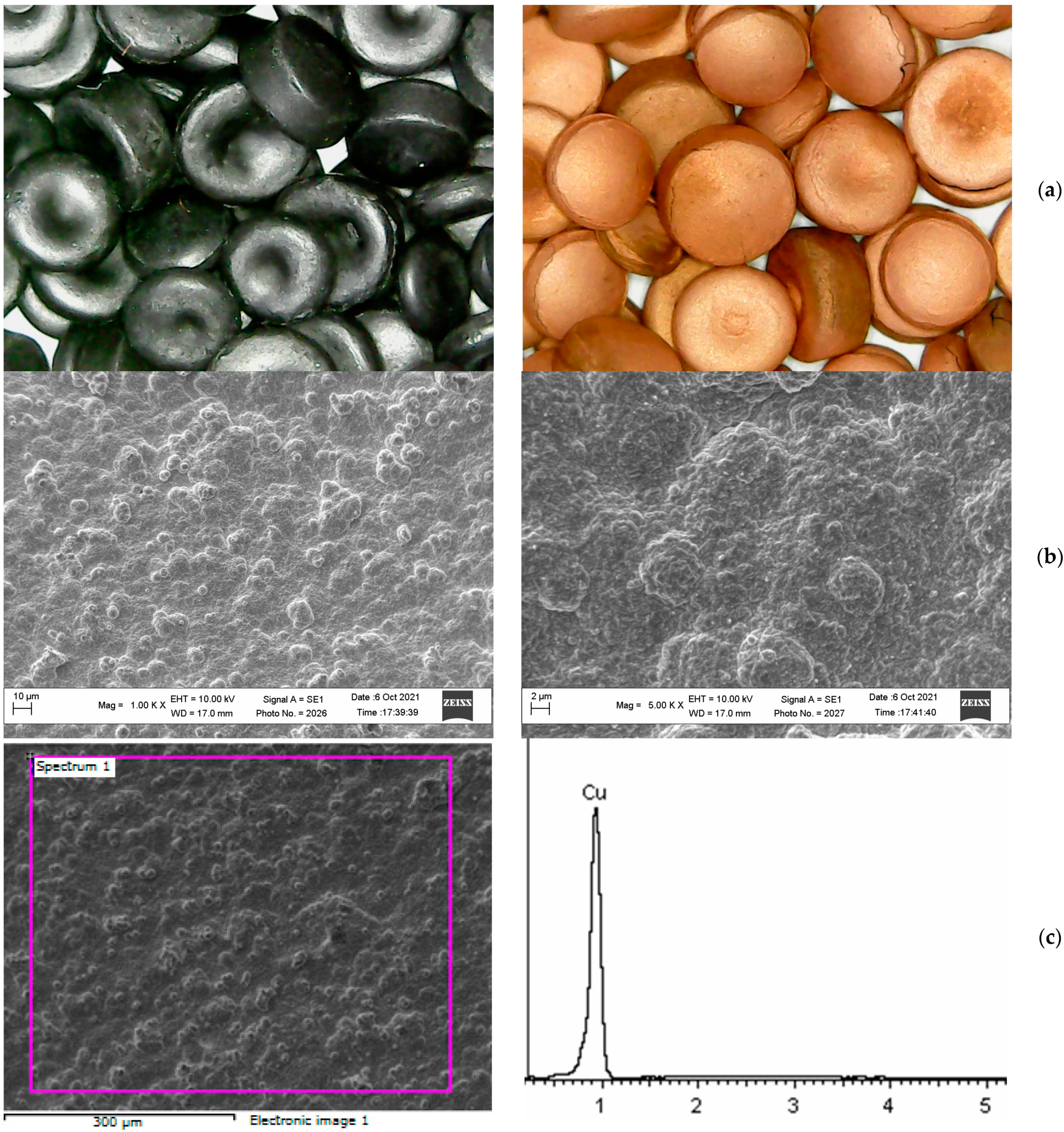



| Element | Mass% | Atomic% |
|---|---|---|
| Cu L | 100.00 | 100.00 |
| Sum | 100.00 |
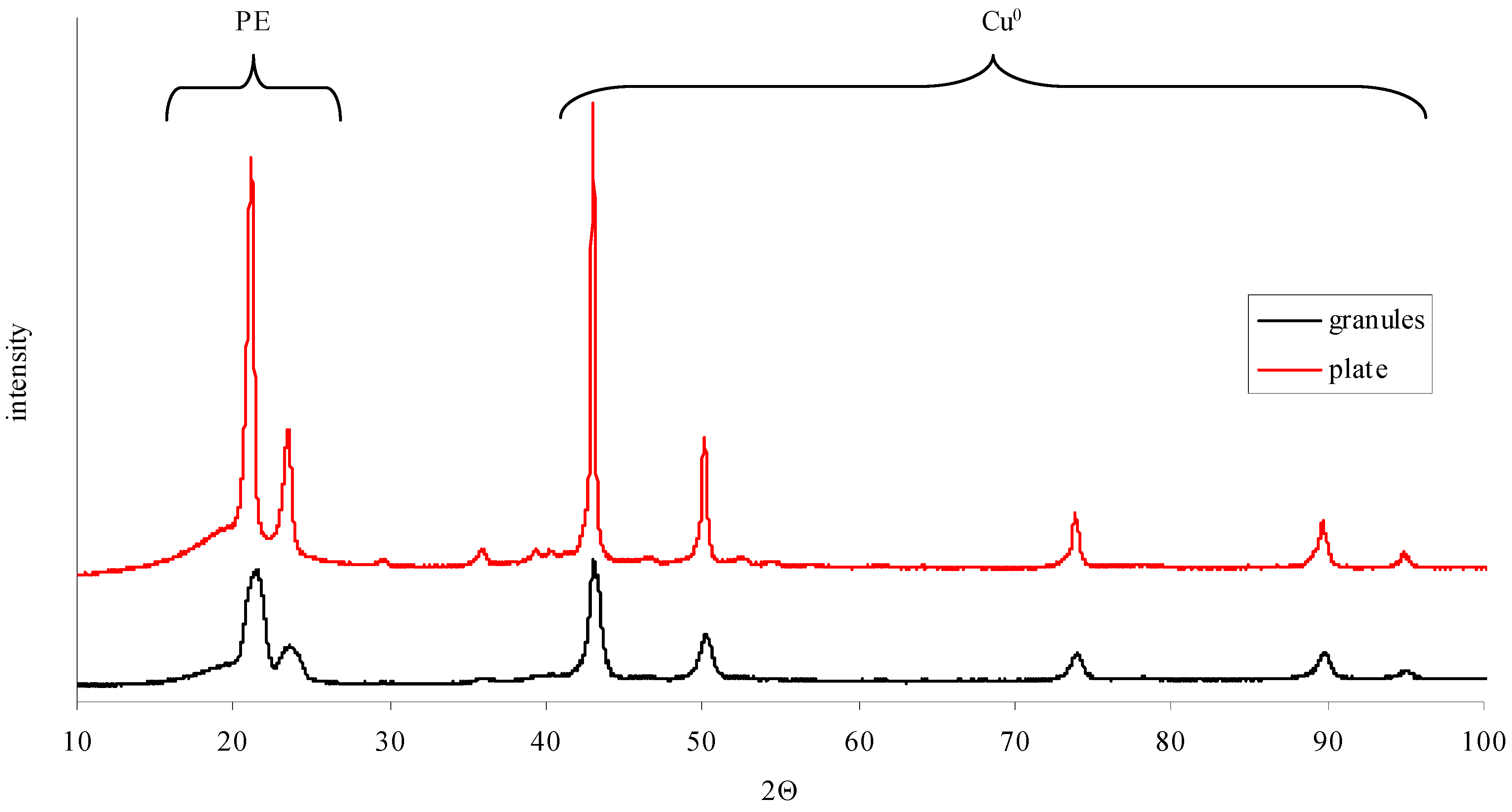
| Sample | Degree of Crystallinity | Position | Height | Width | Size L | Distance d |
|---|---|---|---|---|---|---|
| Initial PE granules | 0.479 | 21.1 | 323.2 | 0.9 | 101.7 | 4.2 |
| 23.4 | 101.1 | 1.0 | 89.2 | 3.8 | ||
| 19.9 * | 65.8 | 5.4 | - | - | ||
| Plate made of initial PE granules | 0.547 | 20.9 | 549.1 | 0.5 | 173.7 | 4.2 |
| 23.3 | 141.6 | 0.7 | 133.0 | 3.8 | ||
| 20.1 * | 75.3 | 4.1 | - | - |
| Sample | Degree of Crystallinity | Position | Height | Width | Size L | Distance d |
|---|---|---|---|---|---|---|
| Metallized PE granules | 0.388 | 21.4 | 191.2 | 1.2 | 73.0 | 4.2 |
| 23.7 | 60.1 | 1.2 | 73.7 | 3.8 | ||
| 19.7 * | 53.0 | 8.9 | - | - | ||
| Plate made of metallized PE granules | 0.501 | 21.1 | 386.4 | 0.5 | 187.5 | 4.2 |
| 23.5 | 128.0 | 0.6 | 151.3 | 3.8 | ||
| 20.1 * | 54.0 | 5.3 | - | - |
| Number of Heating and Cooling Cycles | Degree of Crystallinity | Position | Height | Width | Size L | Distance d | QPE kJ/kg |
|---|---|---|---|---|---|---|---|
| 1 | 0.501 | 21.1 | 386.4 | 0.5 | 187.5 | 4.2 | 996.2 |
| 23.5 | 128.0 | 0.6 | 151.3 | 3.8 | |||
| 20.1 * | 54.0 | 5.3 | - | - | |||
| 100 | 0.494 | 21.2 | 476.6 | 0.5 | 196.6 | 4.2 | 990.5 |
| 23.5 | 117.0 | 0.6 | 124.4 | 3.8 | |||
| 20.0 * | 65.2 | 4.8 | - | - | |||
| 200 | 0.459 | 20.9 | 399.4 | 0.5 | 178.0 | 4.3 | 962.1 |
| 23.2 | 112.5 | 0.7 | 133.8 | 3.8 | |||
| 19.9 * | 75.1 | 4.7 | - | - | |||
| 300 | 0.405 | 21.2 | 336.3 | 0.5 | 179.6 | 4.2 | 918.3 |
| 23.6 | 61.8 | 0.7 | 132.2 | 3.8 | |||
| 20.1 * | 72.4 | 5.6 | - | - | |||
| 400 | 0.284 | 21.1 | 187.2 | 0.6 | 156.6 | 4.2 | 820.0 |
| 23.5 | 50.8 | 0.8 | 118.3 | 3.8 | |||
| 20.0 * | 70.7 | 5.7 | - | - | |||
| 500 | 0.268 | 21.0 | 182.4 | 0.6 | 160.3 | 4.2 | 807.0 |
| 23.4 | 38.0 | 0.8 | 108.7 | 3.8 | |||
| 19.8 * | 73.6 | 6.0 | - | - | |||
| 600 | 0.248 | 21.0 | 175.2 | 0.5 | 165.2 | 4.2 | 790.8 |
| 23.4 | 47.0 | 0.7 | 124.5 | 3.8 | |||
| 19.8 * | 73.9 | 6.5 | - | - | |||
| 700 | 0.224 | 21.0 | 172.8 | 0.6 | 161.6 | 4.2 | 771.3 |
| 23.4 | 40.1 | 0.8 | 113.7 | 3.8 | |||
| 19.8 * | 71.1 | 6,2 | - | - | |||
| 800 | 0.209 | 21.0 | 164.3 | 0.6 | 162.7 | 4.2 | 759.1 |
| 23.3 | 36.0 | 0.8 | 117.7 | 3.8 | |||
| 19.8 * | 75.4 | 6.9 | - | - | |||
| 900 | 0.196 | 21.0 | 153.7 | 0.5 | 163.7 | 4.2 | 748.6 |
| 23.3 | 44.7 | 0.7 | 122.2 | 3.8 | |||
| 19.7 * | 72.6 | 6.8 | - | - | |||
| 1000 | 0.192 | 20.9 | 145.4 | 0.6 | 159.7 | 4.2 | 745.3 |
| 23.3 | 50.2 | 0.8 | 107.7 | 3.8 | |||
| 19.3 * | 62.8 | 6.7 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moravskyi, V.; Kucherenko, A.; Kuznetsova, M.; Dulebova, L.; Spišák, E. X-ray Diffraction Study of Metallized Polyethylene for Creating Heat Storage Systems. Appl. Sci. 2024, 14, 4183. https://doi.org/10.3390/app14104183
Moravskyi V, Kucherenko A, Kuznetsova M, Dulebova L, Spišák E. X-ray Diffraction Study of Metallized Polyethylene for Creating Heat Storage Systems. Applied Sciences. 2024; 14(10):4183. https://doi.org/10.3390/app14104183
Chicago/Turabian StyleMoravskyi, Volodymyr, Anastasiia Kucherenko, Marta Kuznetsova, Ludmila Dulebova, and Emil Spišák. 2024. "X-ray Diffraction Study of Metallized Polyethylene for Creating Heat Storage Systems" Applied Sciences 14, no. 10: 4183. https://doi.org/10.3390/app14104183





